Abstract
Recent studies suggest that epithelial–mesenchymal transition (EMT) correlates with cancer metastasis. In addition, there is growing evidence of the association of EMT with cancer stem cells (CSCs). Recently, we showed that the T-box transcription factor BRACHYURY could be a strong regulator of EMT and the CSC phenotype, which were effectively suppressed by a BRACHYURY knockdown in an adenoid cystic carcinoma cell line. In this study, we further tested whether BRACHYURY is a regulator of cancer stemness by means of forced expression of BRACHYURY in oral cancer cell lines. BRACHYURY, SOX2, or both were stably transfected into oral carcinoma cell lines. We analysed these transfectants with respect to self-renewal phenotypes using a sphere-formation assay, and we assessed the expression levels of EMT markers and stem cell markers using real-time reverse transcription-polymerase chain reaction (RT-PCR). Cell migration and invasiveness in vitro were evaluated using a wound healing assay and a tumour cell dissemination assay, respectively. Forced expression of BRACHYURY or SOX2 slightly increased expression of EMT and stem cell markers and the self-renewal phenotype. The expression levels, however, were much lower compared to those of cancer stem cell-like cells. Forced co-expression of BRACHYURY and SOX2 strongly upregulated EMT and stem cell markers and the self-renewal phenotype. Cell migration and invasiveness in vitro were also remarkably enhanced. These synergistic effects increased expression levels of FIBRONECTIN, SNAIL, SLUG, ZEB1, and TGF-β2. In particular, the effects on FIBRONECTIN and TGF-β2 were significant. We found that BRACHYURY and SOX2 synergistically promote cancer stemness in oral cancer cells. This finding points to the importance of gene or protein networks associated with BRACHYURY and SOX2 in the development and maintenance of the CSC phenotype.
1. Introduction
Metastasis is a multistep cascade involving the migration of tumour cells from their site of origin, evasion from host defence systems, subsequent seeding at distant organs, and growth of secondary tumours. Recent studies have revealed that a specific change in the cancer cell phenotype, epithelial–mesenchymal transition (EMT), is involved in the metastasis mechanism. Accumulating evidence supports the importance of EMT in cancer progression and metastasis in several types of cancer: head and neck, breast, lung, and prostate cancer [1,2,3,4]. We also previously reported clinical evidence that EMT strongly correlates with poor prognosis in patients with oral squamous cell carcinoma [5]. In order to spawn macroscopic metastases, which are often facilitated by EMT, disseminated cancer cells presumably need a self-renewal ability similar to that exhibited by stem cells. From this point of view, the EMT process, which allows for migration and metastasis of cancer cells, would be also the key of a self-renewal capability to disseminated cancer cells. Actually, the accumulated evidences of relationship between EMT and cancer stem cells (CSCs) was reported recently [3,6,7,8,9,10,11]. The EMT or CSC program appears to be controlled by genes normally expressed in early embryos, including TWIST, SNAIL, SLUG, ZEB1, and SOX2 [12,13,14,15]. These genes encode the transcription factors which enables the tumour cells to migrate and invade like mesenchymal cells. CSCs were proved to be resistant to chemotherapy and radiotherapy [16,17], and CSCs should be the real target for the future new concept of cancer therapies [18,19].
The T-box transcription factor BRACHYURY has been reported as a key gene for mesoderm formation during embryonic stage [20]. Recently, BRACHYURY is also recognised to induce EMT in human carcinoma cell lines [21]. In our previous study, we demonstrated that a CSC-like cell line (adenoid cystic carcinoma), ACCS-M GFP undergoes EMT [22], and that small hairpin RNA (shRNA) silencing of BRACHYURY downregulates EMT (and stem cell markers) in that cell line and leads to a loss of CSC-like and EMT characteristics of this cell line [11]. Tumourigenesis and metastasis of ACCS-M GFP in vivo were inhibited completely by BRACHYURY knockdown and partially by knockdown of SOX2, the conventional CSC regulator gene [11]. These data suggest that EMT may be directly linked to CSCs, and that BRACHYURY controls EMT and the CSC phenotype (cancer stemness). We also confirmed that expression of Brachyury protein strongly correlates with EMT and poor prognosis in oral cancer patients [5].
In this regard, BRACHYURY silencing could effectively control cancer stemness and offer a new concept for the development of cancer treatments. In this study, we used forced expression of BRACHYURY and SOX2 in oral cancer cell lines to confirm that BRACHYURY and SOX2 are regulators of the CSC phenotype.
2. Results
2.1. Forced Expression of BRACHYURY Does Not Promote Self-Renewal Capacity, But a BRACHYURYy Knockdown Suppresses the Self-Renewal Capacity in Oral Cancer Cell Lines
We previously reported successful isolation of highly metastatic and tumourigenic CSC-like cells—the ACCS-M GFP cell line—from non-metastatic (0% incidence) and low tumourigenic (22.2% incidence) parental adenoid cystic carcinoma ACCS GFP cells using in vivo selection [22]. We also showed that BRACHYURY knockdown completely inhibits CSC and EMT phenotypes of the ACCS-M GFP cells. These findings support a crucial role of BRACHYURY in the regulation of cancer stemness in adenoid cystic carcinoma cell lines [11]. Therefore, in the present work, we tested the hypothesis that Brachyury can promote CSC features in adenoid cystic carcinoma cells. For this purpose, we established stable Brachyury transfectants of ACCS-GFP and ACCS-Bra cell lines. We also confirmed the effect of a BRACHYURY knockdown on ACCS-M GFP cells by means of Brachyury shRNA (Figure 1A). Forced expression of BRACHYURY slightly increased (2.0-fold) sphere formation (the number of spheres) in the primary sphere assay in comparison to parental ACCS-GFP cells (P = 0.0983, ANOVA), but had no effect in the secondary sphere assay (P = 0.125, ANOVA). In contrast, the BRACHYURY knockdown on ACCS-M GFP cells remarkably inhibited sphere formation in both the primary (P = 0.0001, ANOVA) and the secondary assay (P = 0.0001, ANOVA), with respect to both the diameter and the number of spheres (Figure 1).
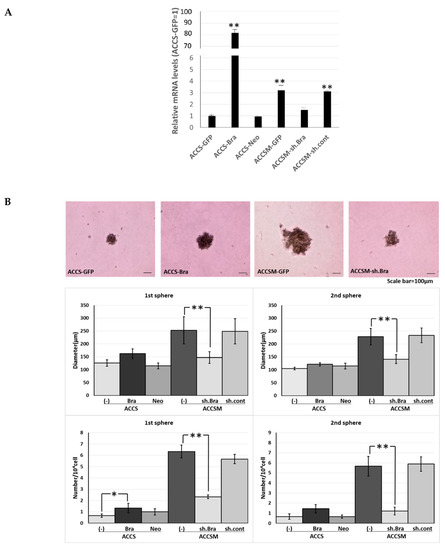
Figure 1.
