IFT80 Improves Invasion Ability in Gastric Cancer Cell Line via ift80/p75NGFR/MMP9 Signaling
Abstract
1. Introduction
2. Results
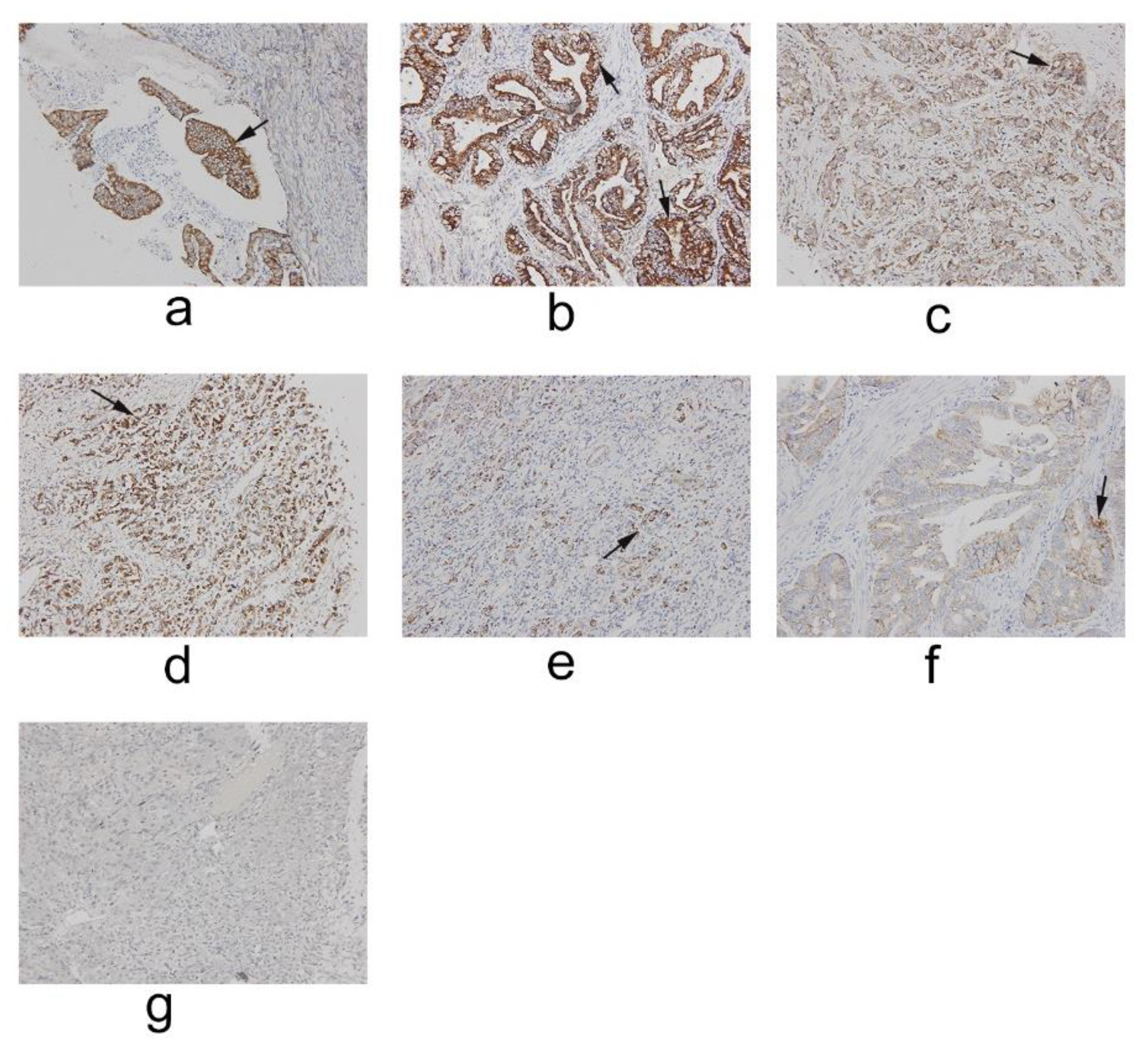
2.1. IFT80 Is Highly Expressed in Clinical Gastric Cancer
2.2. Overexpression of IFT80 Increases IFT80 Expression and Improves Cilia Formation in the SGC-7901 Gastric Cancer Cell Line
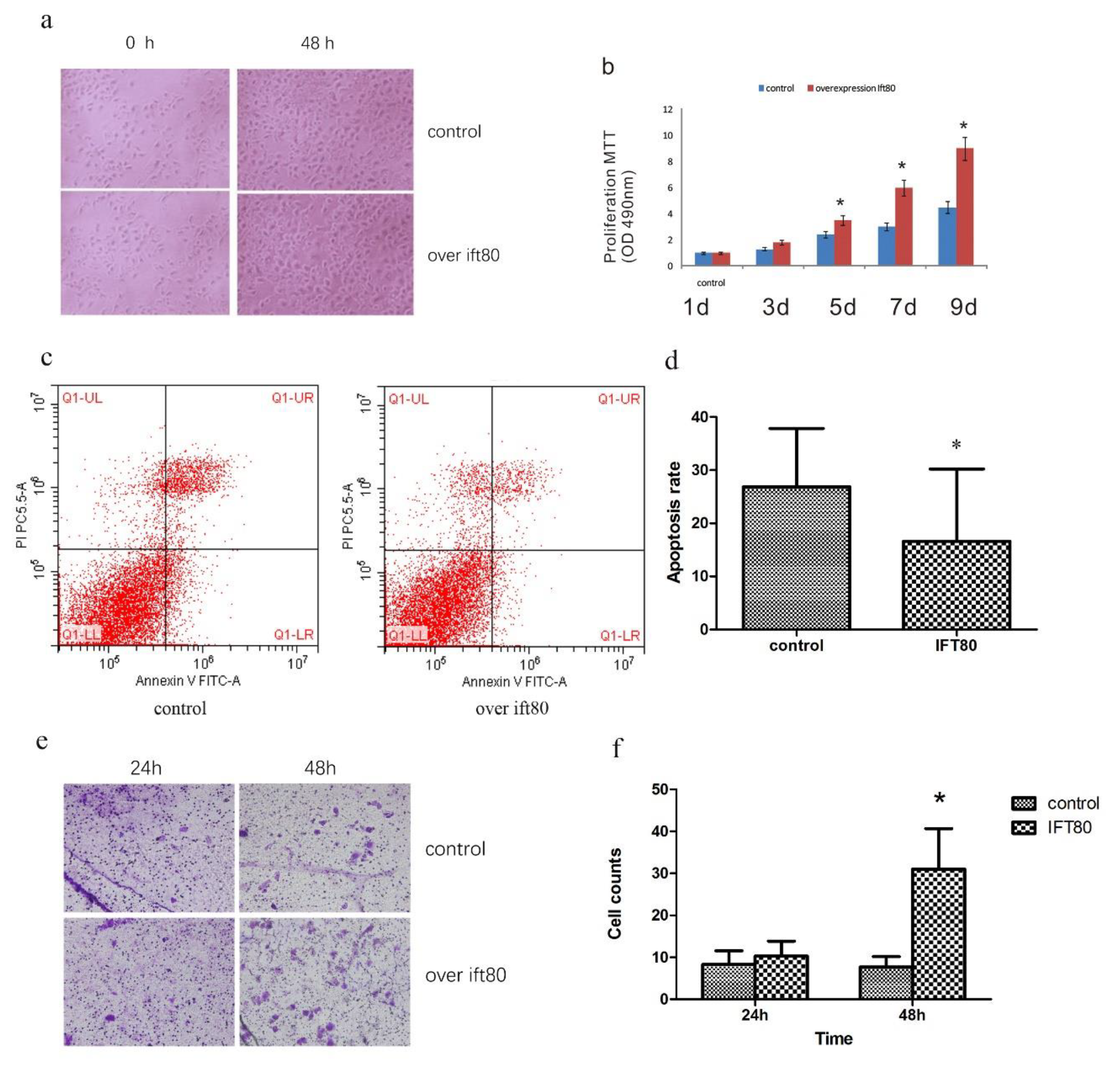
2.3. Overexpression of IFT80 Increases the Proliferation and Invasion Potential of SGC-7901 Cells, but Inhibits Apoptosis
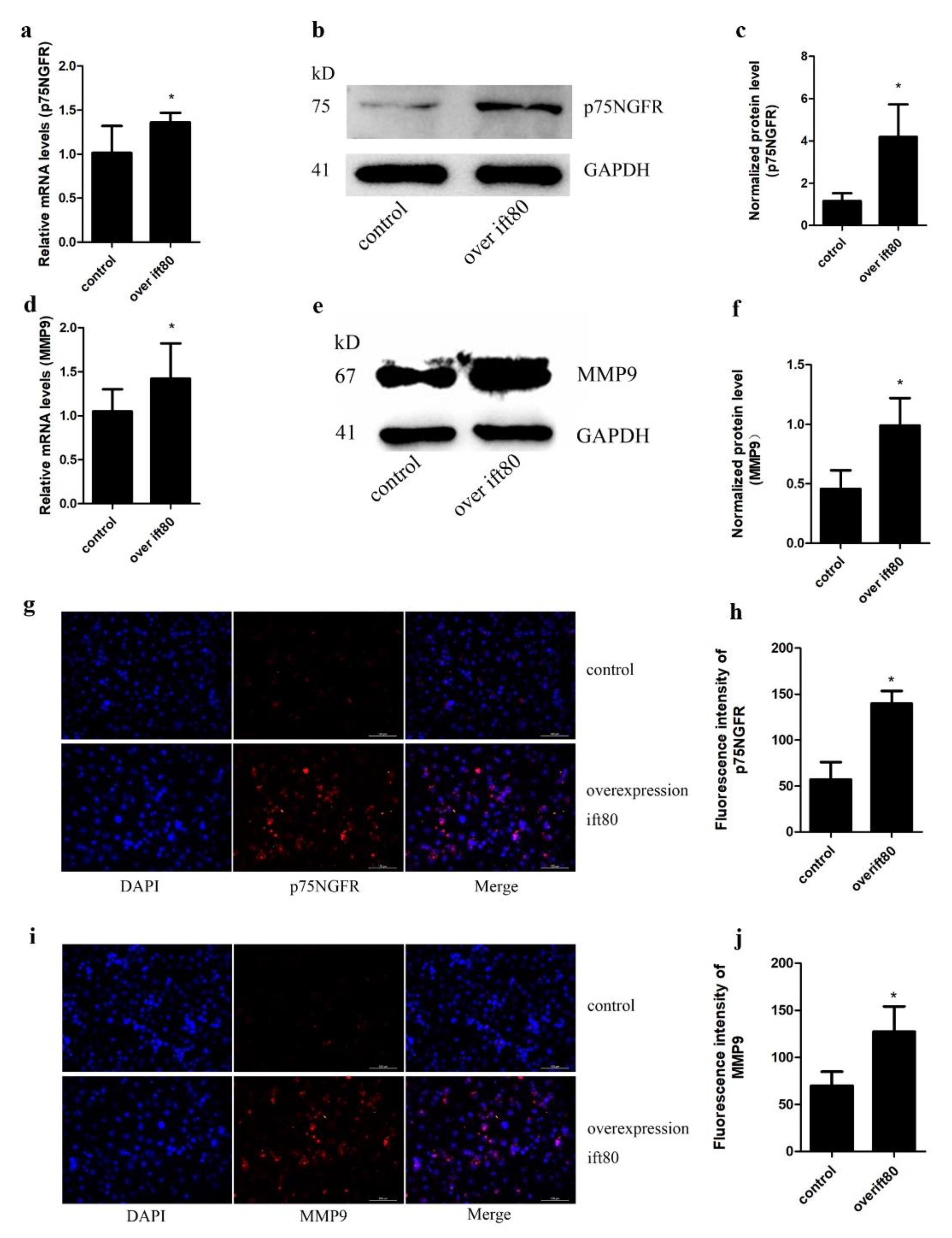
2.4. Overexpression of IFT80 Increases the mRNA and Protein Expression of p75NGFR and MMP9 in SGC-7901 Gastric Cancer Cells
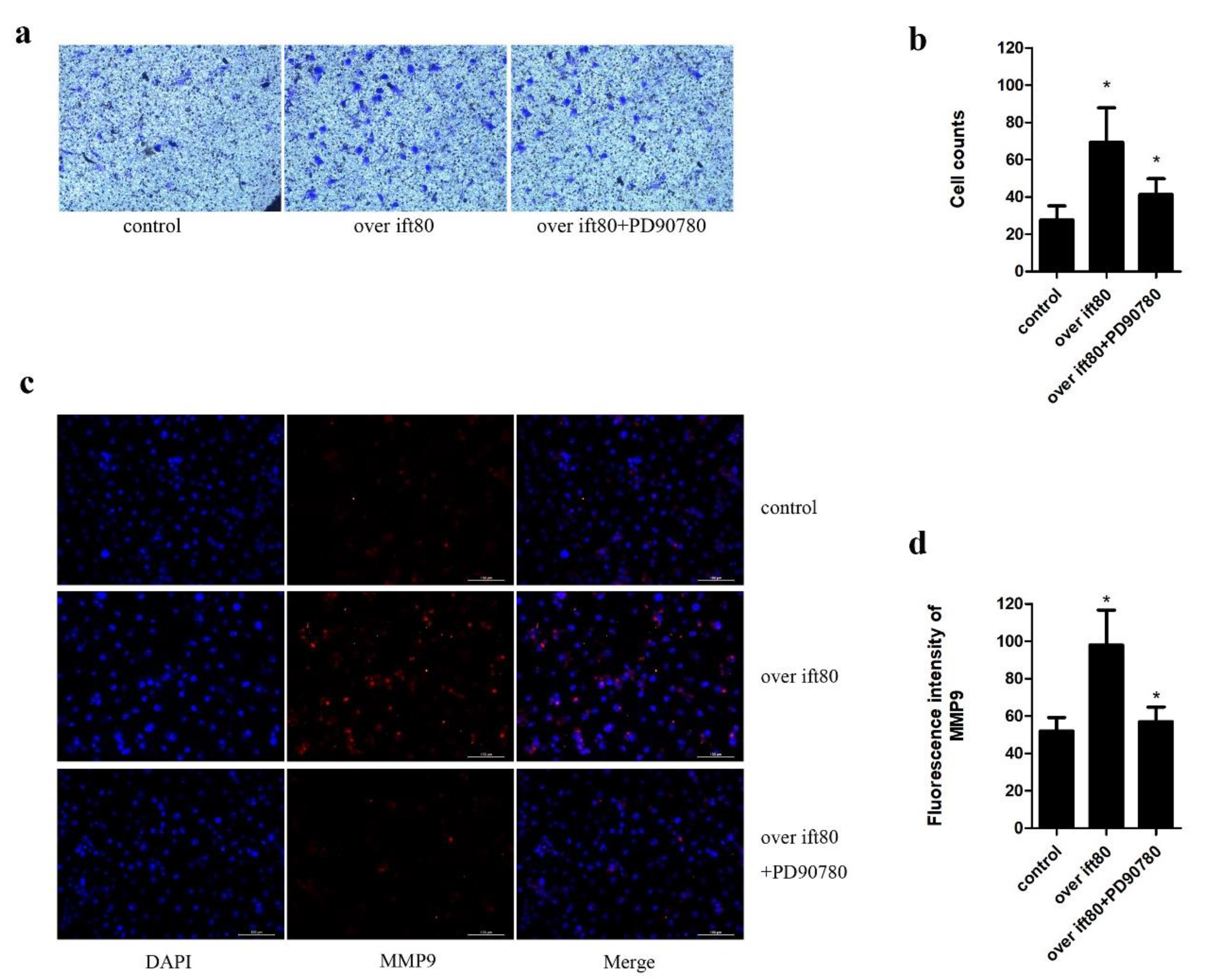
2.5. PD90780, a p75NGFR Antagonist, Inhibits the Increased Invasion Ability Resulting from Overexpression IFT80 in SGC-7901 Gastric Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Clinical Specimens Collected and Immunohistochemistry
4.3. Cell Culture and Treatment
4.4. Apoptosis Assays
4.5. Transwell Invasion Assays
4.6. Immunofluorescence
4.7. qRT-PCR
4.8. Western Blot
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ge, P.B.; Wei, L.T.; Zhang, M.K.; Hu, B.; Wang, K.; Li, Y.; Liu, S.; Wang, J.; Li, Y. TRPC1/3/6 inhibition attenuates the TGF-β1-induced epithelial-mesenchymal transition in gastric cancer via the Ras/Raf1/ERK signaling pathway. Cell Biol. Int. 2018, 42, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, L.; Ajani, J.; Xie, K. Molecular basis of gastric cancer development and progression. Gastric Cancer 2004, 7, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Zhang, S.; Zhao, P.; Zeng, H.; Zou, X. Report of cancer incidence and mortality in China, 2010. Ann. Transl Med. 2014, 2, 61–85. [Google Scholar] [PubMed]
