Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy
Abstract
1. Introduction
2. Results
2.1. Sample Characteristics
2.2. Intestinal Microbiome before and after Hypnosis
2.2.1. Alpha Diversity
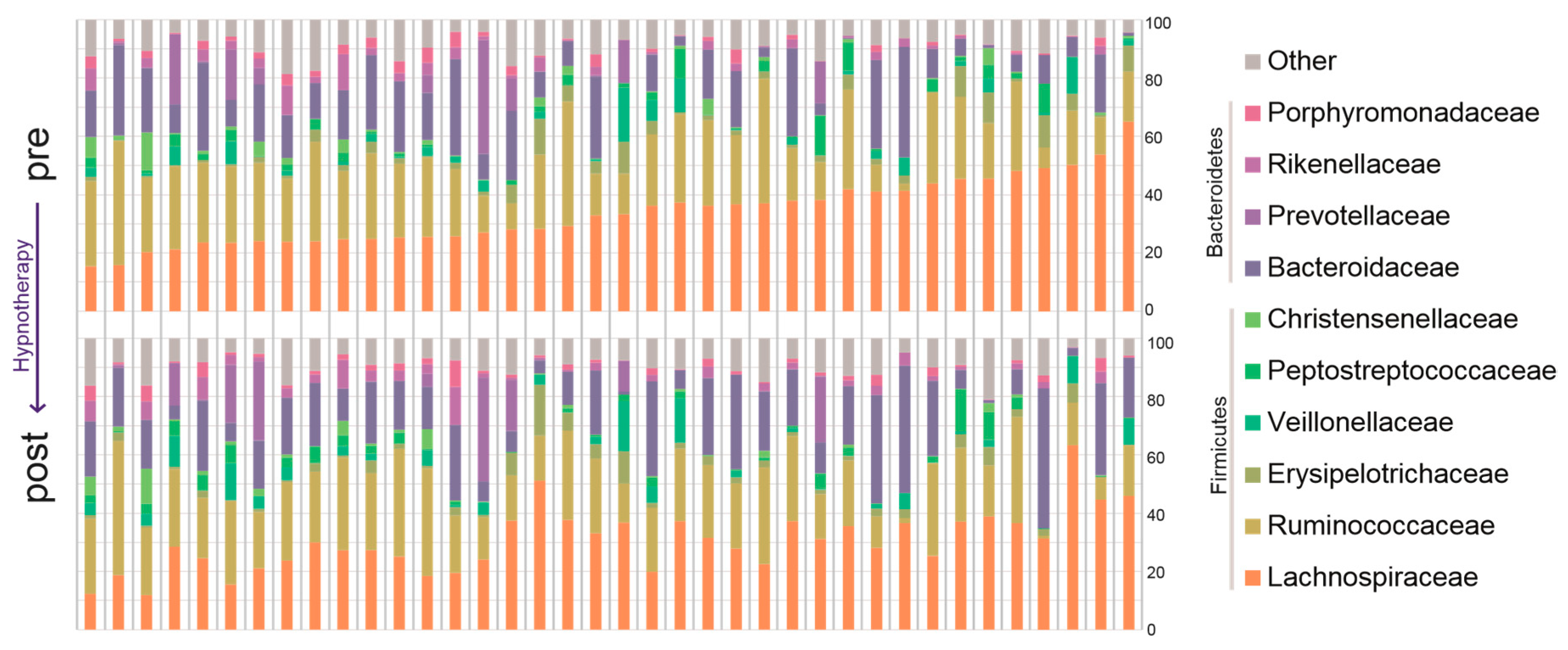
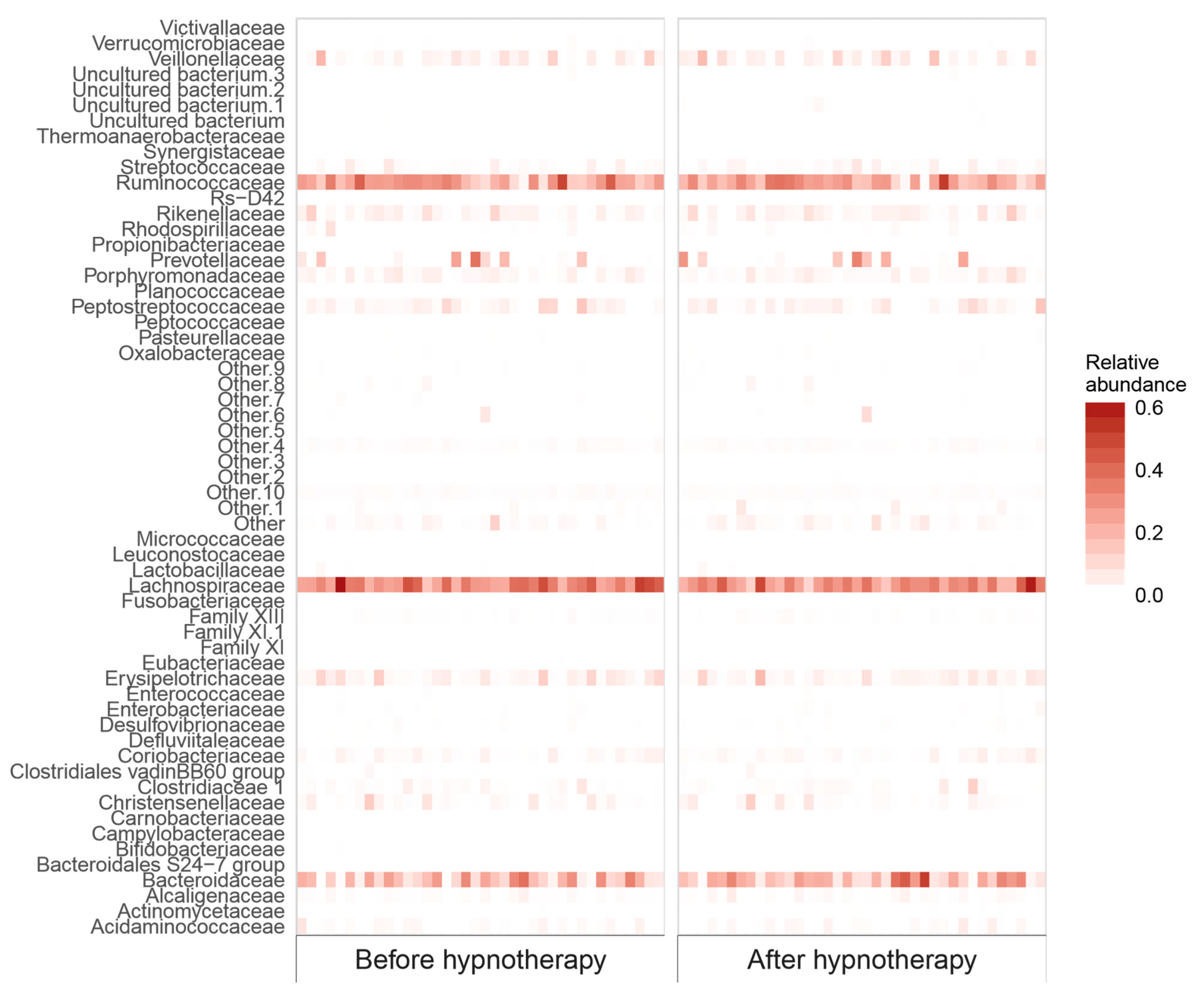
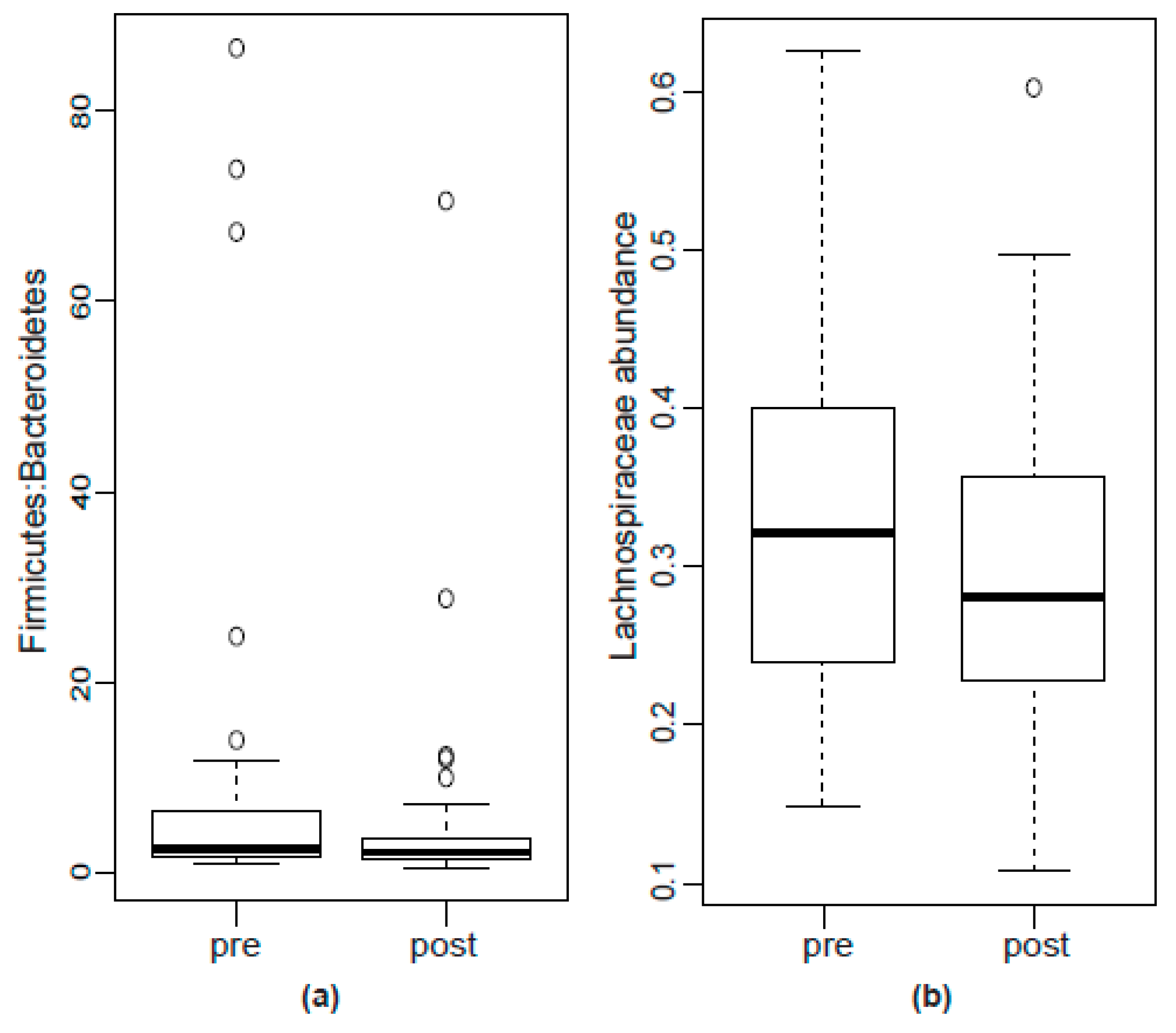
2.2.2. Bacterial Abundance
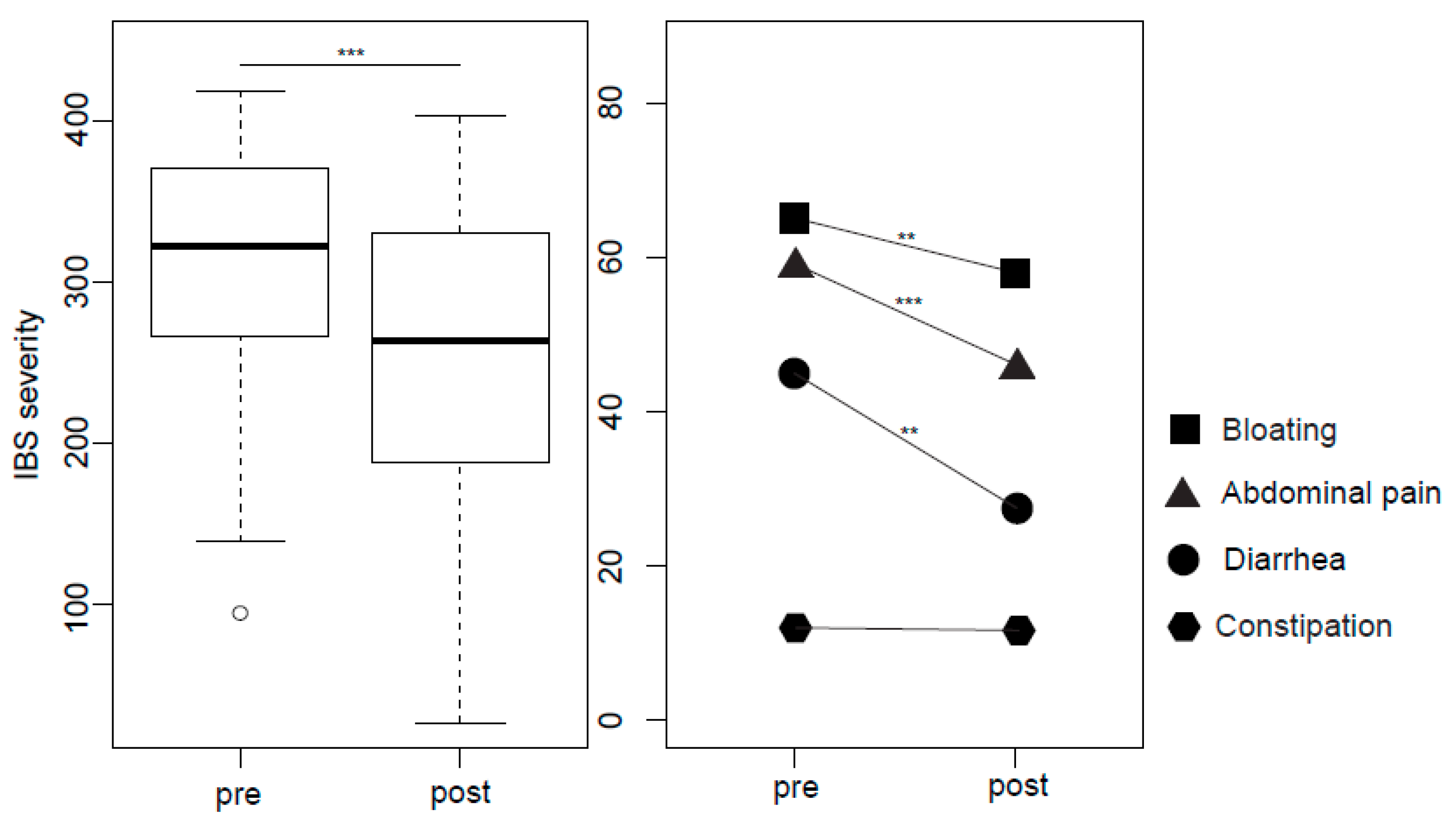
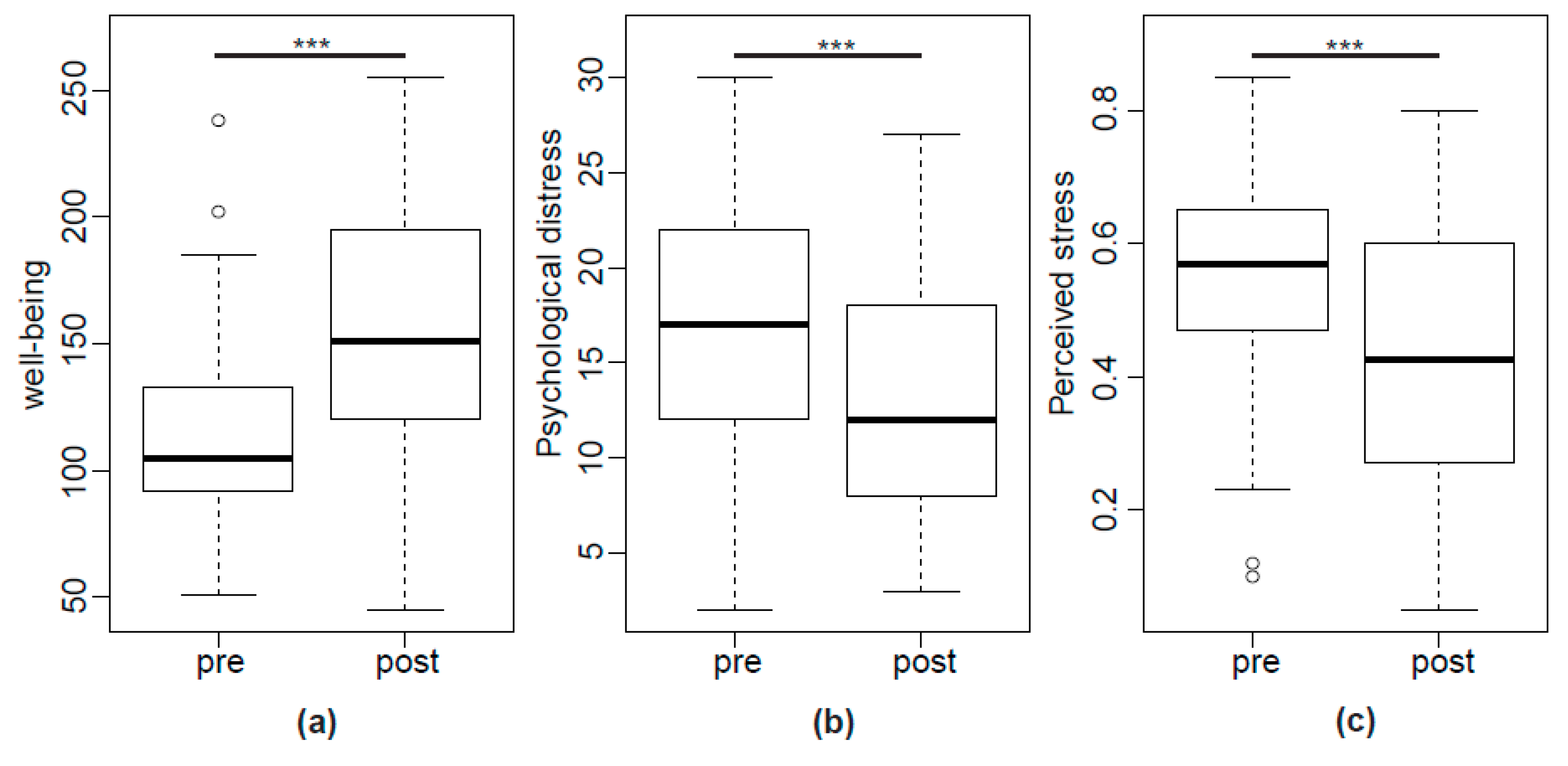
2.3. IBS Symptoms, Wellbeing and Psychological Stress
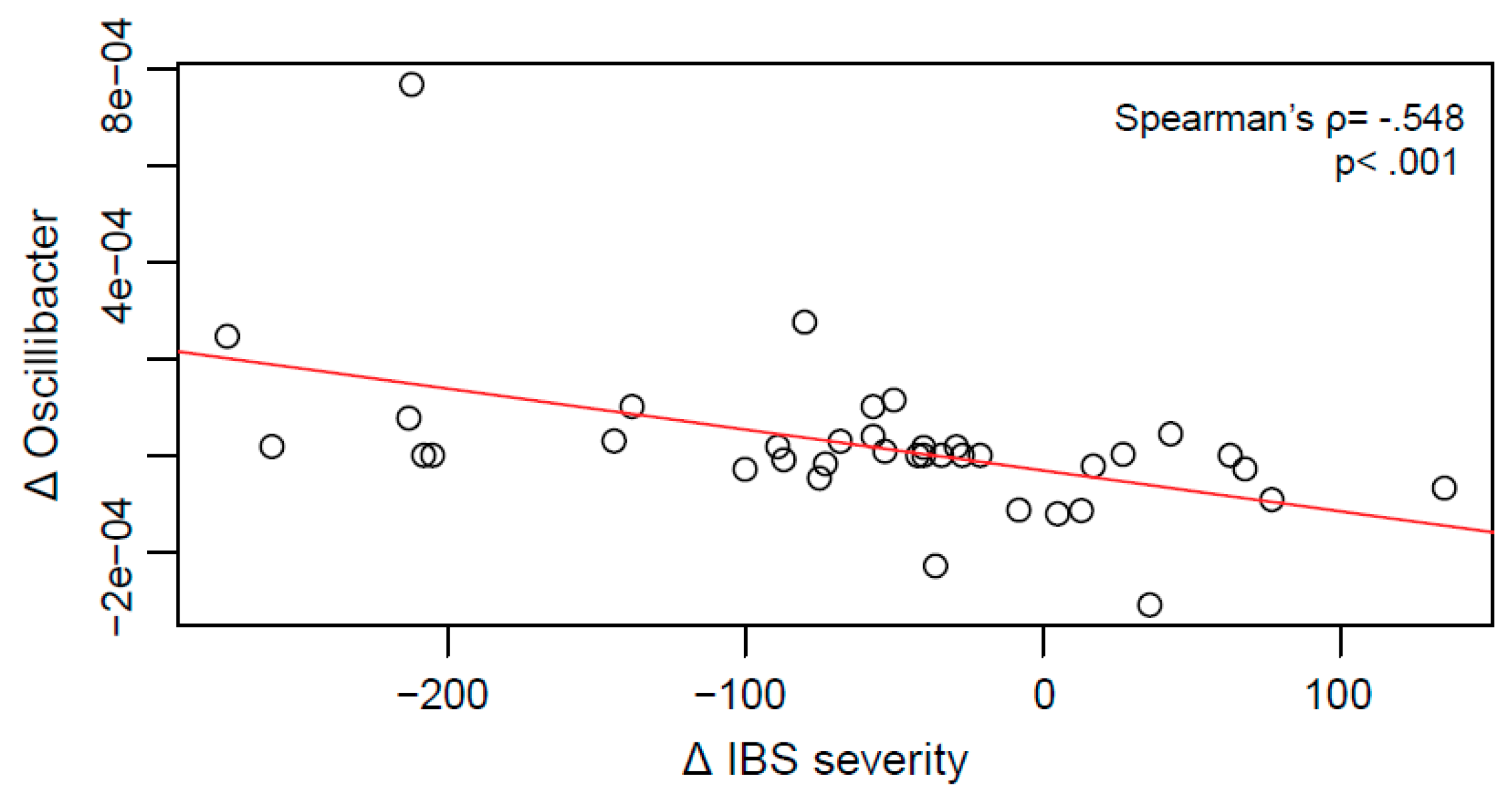
2.4. Relationship between Clinical Improvement and Microbial Changes
2.5. Microbial Analyses of Sample Subgroups
2.6. Diet
3. Discussion
4. Materials and Methods
4.1. Recruitment
4.2. Intervention
4.3. 16S rRNA Sequencing
4.4. Alpha Diversity Analyses
4.5. Analyses of Bacterial Abundance
4.6. Correlational Analyses
4.7. Questionnaires
4.8. Assessment of Diet
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IBS | Irritable bowel syndrome |
| GHT | Gut-directed hypnotherapy |
| FDR | False Discovery Rate |
| OTU | Operational Taxonomic Unit |
| HADS | Hospital Anxiety and Depression Scale |
| VAS | Visual Analogue Scales |
| GABA | Gamma-aminobutyric acid |
References
- Endo, Y.; Shoji, T.; Fukudo, S. Epidemiology of irritable bowel syndrome. Ann. Gastroenterol. 2015, 28, 158–159. [Google Scholar] [PubMed]
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Savidge, T.; Shulman, R.J. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology 2014, 146, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stilling, R.M.; Stanton, C.; Cryan, J.F. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sundin, J.; Öhman, L.; Simren, M. Understanding the Gut Microbiota in Inflammatory and Functional Gastrointestinal Diseases. Psychosom. Med. 2017, 79, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; De Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P. Intestinal microbiota and diet in IBS: Causes, consequences, or epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.; Cryan, J.; Quigley, E.; Dinan, T.; Clarke, G. A sustained hypothalamic–pituitary–adrenal axis response to acute psychosocial stress in irritable bowel syndrome. Psychol. Med. 2014, 44, 3123–3134. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Naliboff, B.; Shih, W.; Presson, A.; Videlock, E.; Ju, T.; Kilpatrick, L.; Gupta, A.; Mayer, E.; Chang, L. Resilience is decreased in irritable bowel syndrome and associated with symptoms and cortisol response. Neurogastroenterol. Motil. 2017, 30, e13155. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Muratsubaki, T.; Oudenhove, L.; Morishita, J.; Yoshizawa, M.; Kohno, K.; Yagihashi, M.; Tanaka, Y.; Mugikura, S.; Dupont, P. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci. Rep. 2017, 7, 12425. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; O’Toole, P.W.; Ohman, L.; Claesson, M.J.; Deane, J.; Quigley, E.M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012, 61, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Sundin, J.; Rangel, I.; Fuentes, S.; Heikamp-de Jong, I.; Hultgren-Hörnquist, E.; Vos, W.; Brummer, R.-J. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment. Pharmacol. Ther. 2015, 41, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.; Fournier, C.; Durdevic, M.; Knoblich, L.; Keip, B.; Dejaco, C.; Trauner, M.; Moser, G. A microbial signature of psychological distress in irritable bowel syndrome. Psychosom. Med. 2018, 80, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Meerveld, G.V.; Cryan, J.F. Stress and the Microbiota–Gut–Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Fourie, N.H.; Wang, D.; Abey, S.K.; Creekmore, A.L.; Hong, S.; Martin, C.G.; Wiley, J.W.; Henderson, W.A. Structural and functional alterations in the colonic microbiome of the rat in a model of stress induced irritable bowel syndrome. Gut Microbes 2017, 8, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Galley, J.D.; Nelson, M.C.; Yu, Z.; Dowd, S.E.; Walter, J.; Kumar, P.S.; Lyte, M.; Bailey, M.T. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Loundou, A.; Hamdani, N.; Boukouaci, W.; Dargel, A.; Oliveira, J.; Roger, M.; Tamouza, R.; Leboyer, M.; Boyer, L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): A systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Naliboff, B.D.; Chang, L.; Coutinho, S.V.V. Stress and irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G519–G524. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Clarke, G.; Quigley, E.M.; Groeger, J.A.; Dinan, T.G.; Cryan, J.F. Gut memories: Towards a cognitive neurobiology of irritable bowel syndrome. Neurosci. Biobehav. Rev. 2012, 36, 310–340. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Endo, Y.; Fukudo, S. Association between alexithymia and functional gastrointestinal disorders. Front. Psychol. 2018, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Thakur, E.; Holmes, H.; Lockhart, N.; Carty, J.; Ziadni, M.; Doherty, H.; Lackner, J.; Schubiner, H.; Lumley, M. Emotional awareness and expression training improves irritable bowel syndrome: A randomized controlled trial. Neurogastroenterol. Motil. 2017, 29, e13143. [Google Scholar] [CrossRef] [PubMed]
- Ballou, S.; Keefer, L. Psychological interventions for irritable bowel syndrome and inflammatory bowel diseases. Clin. Transl. Gastroenterol. 2017, 8, e214. [Google Scholar] [CrossRef] [PubMed]
- Whorwell, P.J.; Prior, A.; Faragher, E.B. Controlled trial of hypnotherapy in the treatment of severe irritable bowel syndrome. Lancet 1984, 324, 1232–1234. [Google Scholar] [CrossRef]
- Gonsalkorale, W.M. Gut-directed hypnotherapy: The Manchester approach for treatment of irritable bowel syndrome. Int. J. Clin. Exp. Hypn. 2006, 54, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Trägner, S.; Gajowniczek, E.E.; Mikulits, A.; Michalski, M.; Kazemi-Shirazi, L.; Kulnigg-Dabsch, S.; Führer, M.; Ponocny-Seliger, E.; Dejaco, C. Long-term success of GUT-directed group hypnosis for patients with refractory irritable bowel syndrome: A randomized controlled trial. Am. J. Gastroenterol. 