Effects of BRACHYURY transfection on the sphere-forming ability of ACCS (adenoid cystic carcinoma) cells. Brachyury mRNA expression levels of the indicated ACCS (adenoid cystic carcinoma) cells and in derivative clones [ACCS-Brachyury (Bra), ACCS-Neomycin (Neo), ACCSM-sh.Brachyury (sh.Bra), and ACCSM-sh.control (sh.cont)] were quantified using real-time RT-PCR. mRNA level was compared with that in ACCS-GFP cells (parental cell line), and the data are shown in arbitrary units as relative mRNA levels (ACCS-GFP = 1.0) (A). ACCS cells were cultured at a density of 5 × 104 cells/mL in a serum-free medium for floating culture for 10 days (primary spheres). Primary spheres (day 10) were dissociated into individual cells and further cultured at a density of 104 cells/mL for 10 days. The spheres were observed under a phase contrast microscope ((B), top panel). Sphere diameters were measured ((B), middle panel), and numbers (diameter > 100 μm) ((B), bottom panel) were counted. Sphere numbers were standardised as a sphere number per 104 cells originally seeded ((B), bottom panel). The experiments were performed in triplicate, and the data were calculated as mean ± SD. Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
We next analysed the effect of Brachyury on the sphere-forming ability using another type of oral carcinoma cell, squamous cell carcinoma (SCC). For this purpose, we used SCCTF cell lines with Green Fluorescence Protein (GFP) expression (TF-GFP), BRACHYURY stable transfectant TF-GFP cells (TF-Bra), and control vector-transfected and selected by Neomycin (TF-Neo). Forced expression of Brachyury slightly induced (1.8-fold) sphere formation (the number of spheres) in the primary sphere assay compared to the parental TF-GFP cells, but had no effect in the secondary sphere assay (Figure 2). This finding is in agreement with the results obtained using the adenoid cystic carcinoma cells described above. We confirmed the cell proliferation rate on cell culture dish did not change in both cell lines (data not shown).
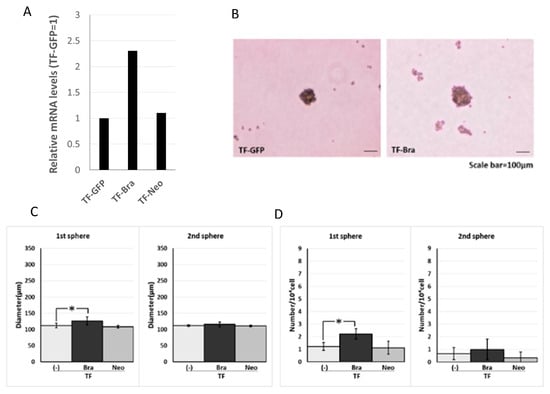
Figure 2.
Effects of BRACHYURY transfection on the sphere-forming ability of TF (squamous cell carcinoma) cells. Brachyury mRNA expression levels of the indicated TF-GFP cells and in derivative clones [TF-Brachyury (Bra), TF-Neomycin (Neo)] were quantified using real-time RT-PCR. mRNA level was compared with that in TF-GFP cells (parental cell line), and the data are shown in arbitrary units as relative mRNA levels (ACCS-GFP = 1.0) (A). Sphere-forming ability of TF-Brachyury (Bra) and TF-Neomycin (Neo) cells were analysed and quantified as described in Figure 1. Image of phase contrast microscope (B). Sphere diameters (C), Sphere numbers (D). The experiments were performed in triplicate, and the data were calculated as mean ± SD. Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
2.2. Forced Expression of BRACHYURY Weakly Induces EMT-Related Markers and Stem Cell Markers in Oral Cancer Cell Lines
We next analysed the effect of BRACHYURY expression on EMT-related markers and stem cell markers in ACCS (Figure 3) and TF (Figure 4) cell lines. EMT and CSC markers were evaluated their expression level using quantitative real-time RT-PCR (Figure 3). Each mRNA level was compared with that in the parental cells, ACCS GFP and TF-GFP cells, and the data are shown as relative mRNA levels (ACCS GFP or TF-GFP levels are set to 1.0 arbitrary unit). We analysed the expression levels of EMT-related genes (Figure 3A and Figure 4A), stem cell markers (Figure 3B and Figure 4B), and differentiation markers (Figure 3C and Figure 4C). In ACCS cells, forced expression of BRACHYURY increased expression of CLAUDIN, OCCULUDIN, VIMENTIN, FIBRONECTIN, SLUG, ZEB1, GSK3β, OCT4, and NANOG and caused a decrease in E-CADHERIN expression. These changes were similar to the changes observed in the CSC-like cell line ACCS-M GFP in all markers, but the changes were smaller in BRACHYURY transfectants (except for CLAUDIN, OCCLUDIN, and NANOG). In contrast, mRNA levels of all markers decreased significantly in the BRACHYURY knockdown cells (ACCS-M shBra) compared to parental ACCS-M GFP cells. In TF (squamous cell carcinoma) cells, forced expression of BRACHYURY showed nearly the same changes in gene expression as in ACCS-M GFP cells. Notably, the increase of vimentin expression as a result of BRACHYURY transfection was significantly higher compared to the other markers that increased in TF-Bra cells.
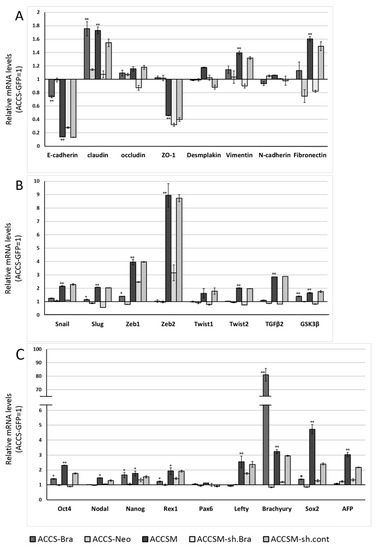
Figure 3.
Effects of BRACHYURY transfection on gene expression related to epithelial–mesenchymal transition (EMT) and the cancer stem cell (CSC) phenotype in ACCS cells. mRNA expression levels of the indicated genes in ACCS cells and in derivative clones were quantified using real-time RT-PCR as described in method section. Each mRNA level was compared with that in ACCS-GFP cells (parental cell line), and the data are shown in arbitrary units as relative mRNA levels (ACCS-GFP = 1.0). The expression levels of EMT-related genes (A,B), stem cell markers, and differentiation markers (C) are shown. The experiments were performed in triplicate, and the data were calculated as mean ± SD. Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
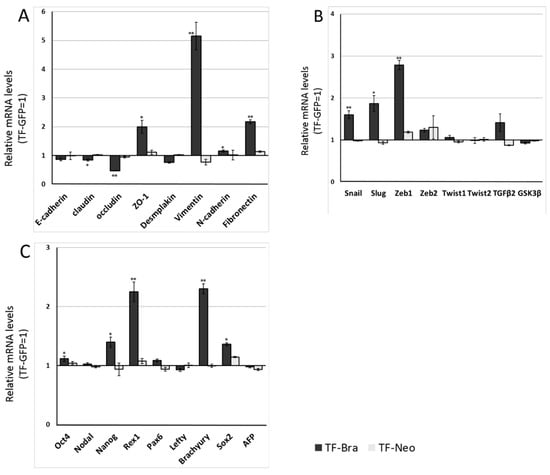
Figure 4.