- Zhang, X.T. Current status of clinical research on gastric cancer in China. Zhonghua Wei Chang Wai Ke Za Zhi 2013, 16, 521–523. [Google Scholar] [PubMed]
- Satir, P.; Søren, T.; Christensen, S.T. Structure and function of mammalian cilia. Histochem. Cell Biol. 2008, 129, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Satir, P.; Pedersen, L.B.; Christensen, S.T. The primary cilium at a glance. J. Cell Sci. 2010, 123, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Berbari, N.F.; O’Connor, A.K.; Haycraft, C.J.; Yoder, B.K. The primary cilium as a complex signaling center. Curr. Biol. 2009, 19, R526–R535. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Ciliogenesis: Building the cell’s antenna. Nat. Rev. Mol. Cell Biol. 2011, 12, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Dickert, B.L.; Vucica, Y.; Seeley, E.S.; Rosenbaum, J.L.; Witman, G.B.; Cole, D.G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000, 151, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Gorivodsky, M.; Mukhopadhyay, M.; Wilsch-Braeuninger, M.; Phillips, M.; Teufel, A.; Kim, C.; Malik, N.; Huttner, W.; Westphal, H. Intraflagellar transport protein 172 is essential for primary cilia formation. Dev. Biol. 2009, 325, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Wang, C.D. The intraflagellar transport protein IFT80 is required for cilia formation and osteogenesis. Bone 2012, 51, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Goetz, S.C.; Anderson, K.V. The primary cilium: A signallingcentre during vertebrate development. Nat. Rev. Genet. 