2013, 108, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Whorwell, P.J. Hypnotherapy for irritable bowel syndrome: The response of colonic and noncolonic symptoms. J. Psychosom. Res. 2008, 64, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, P.; Ljótsson, B.; Bjornsson, E.; Abrahamsson, H.; Simrén, M. Patient satisfaction after gut-directed hypnotherapy in irritable bowel syndrome. Neurogastroenterol. Motil. 2013, 25, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Carruthers, H.; Morris, J.; Hasan, S.; Archbold, S.; Whorwell, P. Hypnotherapy for irritable bowel syndrome: An audit of one thousand adult patients. Aliment. Pharmacol. Ther. 2015, 41, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Gonsalkorale, W.M.; Toner, B.B.; Whorwell, P.J. Cognitive change in patients undergoing hypnotherapy for irritable bowel syndrome. J. Psychosom. Res. 2004, 56, 271–278. [Google Scholar] [CrossRef]
- Peter, J.; Tran, U.S.; Michalski, M.; Moser, G. The structure of resilience in irritable bowel syndrome and its improvement through hypnotherapy: Cross-sectional and prospective longitudinal data. PLoS ONE 2018. [Google Scholar] [CrossRef] [PubMed]
- Lowén, M.B.; Mayer, E.A.; Sjöberg, M.; Tillisch, K.; Naliboff, B.; Labus, J.; Lundberg, P.; Ström, M.; Engström, M.; Walter, S.A. Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2013, 37, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Prior, A.; Colgan, S.; Whorwell, P. Changes in rectal sensitivity after hypnotherapy in patients with irritable bowel syndrome. Gut 1990, 31, 896–898. [Google Scholar] [CrossRef] [PubMed]
- Lea, R.; Houghton, L.; Calvert, E.; Larder, S.; Gonsalkorale, W.; Whelan, V.; Randles, J.; Cooper, P.; Cruickshanks, P.; Miller, V. Gut-focused hypnotherapy normalizes disordered rectal sensitivity in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003, 17, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Turner, M.J.; Johnson, D.A.; Burnett, C.K.; Whitehead, W.E. Hypnosis treatment for severe irritable bowel syndrome: Investigation of mechanism and effects on symptoms. Dig. Dis. Sci. 2002, 47, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.; Arns, P.A.; Hoffman, J.W. The relaxation response and hypnosis. Int. J. Clin. Exp. Hypn. 1981, 29, 259–270. [Google Scholar] [CrossRef]