Effects of BRACHYURY transfection on gene expression related to epithelial–mesenchymal transition (EMT) and cancer stemness in TF cells. The mRNA expression levels of the indicated genes in TF cells and derivative clones were quantified by means of real-time RT-PCR. Each mRNA level was compared with that of TF-GFP cells, and the data are shown in arbitrary units as relative mRNA levels (TF-GFP = 1.0). The expression levels of EMT-related genes (A,B), stem cell markers, and differentiation markers (C) are shown. The experiments were performed in triplicate, and the data were calculated as mean ± SD. Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
2.3. Forced Co-Expression of BRACHYURY and SOX2 Induced the Self-Renewal Phenotype in Oral Cancer Cell Lines
We previously reported that SOX2 knockdown partially inhibits CSC and EMT phenotypes of ACCS-M GFP cells [11]. The data from forced expression and a knockdown of BRACHYURY in oral carcinoma cell lines suggests that Brachyury may need a partner to drive the expression of the CSC phenotype. Therefore, we next analysed the effect of forced co-expression of BRACHYURY and SOX2 on the self-renewal capacity. To this end, we established SOX2 transfectants and BRACHYURY + SOX2 co-expression transfectants of ACCS-GFP and TF-GFP cells: ACCS-Sox2, ACCS-Bra/Sox2, TF-Sox2, and TF-Bra/Sox2. Forced expression of SOX2 enhanced sphere formation (the number of spheres) in the primary sphere assay compared to parental cells ACCS-GFP (9.3-fold) and TF-GFP (2.8-fold), but failed to increase the number of spheres in the secondary sphere assay. In contrast, co-expression of BRACHYURY and SOX2 enhanced sphere formation in both the primary and the secondary sphere assays, with respect to both the diameter and the number of spheres, in ACCS and TF cells. This enhancement reached the level of ACCS-M GFP cells (Figure 5). Forced expression of SOX2 or co-expression of BRACHYURY and SOX2 did not change the cell proliferation rate on cell culture dish (data not shown).
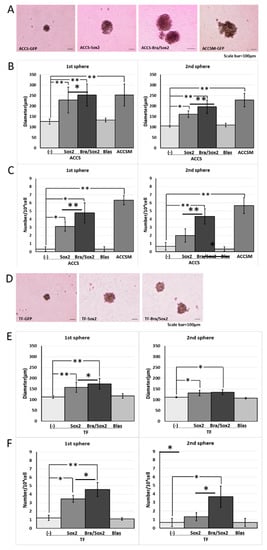
Figure 5.
Effects of BRACHYURY and SOX2 transfection on the sphere-forming ability of ACCS (adenoid cystic carcinoma) and TF (squamous cell carcinoma) cells. ACCS-Sox2, ACCS-Blasticidin (Blas), ACCS-Bra/Sox2, TF-Sox2, TF-Blasticidin (Blas), and TF-Bra/Sox2 cells were cultured, and the sphere-forming ability was quantified as described in the legend of Figure 1. Images of phase contrast microscope (A,D). Sphere diameters (P < 0.0001, ANOVA) (B,E), and Sphere numbers (P < 0.0001, ANOVA) (C,F) were analysed as described in the legend of Figure 1. The experiments were performed in triplicate, and the data were calculated as mean ± SD. Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
2.4. Forced Co-Expression of BRACHYURY and SOX2 Induced EMT-Related Markers and Stem Cell Markers in Oral Cancer Cell Lines
We next analysed the effect of SOX2 and co-expression of BRACHYURY and SOX2 on EMT-related markers and stem cell markers in ACCS (Figure 6A–C) and TF (Figure 6D–F) cell lines in the same way as shown in Figure 3. The expression levels of EMT-related genes (Figure 6A,D), stem cell markers (Figure 6B,E), and differentiation markers (Figure 6C,F) are shown. In ACCS cells, forced expression of SOX2 caused a significant increase in mRNA expression of VIMENTIN, N-CADHERIN, FIBRONECTIN, SNAIL, SLUG, ZEB1, TGF-β2, GSK3β, OCT4, and NANOG and caused a decrease in E-CADHERIN mRNA (Figure 6A–C). In TF cells, forced expression of SOX2 caused a significant increase in mRNA expression of VIMENTIN, N-CADHERIN, FIBRONECTIN, SNAIL, SLUG, ZEB1, OCT4, REX1, and NANOG and decreased E-CADHERIN expression (Figure 6D–F). The pattern of changes was nearly the same in the 2 cell lines. Co-expression of BRACHYURY and SOX2 had synergistic effects on the enhancement of mRNA expression of FIBRONECTIN, SNAIL, SLUG, ZEB1, and TGF-β2 in both cell lines. These synergistic effects on FIBRONECTIN and TGF-β2 were especially strong.
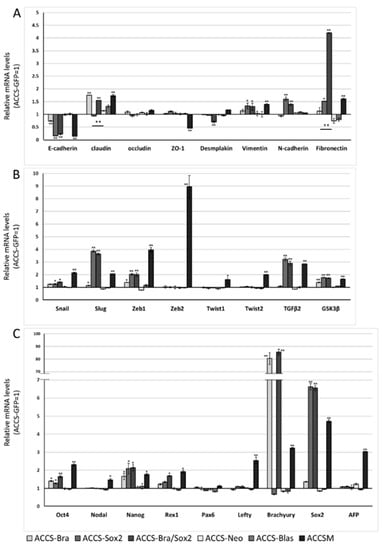
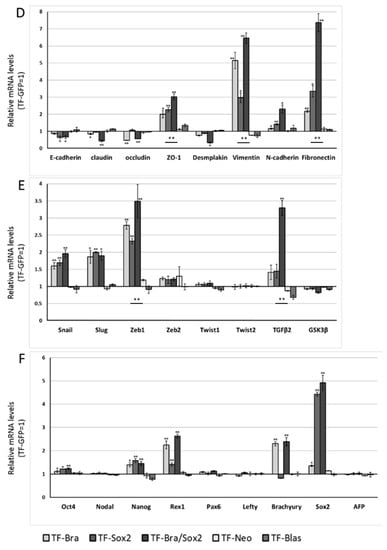
Figure 6.