2010, 11, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Keady, B.T.; Le, Y.Z.; Pazour, G.J. IFT20 is required for opsintraffick-ing and photoreceptor outer segment development. Mol. Biol. Cell 2011, 22, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Lucker, B.F.; Behal, R.H.; Qin, H.; Siron, L.C.; Taggart, W.D.; Rosenbaum, J.L.; Cole, D.G. Characterization of the intraflagellar transport complex B core: Direct interaction of the IFT81 and IFT74/72 subunits. J. Biol. Chem. 2005, 280, 27688–27696. [Google Scholar] [CrossRef] [PubMed]
- Rix, S.; Calmont, A.; Scambler, P.J.; Beales, P.L. An Ift80 mouse model of short rib polydactyly syndromes shows defects in hedgehog signalling without loss or malformation of cilia. Hum. Mol. Genet. 2011, 20, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Beales, P.L.; Bland, E.; Tobin, J.L.; Bacchelli, C.; Tuysuz, B.; Hill, J.; Rix, S.; Pearson, C.G.; Kai, M.; Hartley, J.; et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat. Genet. 2007, 39, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, D.P.; Huber, C.; Sang, K.H.; Baujat, G.; Collins, F.; Delezoide, A.L.; Dagoneau, N.; Le Merrer, M.; Martinovic, J.; Mello, M.F.; et al. Mutation in IFT80 in a fetus with the phenotype of Verma-Naumoff provides molecular evidence for Jeune-Verma-Naumoff dysplasia spectrum. J. Med. Genet. 2011, 48, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Dagoneau, N.; Goulet, M.; Genevieve, D.; Sznajer, Y.; Martinovic, J.; Smithson, S.; Hubei, C.; Baujat, G.; Flori, E.; Tecco, L.; et al. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am. J. Hum. Genet. 2009, 84, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Cao, J.; He, X.N.; Serra, R.; Qu, J.; Cao, X.; Yang, S. Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat. Commun. 2016, 7, 11024. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yan, L.; Lu, C.; Zhang, C.; Zhu, F.; Yang, J.; Jing, H.; Zhang, Y.; Qiao, J.; Guo, H. Activation of hedgehog signaling and its association with cisplatin resistance in ovarian epithelial tumors. Oncol. Lett. 2018, 15, 5569–5576. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Schmalfeldt, B.; Konik, E.; Späthe, K.; Härting, K.; Fenn, A.; Berger, U.; Fridman, R.; Schmitt, M.; Prechtel, D.; et al. Expression of latent matrix metalloproteinase 9 (MMP-9) predicts survival in advanced ovarian cancer. Gynecol. Oncol. 2001, 82, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol. Rep. 2010, 23, 605–614. [Google Scholar] [PubMed]
- Hu, X.; Li, D.; Zhang, W.; Zhou, J.; Tang, B.; Li, L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch. Gynecol. Obstet. 2012, 286, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S. Enhancedcell surface expression of matrix metalloproteinases and their inhibitors, andtumor-inducedhost response in progression of human gastric carcinoma. Dig. Dis. Sci. 2004, 49, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Khasigov, P.Z.; Podobed, O.V.; Gracheva, T.S.; Salbiev, K.D.; Grachev, S.V.; Berezov, T.T. Role of matrix metalloproteinases and their inhibitors in tumor invasion and metastasis. Biochemistry 2003, 68, 711–717. [Google Scholar] [PubMed]
