Transport of Apolipoprotein B-Containing Lipoproteins through Endothelial Cells Is Associated with Apolipoprotein E-Carrying HDL-Like Particle Formation
Abstract
1. Introduction
2. Results
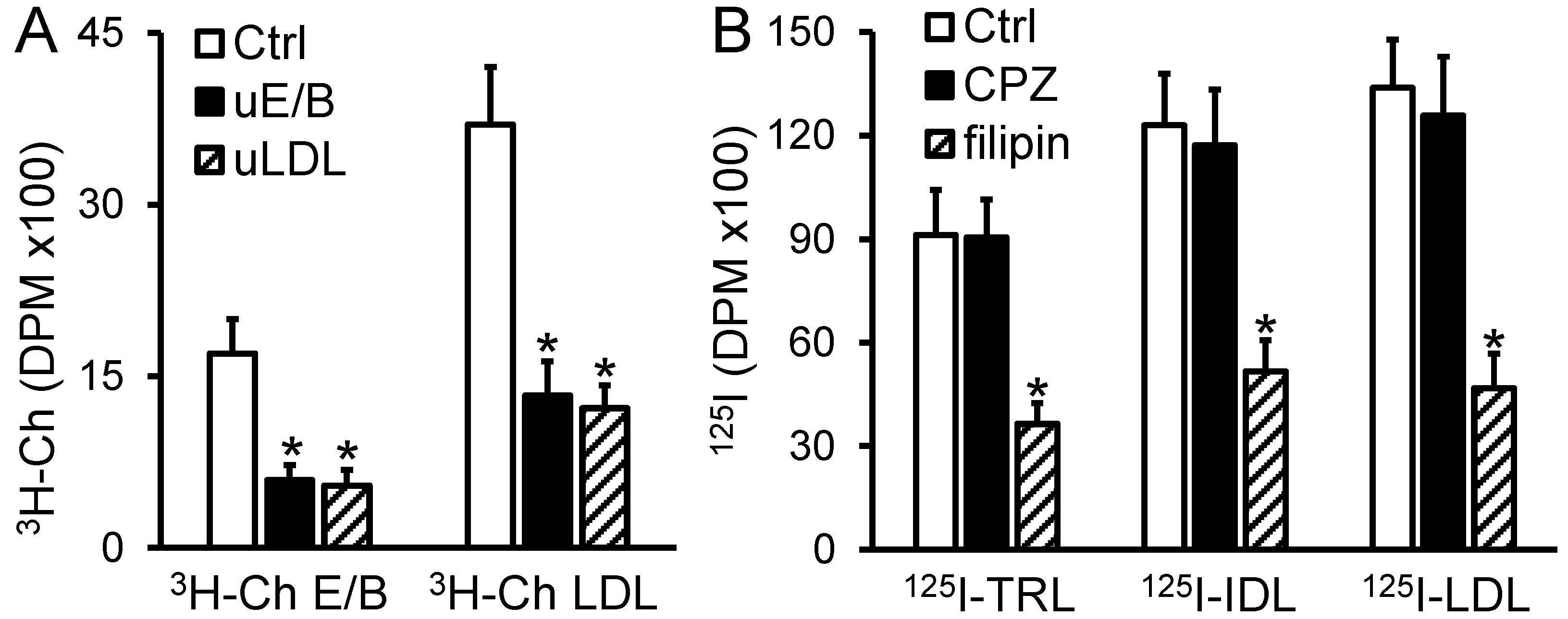
2.1. Transport of apoB-LPs through the MAEC Monolayer Is Competition-Dependent and Filipin Inhibitable
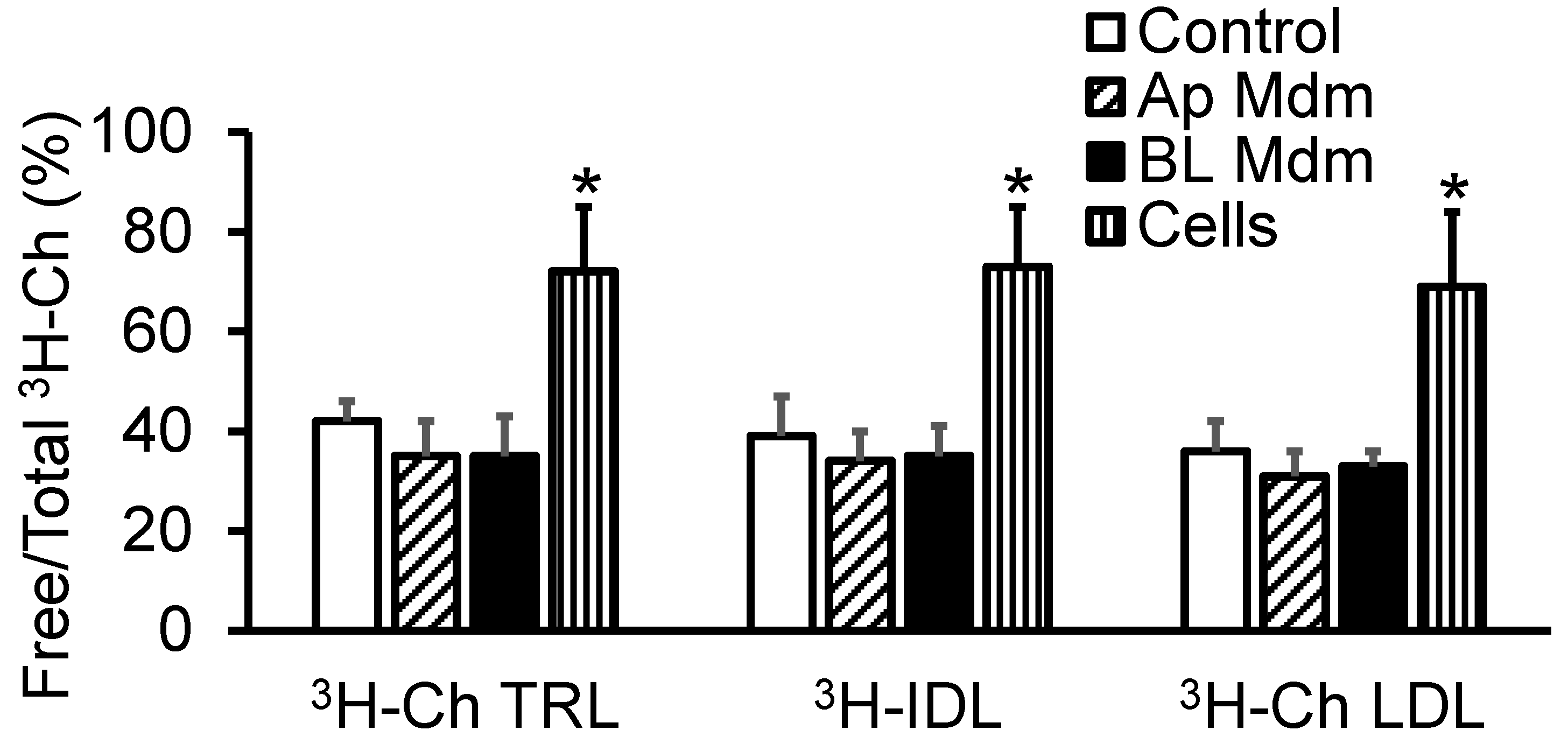
2.2. Transendothelial Transport of apoB-LPs Did Not Alter Their Free/Total Cholesterol Ratio
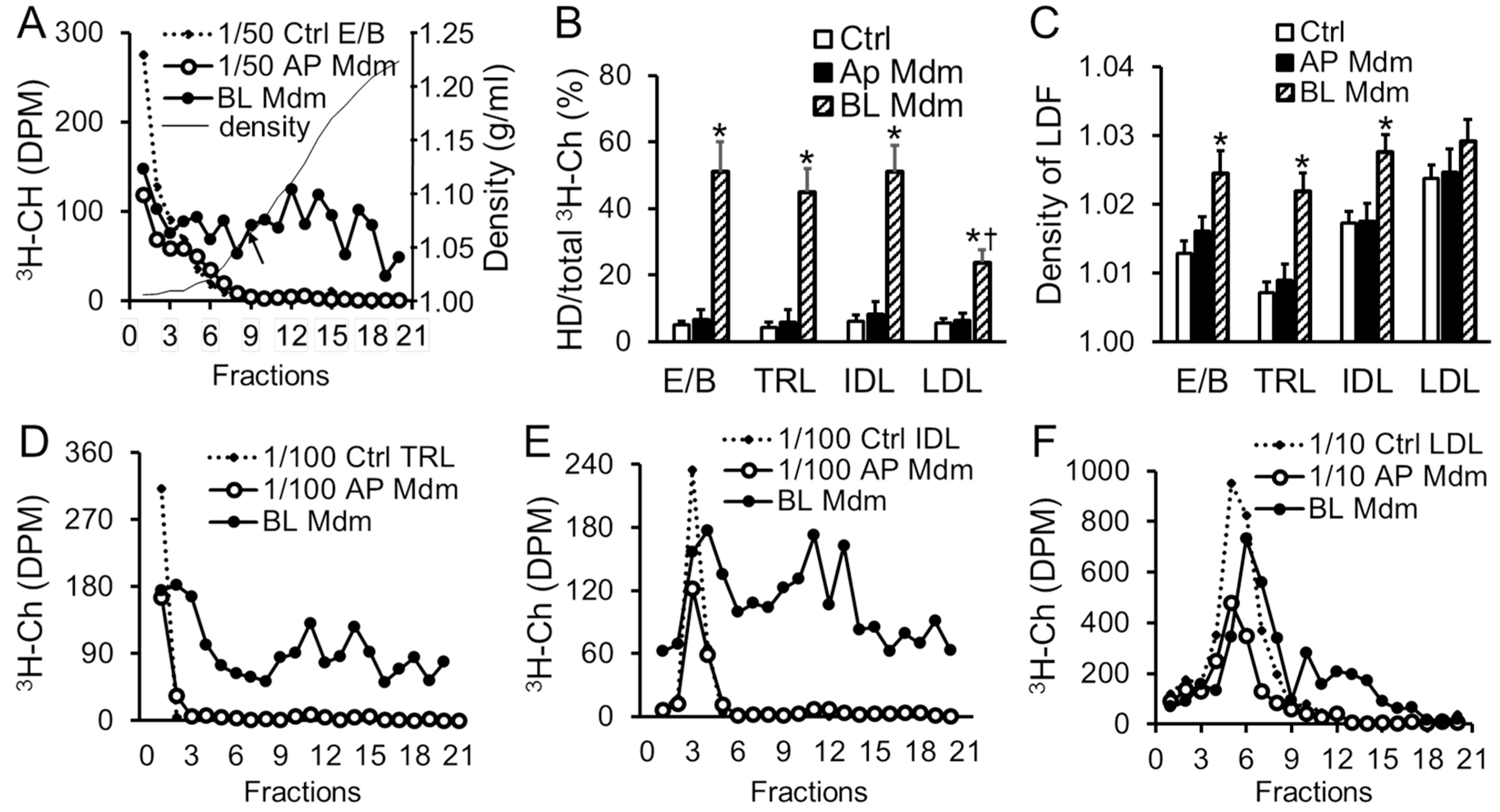
2.3. Transendothelial Transport of apoB-LPs Resulted in Cholesterol Redistribution among Lipoprotein Fractions
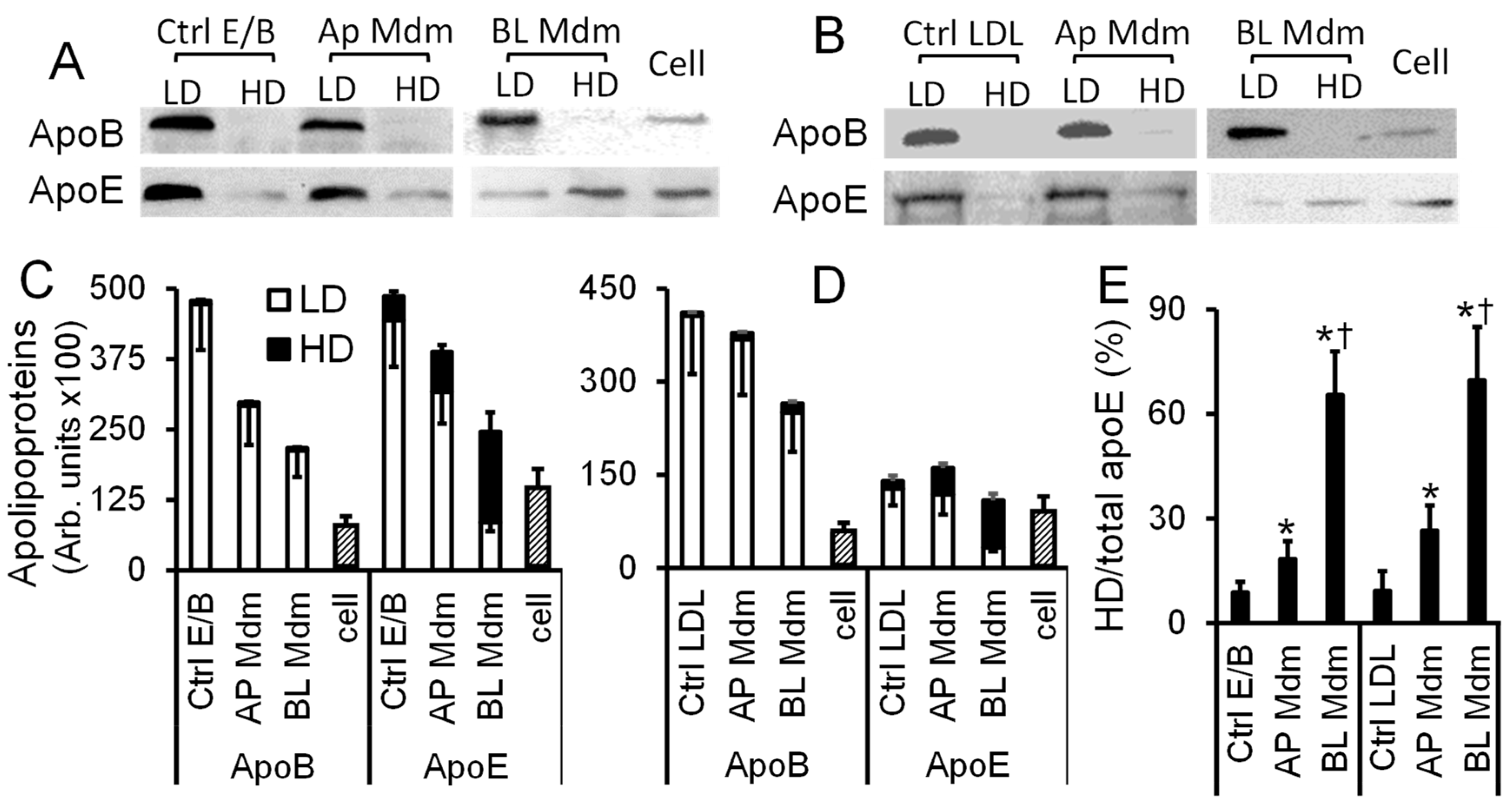
2.4. Transendothelial Transport of apoB-LPs Resulted in apoE Redistribution among Lipoprotein Fractions
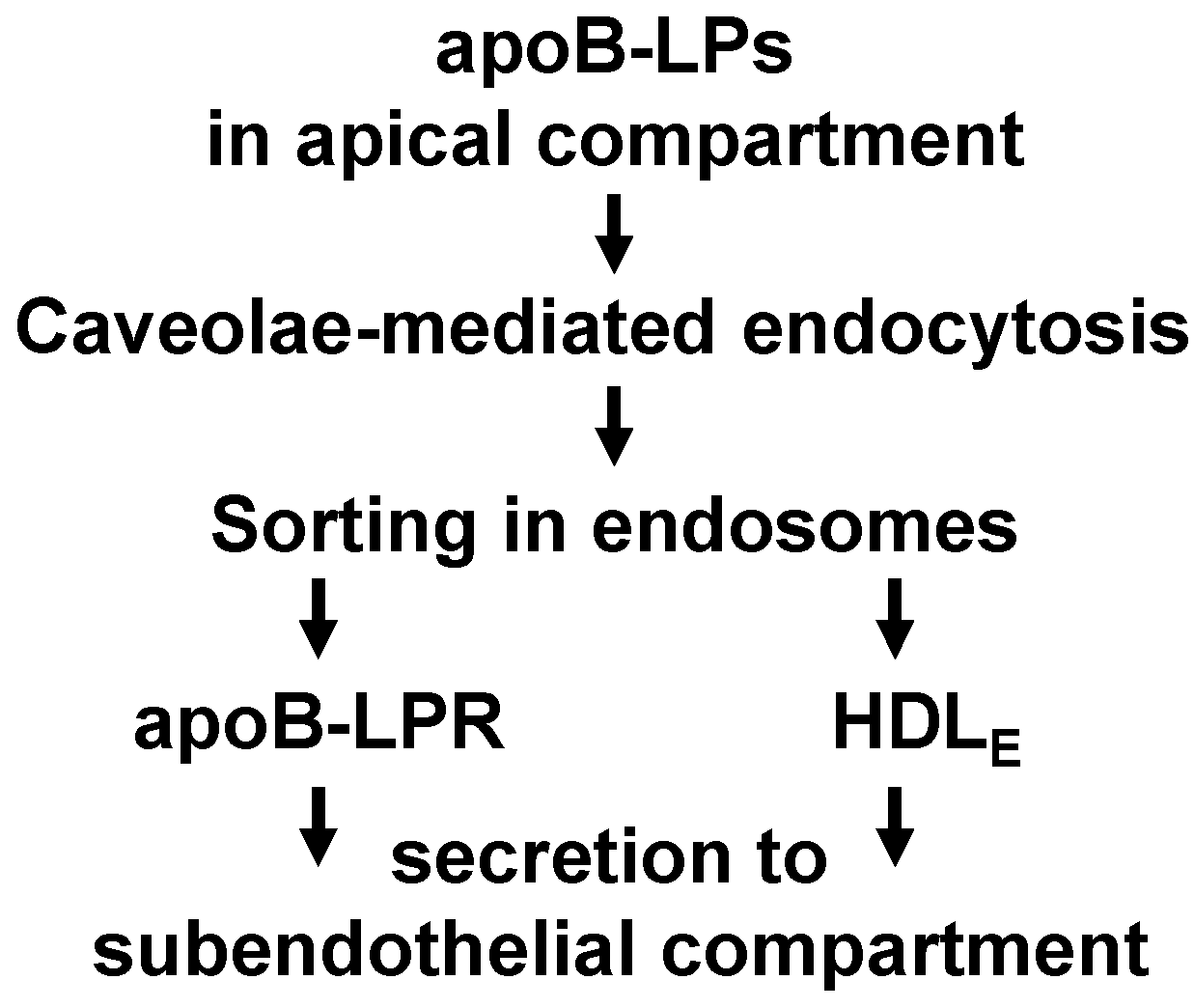
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Isolation and Radiolabeling of Lipoproteins
4.3. Transendothelial Transport of apoB-LPs
4.4. Precipitation of Free Cholesterol by Digitonin