Effects of BRACHYURY and SOX2 transfection on gene expression related to epithelial–mesenchymal transition (EMT) and cancer stem cell (CSC) phenotype in ACCS and TF cells. The mRNA expression levels of the indicated genes in ACCS (adenoid cystic carcinoma) and TF (squamous cell carcinoma) cells and in derivative clones were quantified using real-time RT-PCR. Each mRNA level was compared with that in ACCS-GFP or TF-GFP cells, and the data are shown in arbitrary units as relative mRNA levels (ACCS-GFP, TF-GFP = 1.0). The expression levels of EMT-related genes (A,B,D,E), stem cell markers, and differentiation markers (C,F) are shown. The experiments were performed in triplicate, and the data were calculated as mean ± SD. Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
2.5. Artificial CSC-Like Cells Express an Invasive Phenotype In Vitro
The above results suggested that co-expression of BRACHYURY and SOX2 induces the CSC phenotype. We then tested whether these artificial CSC-like cells show signs of invasiveness in vitro. The effect of forced expression of BRACHYURY or/and SOX2 on cell migration of oral cancer cells in vitro was analysed using a wound healing assay (Figure 7). Cell migration of ACCS-M GFP cells was approximately 2 times faster than that of ACCS GFP cells. Expression of BRACHYURY or SOX2 increase the cell migration (P < 0.01) but failed to reach the level of ACCS-M.
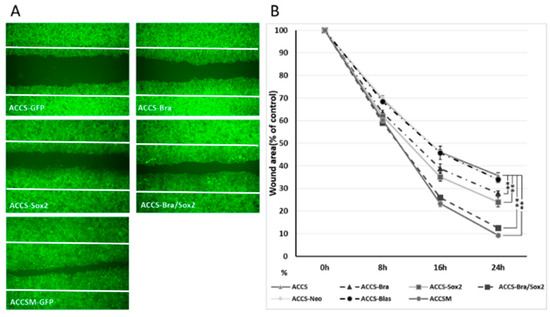
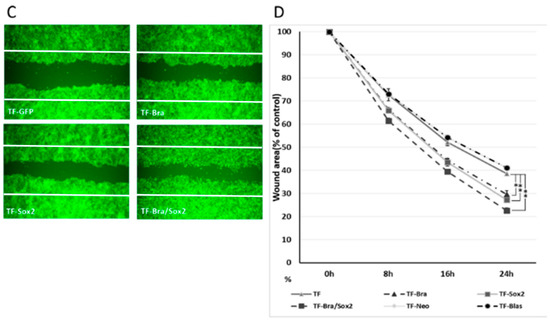
Figure 7.
Effects of BRACHYURY and SOX2 transfection on cell migration of ACCS and TF cells. (A,C) ACCS (adenoid cystic carcinoma) and TF (squamous cell carcinoma) cells and derivative clones were seeded at 3 × 105 cells per well in a 6-well plate and incubated for 24 h so that a confluent monolayer of cells could form. After 24 h, scratch wounds were inflicted on the confluent monolayer of cells (white line) and incubation was continued for a further 24 h. Randomly chosen wound fields were photographed under a fluorescence microscope in the course of 24 h every 8 h. (B,D) The wound areas were evaluated using the following formula: wound area (% of control) = (wound area after the indicated period/initial wound area) × 100. The experiments were performed in triplicate, and the data were calculated as mean ± SD and ANOVA (P < 0.0001). Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
Co-expression of BRACHYURY and SOX2 increased cell migration almost to the level of the CSC-like cells. There was no significance between ACCS-M GFP and ACCS Bra/Sox 2 (P = 0.183) (Figure 7A,B). Cell migration of TF-Bra/Sox2 cells was approximately 3 times faster than that of TF GFP cells (P = 0.001; Figure 7C,D).
We next analysed the effect of forced expression of BRACHYURY or/and SOX2 on cell invasiveness in vitro using a tumour cell dissemination assay that we established previously [23]. In this assay, carcinoma cells invade to artificial stroma (fibroblast embedded collagen gel) are visualised as fluorescent spots migrating from artificial primary cancer nest (cancer cell pellet solidified with collagen gel). Cancer cell invasiveness is evaluated by the number of invasive cells and their distance from the artificial primary cancer nest. As shown in Figure 8A, ACCS-M GFP cells invaded into artificial stroma very aggressive manner. Co-expression of BRACHYURY and SOX2 strongly enhanced the invasiveness of ACCS-GFP cells to the level of the aggressive ACCS-M GFP cells (P = 0.223) (Figure 8A,B). Figure 8B shows the comparison of invasiveness among the ACCS cell lines. Relative invasiveness values (ACCS GFP = 1.0 arbitrary unit) were 3.7 (ACCS-Bra), 4.1 (ACCS-Sox2), 5.0 (ACCS-Bra/Sox2), and 5.6 (ACCS-M GFP). Expression of BRACHYURY or SOX2 increase the cell invasiveness (P < 0.01) but failed to reach the level of ACCS-M.
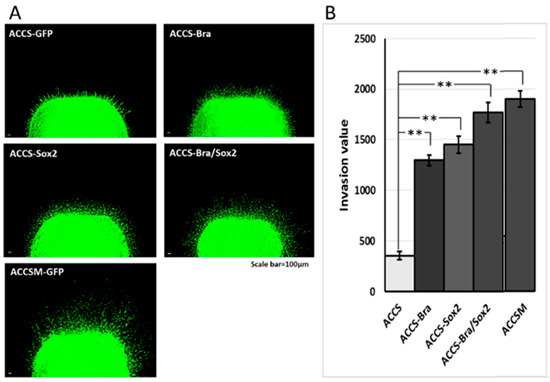
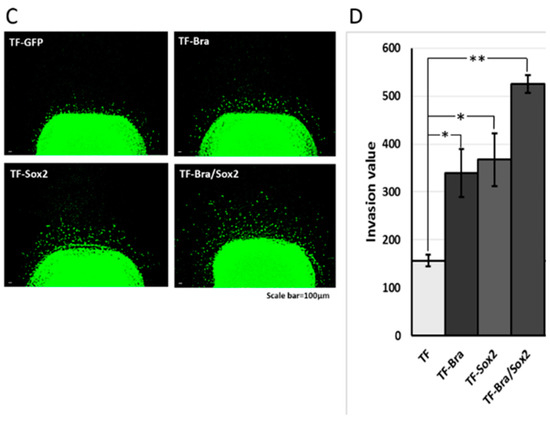
Figure 8.
Effects of BRACHYURY and SOX2 transfection on cell invasiveness of ACCS and TF cells. One million ACCS and TF cells each were pelleted and solidified as described in Materials and Methods. The collagen-embedded tumour cells were embedded in the collagen-embedded fibroblasts and allowed to invade into artificial stroma. After 7 days, tumour dissemination was observed under a fluorescence microscope (A,C). Tumour dissemination from the tumour cell pellet was evaluated in 5 randomly selected standardised rectangular light fields (500 × 100 μm), and the values were summed as described in Materials and Methods (B,D). The experiments were performed in triplicate, and the data were calculated as mean ± SD and ANOVA (P < 0.0001). Statistical significance of differences was analysed using the Student’s t test. * P < 0.05, ** P < 0.01.
Invasiveness of TF-GFP (squamous cell carcinoma) cells was strongly enhanced by co-expression of BRACHYURY and SOX2 (Figure 8C). Figure 8D shows comparison of the invasiveness among the squamous cell carcinoma cell lines. Relative invasiveness values (TF GFP = 1.0) were 2.2 (TF-Bra), 2.3 (TF-Sox2), and 3.3 (TF-Bra/Sox2).