- Kekecs, Z.; Szekely, A.; Varga, K. Alterations in electrodermal activity and cardiac parasympathetic tone during hypnosis. Psychophysiology 2016, 53, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.J.; Bughi, S.; Morrison, J.; Tanavoli, S.; Tanavoli, S.; Zadeh, H.H. Hypnosis, differential expression of cytokines by T-cell subsets, and the hypothalamo-pituitary-adrenal axis. Am. J. Clin. Hypn. 2003, 45, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Tache, Y.; Larauche, M.; Yuan, P.-Q.; Million, M. Brain and gut CRF signaling: Biological actions and role in the gastrointestinal tract. Curr. Mol. Pharmacol. 2018, 11, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Tandon, R.K. Stress and the gastrointestinal tract. J. Gastroenterol. Hepatol. 2005, 20, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Mawdsley, J.E.; Jenkins, D.G.; Macey, M.G.; Langmead, L.; Rampton, D.S. The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am. J. Gastroenterol. 2008, 103, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Beaugerie, L.; Burger, A.; Cadranel, J.; Lamy, P.; Gendre, J.; Le Quintrec, Y. Modulation of orocaecal transit time by hypnosis. Gut 1991, 32, 393–394. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.B.; Spiegel, D. Modulation of gastric acid secretion by hypnosis. Gastroenterology 1989, 96, 1383–1387. [Google Scholar] [CrossRef]
- Simrén, M.; Ringström, G.; Björnsson, E.S.; Abrahamsson, H. Treatment with hypnotherapy reduces the sensory and motor component of the gastrocolonic response in irritable bowel syndrome. Psychosom. Med. 2004, 66, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, P.; Törnblom, H.; Sadik, R.; Björnsson, E.S.; Abrahamsson, H.; Simrén, M. Effects on gastrointestinal transit and antroduodenojejunal manometry after gut-directed hypnotherapy in irritable bowel syndrome (IBS). Scand. J. Gastroenterol. 2012, 47, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Hansen, L.B.; Bahl, M.I.; Frandsen, H.L.; Carvalho, V.; Gøbel, R.J.; Dalgaard, M.D.; Plichta, D.R.; Sparholt, M.H.; Vestergaard, H. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat. Microbiol. 2016, 1, 16093. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.; Morris, J.; Whorwell, P. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Elmadfa, I.; Wagner, K. Oesterreichischer Ernaehrungsbericht; Universität Wien: Vienna, Austria, 2012. [Google Scholar]
- Allen, A.P.; Dinan, T.G.; Clarke, G.; Cryan, J.F. A psychology of the human brain–gut–microbiome axis. Soc. Personal. Psychol. Compass 2017, 11, e12309. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Bachner, H.A. Focus: Microbiome: Integrative Therapies in Anxiety Treatment with Special Emphasis on the Gut Microbiome. Yale J. Boil. Med. 2016, 89, 397–422. [Google Scholar]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Naseribafrouei, A.; Hestad, K.; Avershina, E.; Sekelja, M.; Linløkken, A.; Wilson, R.; Rudi, K. Correlation between the human fecal microbiota and depression. Neurogastroenterol. Motil. 2014, 26, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Holzl, J.; Godau, P. Receptor bindings studies with Valeriana officinalis on the benzodiazepine receptor. Planta. Med 1989, 55, 642. [Google Scholar] [CrossRef]
- Katano, Y.; Fujinami, S.; Kawakoshi, A.; Nakazawa, H.; Oji, S.; Iino, T.; Oguchi, A.; Ankai, A.; Fukui, S.; Terui, Y. Complete genome sequence of Oscillibacter valericigenes Sjm18–20T (=NBRC 101213T). Stand. Genom. Sci. 2012, 6, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Demertzi, A.; Vanhaudenhuyse, A.; Noirhomme, Q.; Faymonville, M.-E.; Laureys, S. Hypnosis modulates behavioural measures and subjective ratings about external and internal awareness. J. Physiol. Paris 2015, 109, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Patterson, D.R. Hypnotic approaches for chronic pain management: Clinical implications of recent research findings. Am. Psychol. 2014, 69, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Betz, C.; Mannsdörfer, K.; Bischoff, S. Validation of the IBS-SSS. Z. Gastroenterol. 2013, 51, 1171–1176. [Google Scholar] [PubMed]