- Hudak, L.M.; Lunt, S.; Chang, C.H.; Winkler, E.; Flammer, H.; Lindsey, M.; Perkins, B.D. The intraflagellar transport protein ift80 is essential for photoreceptor survival in a zebrafish model of jeune asphyxiating thoracic dystrophy. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3792–3799. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, X.; Yang, S. IFT80 is essential for chondrocyte differentiation by regulating Hedgehog and Wnt signaling pathways. Exp. Cell Res. 2013, 319, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Pazour, G.J.; Witman, G.B. The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 2003, 15, 105–110. [Google Scholar] [CrossRef]
- Eggenschwiler, J.T.; Anderson, K.V. Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 2007, 23, 345–373. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Reiter, J.F. The primary cilium as the cell’s antenna: Signaling at a sensory organelle. Science 2006, 313, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R. The nerve growth factor 35 years later. Science 1987, 237, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Ehrhard, P.; Erb, P.; Graumann, U.; Otten, U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase TRK in activated CD-4 positive Tcell clones. Proc. Natl. Acad. Sci. USA 1993, 90, 10984–10988. [Google Scholar] [CrossRef] [PubMed]
- Ehrhard, P.B.; Erb, P.; Graumann, U.; Schmutz, B.; Otten, U. Expression of functional trk tyrosine kinase receptors after T cell activation. J. Immunol. 1994, 152, 2705–2709. [Google Scholar] [PubMed]
- Otten, U.; Ehrhard, P.; Peck, R. Nerve growth factor and differentiation of human B lymphocytes. Proc. Natl. Acad. Sci. USA 1989, 86, 10059–10063. [Google Scholar] [CrossRef] [PubMed]
- Torcia, M.; Bracci-Laudiero, L.; Lucibello, M.; Nencioni, L.; Labardi, D.; Rubartelli, A.; Cozollino, F.; Aloe, L.; Garaci, E. Nerve growth factor is an autocrine survival factor for memory B lyphocytes. Cell 1996, 85, 345–356. [Google Scholar] [CrossRef]