3. Discussion
Metastasis is a most life-threatening event which directly influencing prognosis in cancer progression. Accumulated evidences suggest that cancer invasion and metastasis is strongly regulated by EMT in cancer cells [24,25,26]. Similarly, CSCs have attracted more and more attention as targets for cancer therapy because they have been reported to exhibit chemo- and radio-resistance [16,17,27,28,29]. More recently, the direct link between EMT and CSC phenotype was reported [6,24,30,31], but the regulatory mechanisms behind cancer stemness and EMT are still unclear.
We recently showed that CSC phenotypes, including EMT, could be blocked by knocking down the transcriptional factor BRACHYURY in vitro and in vivo [11]. We also showed downregulation of EMT-related genes, stem cell markers, and loss of tumourigenic and metastatic potential in vivo as a result of knockdown of BRACHYURY in a CSC-like cell line, ACCS-M GFP [11]. This finding suggests that BRACHYURY is a key regulator of the CSC phenotype. Sarkar et al. [32] also reported that a BRACHYURY knockdown via siRNA resulted in downregulation of expression of CD44, CD166, CD133, ALDH1, and NANOG in colon cancer, and they concluded that BRACHYURY participates in establishment of CSC characteristics by inducing NANOG expression. In the present study, we showed that overexpression of BRACHYURY upregulated NANOG expression slightly, but failed to promote self-renewal capacity not only in ACCS GFP (adenoid cystic carcinoma) cells but also in TF GFP, a squamous cell carcinoma cell line. Fernando et al. [21] reported that forced expression of BRACHYURY caused induction of EMT (gain of mesenchymal markers and loss of epithelial markers) and increased in cell migration and invasion in human carcinoma cell line. In this regard, our results completely match their findings. It is thought that NANOG is not absolutely required for self-renewal of embryonic stem cells but is required for pluripotency [33]. In line with this notion, in our present experiments, BRACHYURY overexpression promoted only the EMT phenotype, a type of invasive phenotype. Some authors proposed to classify EMT into 3 subtypes based on biological status with biomarkers [34,35]. Type 1; EMT associated with organ development, Type 2; EMT related to wound healing and regeneration. EMT in cancer progression and metastasis is categorized as type 3 EMT and specific signaling or biomarkers has yet to be determined. TGF-β would be the most responsible factor for induction of EMT by inducing EMT-inducing transcription factors in cancer cells, notably SNAIL, SLUG, ZEB1, TWIST, GOOSECOID, and FOXC2 [36].
SOX2 (SRY Sex Determining Region Y-Box2) is a member of the Sox (SRY-related HMG box) family of transcription factors. SOX2 maintains stemness and pluripotency of normal stem cells by regulating gene transcription. Recent reports revealed the association between SOX2 and EMT [37,38]. In line with these reports, SOX2 overexpression resulted in specific changes in EMT of ACCS-Sox2 cells in the present work. It is also well known that SOX2 promotes metastasis of breast and prostate cancer cells via activation of the Wnt/β-catenin pathway [39]. Sox2 protein binds to the promoter region of β-catenin and regulates its expression. In addition, Sox2 protein regulates the protein expression of downstream elements in Wnt signaling, DKK3, DVL1, and DVL3 [39]. Aberrant activation of Wnt/β-catenin signaling has been described in a variety of cancers, and inhibition of Wnt/β-catenin by DKK-1 resulted in diminution of the self-renewal capacity of a gastric cancer cell line [40]. Overexpression of SOX2 increased the self-renewal capacity in both ACCS GFP and TF GFP cells in the present work. Nonetheless, the self-renewal capacity of ACCS-Sox2 cells was approximately 50% in size and 30% in number compared to ACCS-M GFP cells, even though SOX2 expression was 150% higher in ACCS-Sox2 cells. These data suggest that SOX2 promotes EMT and self-renewal capacity, but it is not a key player in these processes.
Several factors have been reported as indispensable for stem cell maintenance and differentiation. For example, OCT3/4, SOX2, KLF-4, and C-MYC are necessary for expression of the phenotype of induced pluripotent stem cells in human and mouse fibroblasts [41,42]. These factors have been reported to physically or synergistically interact with each other during development [43]. The same kind of protein or gene network may exist in cancer stem cells. Chen et al. reported that silencing of the SOX2 gene reduces the tumourigenic potential of a lung cancer cell line (A549 cells) with downregulation of C-MYC, WNT1, WNT2, and NOTCH1 in mice; these authors uncovered 246 additional target cancer genes of SOX2 [44]. In the present work, we found significant upregulation of TGF-β and fibronectin as a result of simultaneous overexpression of SOX2 and BRACHYURY. This synergistic effect of SOX2 and BRACHYURY in the promotion of the invasive phenotype of CSCs, especially when it comes to TGF-β and FIBRONECTIN expression, could be explained by the same kind of protein or signaling network as mentioned above.
SOX2 activates the Wnt/β-catenin pathway and causes β-catenin activation [39], and the target gene of this activated β-catenin is BRACHYURY [45]. It was shown that BRACHYURY promotes the EMT phenotype and positively relate each other with TGF-β in mesenchymal-like tumour cells [46,47]. BRACHYURY stimulates TGF-β1 expression, and overexpressed TGF-β1 induces SOX2 in turn [48]. SOX2 also positively regulates FIBRONECTIN expression [49], and FIBRONECTIN enhances the EMT phenotype and self-renewal capacity [50]. Therefore, a potentially unlimited autocrine loop is expected to be established among SOX2, BRACHYURY, TGF-β, and FIBRONECTIN. As discussed above, BRACHYURY mainly plays a role in EMT, and SOX2 is involved in EMT and self-renewal. Their synergistic effect, however, may be more crucial because the interaction of the SOX2 and BRACHYURY gene networks activates a vast network of genes/proteins that drives the development of cancer invasiveness. In other words, the closer to the centre of the network (SOX2 and BRACHYURY) a target factor is, the greater the impact of its inactivation on EMT and cancer stemness. This is why silencing of BRACHYURY is observed to be so effective.
In this study, we showed that co-expression of BRACHYURY and SOX2 promotes both EMT and self-renewal phenotypes in ACCS-GFP cells; phenotypes that resemble ACCS-M GFP, the CSC-like cell line from in vivo selection. Nonetheless, the patterns of the expression of some genes (e.g., ZEB2) are still different between ACCS-M GFP cells and ACCS Bra/Sox2. We could not enhance ZEB2 expression by co-expressing BRACHYURY and SOX2. The role of ZEB2 in CSCs is not clear, and further research is needed to identify which factor(s) control ZEB2 expression and its function in cancer stemness.