4.5. Western Blot Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Foley, E.M.; Esko, J.D. Hepatic heparan sulfate proteoglycans and endocytic clearance of triglyceride-rich lipoproteins. Prog. Mol. Biol. Transl. Sci. 2010, 93, 213–233. [Google Scholar] [PubMed]
- Feingold, K.R.; Grunfeld, C. Introduction to Lipids and Lipoproteins. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Heeren, J.; Grewal, T.; Laatsch, A.; Becker, N.; Rinninger, F.; Rye, K.A.; Beisiegel, U. Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J. Biol. Chem. 2004, 279, 55483–55492. [Google Scholar] [CrossRef] [PubMed]
- Farkas, M.H.; Swift, L.L.; Hasty, A.H.; Linton, M.F.; Fazio, S. The recycling of apolipoprotein E in primary cultures of mouse hepatocytes. Evidence for a physiologic connection to high density lipoprotein metabolism. J. Biol. Chem. 2003, 278, 9412–9417. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Beisiegel, U.; Grewal, T. Apolipoprotein E recycling: Implications for dyslipidemia and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Hasty, A.H.; Plummer, M.R.; Weisgraber, K.H.; Linton, M.F.; Fazio, S.; Swift, L.L. The recycling of apolipoprotein E in macrophages: Influence of HDL and apolipoprotein A-I. J. Lipid Res. 2005, 46, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Roses, A.D. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu. Rev. Med. 1996, 47, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Mora, R.; Lupu, F.; Simionescu, N. Prelesional events in atherogenesis. Colocalization of apolipoprotein B, unesterified cholesterol and extracellular phospholipid liposomes in the aorta of hyperlipidemic rabbit. Atherosclerosis 1987, 67, 143–154. [Google Scholar] [CrossRef]
- Steender, S.; Zilversmit, D.B. Arterial influx of esterified cholesterol from two plasma lipoprotein fractions and its hydrolysis in vivo in hypercholesterolemic rabbits. Atherosclerosis 1981, 39, 97–109. [Google Scholar] [CrossRef]
- Tarbell, J.M. Mass transport in arteries and the localization of atherosclerosis. Annu. Rev. Biomed. Eng. 2003, 5, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Weinbaum, S.; Tzeghai, G.; Ganatos, P.; Pfeffer, R.; Chien, S. Effect of cell turnover and leaky junctions on arterial macromolecular transport. Am. J. Physiol. 