- Saika, T.; Senba, E.; Noguchi, K.; Sato, M.; Yoshida, S.; Kubo, T.; Matsunaga, T.; Tohyama, M. Effects of nerve crush and transsection on mRNA levels for nerve growth factor receptor in the facial motoneurons. Mol. Brain Res. 1991, 9, 157. [Google Scholar] [CrossRef]
- Rocha, A.S.; Risberg, B.; Magalhaes, J.; Trovisco, V.; de Castro, I.V.; Lazarovici, P.; Soares, P.; Davidson, B.; Sobrinho-Simões, M. The p75 neurotrophin receptor is widely expressed in conventional papillary thyroid carcinoma. Hum. Pathol. 2006, 37, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, F.; Marconi, A.; Lotti, R.; Dallaglio, K.; French, L.E.; Hempstead, B.L.; Pincelli, C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J. Investig. Dermatol. 2008, 128, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Passino, M.A.; Adams, R.A.; Sikorski, S.L.; Akassoglou, K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science 2007, 315, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Quann, E.J.; Khwaja, F.; Djakiew, D. The p38 MAPK pathway mediates aryl propionic acid induced messenger RNA stability of p75 NTR in prostate cancer cells. Cancer Res. 2007, 67, 11402–11410. [Google Scholar] [CrossRef] [PubMed]
- Parrens, M.; Dubus, P.; Groppi, A.; Labouyrie, E.; de Mascarel, A.; Merlio, J.P. Differential Expression of NGF Receptors in Human Thymic Epithelial Tumors. Pathol. Res. Pract. 1999, 195, 549–553. [Google Scholar] [CrossRef]