Our results also revealed that the regulation of CSC related genes by BRACHYURY and SOX2 is different by cancer cell origin (stratified squamous epithelial cells and salivary gland cell). Effect of BRACHYURY and SOX2 expression on EMT and pluripotency related genes varied in degree of changes between cell types. As mentioned above, BRACHYURY and SOX2 were not sufficient for initiation of cancer stem cell property, especially pluripotency related genes. However, it is also true that BRACHYURY is required for tumourigenesis and metastasis, the most important property for cancer stem cell in vivo. Because it has been described in our previous study, tumourigenesis and metastasis of ACCSM cell were prevented completely by silencing of BRACHYURY and partially by silencing of SOX2 in vivo [11]. It was also confirmed that protein expression of Brachyury strongly correlates with EMT and metastasis involvement in oral cancer patients [5].
These results will provide important clues as to how the CSC phenotype is developed via accumulating alterations in gene networks and why this phenomenon is very similar to developmental processes with respect to their effects, but is completely different with respect to their mechanisms. When all pieces of the puzzle are in place, researchers will be able to explain why cells expressing the EMT phenotype sometimes fail to metastasise [51,52,53] and why cancer stemness exhibits heterogeneity.
We conclude that BRACHYURY and SOX2 synergistically promote EMT (invasiveness) and self-renewal property, and one of the members of cancer stemness related genes. This new knowledge is expected to lead to more specific and effective therapeutic targets in oral cancer, which may include SOX2 and BRACHYURY, either alone or in combination.
4. Materials and Methods
4.1. Cells and Culture
ACCS, ACCS GFP, and ACCS-M GFP (human oral adenoid cystic carcinoma cell lines) were established and reported previously in our laboratory [22]. In brief, ACCS-GFP was established from the parental cell line ACCS by transfecting green fluorescence protein (GFP) gene. These cell lines had similar morphological characteristics, growth rates, and tumourigenicity in vitro and in vivo. Tumourigenicity of ACCS-GFP was similar to the parental ACCS, (22.2% incidence). ACCS-GFP cells were injected to the tongues of nude mice and the developed tumour was clearly detectable with green fluorescence under excitation light. ACCS-M GFP cells was established by in vivo selection process repeatedly. ACCS-M GFP cells exhibited high tumourigenicity (100% incidence) and spontaneous metastases to submandibular lymph nodes (100% incidence).
SCCTF is a BRACHYURY- and SOX2-negative human oral squamous cell carcinoma cell line. TF-GFP and TF-Neo transfectant cell lines were generated via transfection with the pEGFP-N1 vector and a control vector (Clontech; Palo Alto, CA, USA) for visualisation, using Lipofectamine LTX (Invitrogen; Carlsbad, CA, USA) according to the manufacturer’s instructions. Colony selection after individual transfection procedure was performed using resistance to neomycin (G418; Sigma-Aldrich; St. Louis, MO, USA). The cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM; Sigma-Aldrich, St. Lois, MO, USA) supplemented with 10% fetal bovine serum (FBS; ICN Biomedicals; Aurora, OH, USA), 2 mM L-glutamine, penicillin G, and streptomycin in a humidified incubator with an atmosphere of 5% CO2 at 37 °C.
4.2. Transfection and Knockdown of BRACHYURY and SOX2
For forced expression of BRACHYURY, full-length human Brachyury cDNA was cloned into the pCMV6 vector (ORIGENE Technologies Inc., Rockville, MD, USA), resulting in the pCMV6-Brachyury vector. ACCS-Bra and TF-Bra cells were generated via transfection of the pCMV6-Brachyury vector into ACCS-GFP and TF-GFP cells, respectively. As controls, ACCS-Neo and TF-Neo cells were generated by means of transfection of the pCMV6-Entry vector into ACCS-GFP and TF-GFP cells, respectively.
For forced expression of SOX2, full-length human SOX2 was cloned into the pUNO1 vector (Invitrogen, Carlsbad, CA, USA), resulting in the pUNO1-hSox2 vector. ACCS-Sox2 and TF-Sox2 cells were generated via transfection of the pUNO1-hSox2 vector into ACCS-GFP and TF-GFP cells, respectively. As controls, ACCS-Blas and TF-Blas cells were generated by means of transfection of the pUNO-mcs vector into ACCS-GFP and TF-GFP cells, respectively.
ACCS-Bra/Sox2 and TF-Bra/Sox2 cells were generated via co-transfection of the pCMV6-Brachyury and pUNO1-hSox2 vectors into ACCS-GFP and TF-GFP cells, respectively. The transfection was performed using Lipofectamine LTX (Invitrogen) according to the manufacturer’s instructions.
Gene knockdown experiment was performed on Brachyury express ACCSM-GFP cells by transfecting an shRNA lentiviral plasmid (pLKO.1-puro; Sigma-Aldrich) using Lipofectamine LTX (Invitrogen). Control cells (ACCS-sh.control and ACCS-M-sh.control) were generated by transfection of ACCS GFP and ACCS-M GFP cells with the pLKO.1-puro Control Vector (Sigma-Aldrich). Brachyury knockdown cells (ACCS-shBra and ACCS-M-shBra) were generated by transfection of ACCS GFP and ACCS-M GFP cells with pLKO.1-puro/sh.Brachyury (Sigma-Aldrich). The expression level of Brachyury in shRNA-transfected ACCS cells was monitored using real-time RT-PCR. All transfected cells were maintained in DMEM containing 10% FBS and 2 μg/mL puromycin (Sigma-Aldrich).
4.3. Real-Time RT-PCR
Real-time RT-PCR was employed to quantify the mRNA expression levels of the indicated EMT-related genes, embryonic stem cell markers, and differentiation markers in ACC cells and TF cells. Total RNA was extracted and purified using TRIzol Reagent (Invitrogen). The first-strand cDNA synthesis and amplification of target mRNA for quantification were performed using a real-time PCR system: LightCycler FastStart DNA Master SYBER Green 1 kit (Roche Diagnostics; Mannheim, Germany). The mRNA levels were quantified in triplicate. The PCR cycling conditions are as follows; 10 min at 95 °C for 1 cycle followed by 45 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 60 s. Dissociation curve analysis, normalisation by β-actin mRNA levels and quantification of target mRNA expression level were analysed using the LightCycler 2.0 System software package (Roche Applied Science; Indianapolis, IN, USA). The specific primers for EMT, stem cell markers, and differentiation markers are shown in Table 1. All primers were purchased from NIHON Gene Research Laboratories, Inc. (Sendai, Japan).

Table 1.
Primers used in real-time RT-PCR.
4.4. The Sphere-Formation Assay
Cultured cells were recovered and plated for floating cultures at a density of 5 × 104 cells/mL in 60-mm non-coated dishes with serum-free DMEM containing basic fibroblast growth factor (bFGF, 40 ng/mL) and epidermal growth factor (EGF, 20 ng/mL). The cells were incubated in a humidified atmosphere at 37 °C and 5% CO2, and bFGF and EGF were added to the medium every other day during culture period. After 10 days, the diameters of each cell sphere were measured, and the numbers of sphere with a diameter >100 μm were counted as primary spheres. For passaging of the primary spheres, the spheres were collected by centrifuge and treated with 0.05% trypsin/0.02% ethylendiaminetetraacetic acid (EDTA) and dissociated into single cells. The dissociated cells were plated to 24-well culture plates at a density of 104 cells/mL in a serum-free medium and allowed to grow for further 10 days in a serum-free medium to obtain secondary spheres.