- Spiegel, B.; Camilleri, M.; Bolus, R.; Andresen, V.; Chey, W.D.; Fehnel, S.; Mangel, A.; Talley, N.J.; Whitehead, W.E. Psychometric evaluation of patient-reported outcomes in irritable bowel syndrome randomized controlled trials: A Rome Foundation report. Gastroenterology 2009, 137, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Mujagic, Z.; Keszthelyi, D.; Aziz, Q.; Reinisch, W.; Quetglas, E.; De Leonardis, F.; Segerdahl, M.; Masclee, A. Systematic review: Instruments to assess abdominal pain in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 42, 1064–1081. [Google Scholar] [CrossRef] [PubMed]
- Petermann, F. Hospital Anxiety and Depression Scale, Deutsche Version (HADS-D). Z. Klin. Psychol. Psychiatr. Psychother. 2015, 59, 251–253. [Google Scholar] [CrossRef]
- Sellick, S.M.; Edwardson, A.D. Screening new cancer patients for psychological distress using the hospital anxiety and depression scale. Psychooncology 2007, 16, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Fliege, H.; Rose, M.; Arck, P.; Levenstein, S.; Klapp, B. Validierung des “Perceived Stress Questionnaire” (PSQ) an einer deutschen Stichprobe. Diagnostica 2001, 47, 142–152. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Goecks, J.; Nekrutenko, A.; Taylor, J. Galaxy: A comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010, 11, R86. [Google Scholar] [CrossRef] [PubMed]
- R Core Team (RC Team). R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
| IBS-D | 22 (58%) |
| IBS-mix | 12 (32%) |
| IBS-C | 4 (10%) |
| Post-infectious IBS | 7 (18%) |
| mild/moderate/severe IBS | 1 (2%)/12 (32%)/25 (66%) |
| Disease duration | 7 (3.38–14.25) |
| Presence of psychological distress | 25 (66%) |
| Taxonomy | Before GHT | After GHT | p | q |
|---|---|---|---|---|
| Families | ||||
| Other Bacteroidetes | 0.0465 (0.0092–0.1553) | 0.0771 (0.0205–0.2472) | 0.0035 | 0.154 |
| Clostridiales XI | 0 (0–0) | 0 (0–0.0024) | 0.0053 | 0.154 |
| Lachnospiraceae | 32.18 (24.14–39.89) | 28.11 (22.85–35.55) | 0.0199 | 0.384 |
| Genera | ||||
| Other Bacteroidetes | 0.0474 (0.0093–0.1725) | 0.0568 (0.0205–0.2211) | 0.0045 | 0.549 |