- Barkhash, A.V.; Yurchenko, A.A.; Yudin, N.S.; Romaschenko, A.G.; Ignatieva, E.V.; Kozlova, I.V.; Borishchuk, I.A.; Pozdnyakova, L.L.; Voevoda, M.I. A matrix metalloproteinase 9 (MMP9) gene single nucleotide polymorphism is associated with predisposition to tick-borne encephalitis virus-induced severe central nervous system disease. Ticks Tick-Borne Dis. 2018, 9, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Min, K.W.; Kim, D.H.; Do, S.I.; Kim, K.; Lee, H.J.; Chae, S.W.; Sohn, J.H.; Pyo, J.S.; Oh, Y.H.; Kim, W.S.; et al. Expression patterns of stromal MMP-2 and tumoural MMP-2 and -9 are significant prognostic factors in invasive ductal carcinoma of the breast. APMIS 2014, 122, 1196–1206. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Deng, X.; Yuan, C.; Xin, H.; Liu, G.; Zhu, Y.; Jiang, X.; Wang, C. IFT80 Improves Invasion Ability in Gastric Cancer Cell Line via ift80/p75NGFR/MMP9 Signaling. Int. J. Mol. Sci. 2018, 19, 3616. https://doi.org/10.3390/ijms19113616
Wang R, Deng X, Yuan C, Xin H, Liu G, Zhu Y, Jiang X, Wang C. IFT80 Improves Invasion Ability in Gastric Cancer Cell Line via ift80/p75NGFR/MMP9 Signaling. International Journal of Molecular Sciences. 2018; 19(11):3616. https://doi.org/10.3390/ijms19113616
Chicago/Turabian StyleWang, Rui, Xiaoyan Deng, Chengfu Yuan, Hongmei Xin, Geli Liu, Yong Zhu, Xue Jiang, and Changdong Wang. 2018. "IFT80 Improves Invasion Ability in Gastric Cancer Cell Line via ift80/p75NGFR/MMP9 Signaling" International Journal of Molecular Sciences 19, no. 11: 3616. https://doi.org/10.3390/ijms19113616
APA StyleWang, R., Deng, X., Yuan, C., Xin, H., Liu, G., Zhu, Y., Jiang, X., & Wang, C. (2018). IFT80 Improves Invasion Ability in Gastric Cancer Cell Line via ift80/p75NGFR/MMP9 Signaling. International Journal of Molecular Sciences, 19(11), 3616. https://doi.org/10.3390/ijms19113616