4.5. The Wound Healing Assay
Cancer cells were plated at the density of 6 × 10⁵ cells per well in a 6-well plate (BD Falcon) in DMEM with 10% FBS and allowed to attach for 24 h to obtain confluent monolayer of cells. Then monolayer cells were treated with 25 µg/mL of Mitomycin C for 25min at 37 °C to prevent cell growth. The confluent monolayer of cells is scratched to make wounds using a plastic pipette tip (200 µL), and the cells were washed with phosphate-buffered saline (PBS) and arrowed to migrate for 24 h. Randomly chosen wound fields were photographed under a fluorescence microscope (BZ-8000; Keyence; Osaka, Japan) every 8 h for 24 h. The wound areas were analysed using the following formula:
Wound area (% of control) = (wound area after the indicated period/initial wound area) × 100
4.6. Evaluation of Tumour Dissemination from the Primary Cancer Nest
Evaluation of tumour dissemination from a primary cancer nest was performed as described previously [23]. One million cells were pelleted and resuspended in 25 µL of DMEM and 25 µL of type I collagen (AteloCell; IAC-30; Koken; Tokyo, Japan) to form a solid cell cluster. The collagen-embedded tumour cell pellets were incubated to solidify for 30 min at 37 °C in a 1.5-mL microcentrifuge tube. Five hundred microliters of type I collagen containing fibroblasts (105 cells/mL) were added to a 6-well plate (BD Falcon, Franklin Lakes, NJ, USA). The collagen-embedded tumour cells were embedded in the collagen-embedded fibroblasts and incubated to solidify for 30 min at 37 °C. DMEM was added on top of the collagen gels, and the incubation was continued. After 7 days of culture, tumour migration was observed as green fluorescence under a fluorescence microscope (BZ-8000; Keyence, Osaka, Japan). Cancer cell invasiveness was evaluated by tumour dissemination from the tumour cell pellet (mimics a primary tumour nest). Evaluation were made by measuring the distance of all cells from the edge of the nest in 5 randomly selected standardised rectangular light fields (500 µm × 100 µm), and the values were summed (invasion value).
4.7. Statistical Analysis
All data were calculated as mean ± SD and processed using analysis of variance (ANOVA) for multiple group comparison. When ANOVA is significant, two groups comparison using the Student’s t-test is performed. Statistical analyses were calculated by means of the statistical software SPSS 13.0. Statistical significance was assumed at P < 0.05.
Author Contributions
conceptualisation, T.S., K.N. and N.A.; methodology, N.A., K.N., H.H., K.K., Y.M. and T.S.; validation, N.A., K.N. and T.S.; writing—original draft preparation, N.A., K.N. and T.S.; writing—review and editing, T.S.; visualisation, N.A. and K.N.; supervision, T.S.; project administration, T.S.; funding acquisition, T.S.
Funding
This research was funded by JSPS KAKENHI, grant number JP23390465 and JP15H5045 to T.S.
Acknowledgments
This work was supported by Professor Yoshihide Mori, Kyushu University for administrative and technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| EMT | Epithelial–mesenchymal transition |
| CSCs | Cancer stem cells |
| shRNA | Small hairpin RNA |
| GFP | Green fluorescence protein |
| EDTA | Ethylendiaminetetraacetic acid |
References
- Chang, C.C.; Hsu, W.H.; Wang, C.C.; Chou, C.H.; Kuo, M.Y.; Lin, B.R.; Chen, S.T.; Tai, S.K.; Kuo, M.L.; Yang, M.H. Connective tissue growth factor activates pluripotency genes and mesenchymal-epithelial transition in head and neck cancer cells. Cancer Res. 2013, 73, 4147–4157. [Google Scholar] [CrossRef] [PubMed]
- Drasin, D.J.; Robin, T.P.; Ford, H.L. Breast cancer epithelial-to-mesenchymal transition: Examining the functional consequences of plasticity. Breast Cancer Res. 2011, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, G.; Tirino, V.; Camerlingo, R.; La Rocca, A.; Martucci, N.; Scognamiglio, G.; Franco, R.; Cantile, M.; Normanno, N.; Rocco, G. Prognostic value of cancer stem cells, epithelial-mesenchymal transition and circulating tumor cells in lung cancer. Oncol. Rep. 2013, 29, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Zubeldia, I.G.; Bleau, A.M.; Redrado, M.; Serrano, D.; Agliano, A.; Gil-Puig, C.; Vidal-Vanaclocha, F.; Lecanda, J.; Calvo, A. Epithelial to mesenchymal transition and cancer stem cell phenotypes leading to liver metastasis are abrogated by the novel TGFbeta1-targeting peptides P17 and P144. Exp. Cell Res. 2013, 319, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Imajyo, I.; Sugiura, T.; Kobayashi, Y.; Shimoda, M.; Ishii, K.; Akimoto, N.; Yoshihama, N.; Kobayashi, I.; Mori, Y. T-box transcription factor Brachyury expression is correlated with epithelial-mesenchymal transition and lymph node metastasis in oral squamous cell carcinoma. Int. J. Oncol. 2012, 41, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Hindriksen, S.; Bijlsma, M.F. Cancer stem cells, EMT, and developmental pathway activation in pancreatic tumors. Cancers 2012, 4, 989–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Han, Z.P.; Jing, Y.Y.; Tao, S.F.; Li, T.J.; Wang, H.; Wang, Y.; Li, R.; Yang, Y.; Zhao, X.; et al. CD133(+)CXCR4(+) colon cancer cells exhibit metastatic potential and predict poor prognosis of patients. BMC Med. 2012, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Mallini, P.; Lennard, T.; Kirby, J.; Meeson, A. Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Cancer Treat. Rev. 2014, 40, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.L.; Pernemalm, M.; Crosbie, P.A.; Whetton, A.D. The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat. Rev. 2014, 40, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, M.; Sugiura, T.; Imajyo, I.; Ishii, K.; Chigita, S.; Seki, K.; Kobayashi, Y.; Shirasuna, K. The T-box transcription factor Brachyury regulates epithelial-mesenchymal transition in association with cancer stem-like cells in adenoid cystic carcinoma cells. BMC Cancer 2012, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Chaffer, C.L.; Weinberg, R.A. Cancer stem cells: Mirage or reality? Nat. Med. 2009, 15, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, S.; Brabletz, T. The ZEB/miR-200 feedback loop—A motor of cellular plasticity in development and cancer? EMBO Rep. 2010, 11, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, J.; Vincent, T.; Garcia de Herreros, A. Transcriptional crosstalk between TGFβ and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010, 9, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Kang, Y. Multilayer control of the EMT master regulators. Oncogene 2014, 33, 1755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Ahmad, A.; Banerjee, S.; Azmi, A.S.; Kong, D.; Sarkar, F.H. Pancreatic cancer: Understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Shekhani, M.T.; Jayanthy, A.S.; Maddodi, N.; Setaluri, V. Cancer stem cells and tumor transdifferentiation: Implications for novel therapeutic strategies. Am. J. Stem Cells 2013, 2, 52–61. [Google Scholar] [PubMed]