| Coprococcus 3 | 0.0359 (0.0118–0.1134) | 0.0310 (0.0102–0.0699) | 0.0057 | 0.549 |
| Uc Lachnospiraceae | 0.8217 (0.4973–1.2215) | 0.8329 (0.4190–1.1993) | 0.0149 | 0.743 |
| Clostridiales vadinBB60 group | 0 (0–0.0074) | 0.0016 (0–0.0094) | 0.0156 | 0.743 |
| Other Lachnospiraceae | 7.3539 (5.0824–10.7166) | 6.5448 (4.16041–9.1448) | 0.0263 | 0.743 |
| Lachnospiraceae UCG9 | 0 (0–0.0090) | 0 (0–0.0033) | 0.0328 | 0.743 |
| Intestinimonas | 0 (0–0.0032) | 0.0014 (0–0.0059) | 0.0358 | 0.743 |
| Anaerofustis | 0 (0–0) | 0 (0–0) | 0.0376 | 0.743 |
| Lachnospiraceae UCG10 | 0.0049 (0–0.0251) | 0.0052 (0–0.0142) | 0.0385 | 0.743 |
| Blautia | 5.3194 (3.4527–7.3214) | 4.6071 (2.6657–7.6441) | 0.0445 | 0.743 |
| Coprococcus 2 | 0 (0–0.0016) | 0 (0–0.0109) | 0.0458 | 0.743 |
| Eubacterium ventriosum group | 0.1553 (0.0559–0.4382) | 0.1336 (0.0344–0.2815) | 0.0464 | 0.743 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, J.; Fournier, C.; Keip, B.; Rittershaus, N.; Stephanou-Rieser, N.; Durdevic, M.; Dejaco, C.; Michalski, M.; Moser, G. Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy. Int. J. Mol. Sci. 2018, 19, 3619. https://doi.org/10.3390/ijms19113619
Peter J, Fournier C, Keip B, Rittershaus N, Stephanou-Rieser N, Durdevic M, Dejaco C, Michalski M, Moser G. Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy. International Journal of Molecular Sciences. 2018; 19(11):3619. https://doi.org/10.3390/ijms19113619
Chicago/Turabian StylePeter, Johannes, Camille Fournier, Bettina Keip, Nina Rittershaus, Nicola Stephanou-Rieser, Marija Durdevic, Clemens Dejaco, Maria Michalski, and Gabriele Moser. 2018. "Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy" International Journal of Molecular Sciences 19, no. 11: 3619. https://doi.org/10.3390/ijms19113619
APA StylePeter, J., Fournier, C., Keip, B., Rittershaus, N., Stephanou-Rieser, N., Durdevic, M., Dejaco, C., Michalski, M., & Moser, G. (2018). Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy. International Journal of Molecular Sciences, 19(11), 3619. https://doi.org/10.3390/ijms19113619