- Smalley, M.; Piggott, L.; Clarkson, R. Breast cancer stem cells: Obstacles to therapy. Cancer Lett. 2013, 338, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Palena, C.; Polev, D.E.; Tsang, K.Y.; Fernando, R.I.; Litzinger, M.; Krukovskaya, L.L.; Baranova, A.V.; Kozlov, A.P.; Schlom, J. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin. Cancer Res. 2007, 13, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.I.; Litzinger, M.; Trono, P.; Hamilton, D.H.; Schlom, J.; Palena, C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J. Clin. Investig. 2010, 120, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Shimoda, M.; Sugiura, T.; Seki, K.; Takahashi, M.; Abe, M.; Matsuki, R.; Inoue, Y.; Shirasuna, K. Involvement of epithelial-mesenchymal transition in adenoid cystic carcinoma metastasis. Int. J. Oncol. 2011, 38, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Sugiura, T.; Takahashi, M.; Ishii, K.; Shimoda, M.; Shirasuna, K. A novel function of CD82/KAI-1 on E-cadherin-mediated homophilic cellular adhesion of cancer cells. Cancer Lett. 2008, 266, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Biddle, A.; Mackenzie, I.C. Cancer stem cells and EMT in carcinoma. Cancer Metast. Rev. 2012, 31, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Rhim, A.D.; Mirek, E.T.; Aiello, N.M.; Maitra, A.; Bailey, J.M.; McAllister, F.; Reichert, M.; Beatty, G.L.; Rustgi, A.K.; Vonderheide, R.H.; et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012, 148, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Wellner, U.; Schubert, J.; Burk, U.C.; Schmalhofer, O.; Zhu, F.; Sonntag, A.; Waldvogel, B.; Vannier, C.; Darling, D.; zur Hausen, A.; et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 2009, 11, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.; Ferrari, P.; Fini, M.; Borsari, V.; Fallahi, P.; Antonelli, A.; Berti, P.; Carpi, A.; Miccoli, P. Stem cells: Their role in breast cancer development and resistance to treatment. Curr. Pharm. Biotechnol. 2011, 12, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D.; Parvani, J.G.; Schiemann, W.P. The relevance of the TGF-beta Paradox to EMT-MET programs. Cancer Lett. 2013, 341, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T. EMT and MET in metastasis: Where are the cancer stem cells? Cancer Cell 2012, 22, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Banerjee, S.; Ahmad, A.; Li, Y.; Wang, Z.; Sethi, S.; Sarkar, F.H. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE 2010, 5, e12445. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Shields, B.; Davies, M.L.; Muller, J.; Wakeman, J.A. BRACHYURY confers cancer stem cell characteristics on colorectal cancer cells. Int. J. Cancer 2012, 130, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Silva, J.; Colby, D.; Nichols, J.; Nijmeijer, B.; Robertson, M.; Vrana, J.; Jones, K.; Grotewold, L.; Smith, A. Nanog safeguards pluripotency and mediates germline development. Nature 2007, 450, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Vanlandewijck, M.; Moustakas, A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012, 586, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Villanueva, M.; Zhang, J.S.; Koenig, A.; Abel, E.V.; Smyrk, T.C.; Bamlet, W.R.; de Narvajas, A.A.; Gomez, T.S.; Simeone, D.M.; Bujanda, L.; et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis 2013, 2, e61. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fang, X.; Lou, X.; Hua, D.; Ding, W.; Foltz, G.; Hood, L.; Yuan, Y.; Lin, B. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of CRC patients. PLoS ONE 2012, 7, e41335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Chen, Y.; Chen, S.; Jia, X.; Sun, T.; Liu, Y.; Li, X.; Xiang, R.; Li, N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/β-catenin signal network. Cancer Lett. 2013, 336, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhu, X. The Wnt/β-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol. Med. Rep. 2012, 5, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Rao, S.; Chu, J.; Shen, X.; Levasseur, D.N.; Theunissen, T.W.; Orkin, S.H. A protein interaction network for pluripotency of embryonic stem cells. Nature 2006, 444, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y.; Chen, Y.; Li, X.; Mou, W.; Wang, L.; Liu, Y.; Reisfeld, R.A.; Xiang, R.; Lv, D.; et al. SOX2 gene regulates the transcriptional network of oncogenes and affects tumorigenesis of human lung cancer cells. PLoS ONE 2012, 7, e36326. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.J.; Stappert, J.; Bauer, A.; Kispert, A.; Herrmann, B.G.; Kemler, R. Brachyury is a target gene of the Wnt/β-catenin signaling pathway. Mech. Dev. 2000, 91, 249–258. [Google Scholar] [CrossRef]
- Larocca, C.; Cohen, J.R.; Fernando, R.I.; Huang, B.; Hamilton, D.H.; Palena, C. An autocrine loop between TGF-β1 and the transcription factor brachyury controls the transition of human carcinoma cells into a mesenchymal phenotype. Mol. Cancer Ther. 2013, 12, 1805–1815. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Takahashi, H.; Satoh, N.; Gojobori, T. Brachyury-downstream gene sets in a chordate, Ciona intestinalis: Integrating notochord specification, morphogenesis and chordate evolution. Evol. Dev. 2008, 10, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Todo, T.; Ino, Y.; Takahashi, M.; Miyazawa, K.; Miyazono, K. Autocrine TGF-β signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell 2009, 5, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Han, X.; Jin, C.; Tian, W.; Yu, W.; Ding, D.; Cheng, L.; Huang, B.; Jiang, H.; Lin, B. SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer: New molecular leads for therapeutic intervention. Omics J. Integr. Biol. 2013, 17, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.C.; Singh, P.; Schwarzbauer, J.E. Endogenous production of fibronectin is required for self-renewal of cultured mouse embryonic stem cells. Exp. Cell Res. 2012, 318, 1820–1831. [Google Scholar] [CrossRef] [PubMed]
- Gavert, N.; Vivanti, A.; Hazin, J.; Brabletz, T.; Ben-Ze’ev, A. L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol. Cancer Res. 2011, 9, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Preobrazhenska, O.; auf dem Keller, U.; Sutcliffe, M.; Barclay, L.; McDonald, P.C.; Roskelley, C.; Overall, C.M.; Dedhar, S. Epithelial-mesenchymal transition (EMT) is not sufficient for spontaneous murine breast cancer metastasis. Dev. Dyn. 2008, 237, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Chikaishi, Y.; Uramoto, H.; Tanaka, F. The EMT status in the primary tumor does not predict postoperative recurrence or disease-free survival in lung adenocarcinoma. Anticancer Res. 2011, 31, 4451–4456. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).