1985, 248, H945–H960. [Google Scholar] [CrossRef] [PubMed]
- Hashida, R.; Anamizu, C.; Kimura, J.; Ohkuma, S.; Yoshida, Y.; Takano, T. Transcellular transport of lipoprotein through arterial endothelial cells in monolayer culture. Cell Struct. Funct. 1986, 11, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Pavlides, S.; Gutierrez-Pajares, J.L.; Iturrieta, J.; Lisanti, M.P.; Frank, P.G. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. 2014, 356, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M.; Sugiyama, M.G.; Fung, K.Y.; Gao, Y.; Wang, C.; Levy, A.S.; Azizi, P.; Roufaiel, M.; Zhu, S.N.; Neculai, D.; et al. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc. Res. 2015, 108, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kraehling, J.R.; Chidlow, J.H.; Rajagopal, C.; Sugiyama, M.G.; Fowler, J.W.; Lee, M.Y.; Zhang, X.; Ramirez, C.M.; Park, E.J.; Tao, B.; et al. Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells. Nat. Commun. 2016, 7, 13516. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Pavlides, S.; Cheung, M.W.; Daumer, K.; Lisanti, M.P. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am. J. Physiol. Cell Physiol. 2008, 295, C242–C248. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Lee, H.; Park, D.S.; Tandon, N.N.; Scherer, P.E.; Lisanti, M.P. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 98–105. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Yan, X.; Li, N.; Dang, S.; Xu, L.; Zhao, B.; Li, Z.; Lv, Z.; Fang, X.; Zhang, Y.; et al. Internalization of the TGF-β type I receptor into caveolin-1 and EEA1 double-positive early endosomes. Cell Res. 2015, 25, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.; Tallis, G.; Yalamoori, V.; Anantharamaiah, G.M.; Segrest, J.P. Liposome-like particles isolated from human atherosclerotic plaques are structurally and compositionally similar to surface remnants of triglyceride-rich lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1994, 14, 622–635. [Google Scholar] [CrossRef]
- Hoff, H.F.; Bradley, W.A.; Heideman, C.L.; Gaubatz, J.W.; Karagas, M.D.; Gotto, A.M., Jr. Characterization of low density lipoprotein-like particle in the human aorta from grossly normal and atherosclerotic regions. Biochim. Biophys. Acta 1979, 573, 361–374. [Google Scholar] [CrossRef]
- Dutta, D.; Donaldson, J.G. Search for inhibitors of endocytosis: Intended specificity and unintended consequences. Cell. Logist. 2012, 2, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Weber, W.; Beisiegel, U. Intracellular processing of endocytosed triglyceride-rich lipoproteins comprises both recycling and degradation. J. Cell Sci. 1999, 112 Pt 3, 349–359. [Google Scholar]
- Braun, N.A.; Mohler, P.J.; Weisgraber, K.H.; Hasty, A.H.; Linton, M.F.; Yancey, P.G.; Su, Y.R.; Fazio, S.; Swift, L.L. Intracellular trafficking of recycling apolipoprotein E in Chinese hamster ovary cells. J. Lipid Res. 2006, 47, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Prosser, H.C.; Ng, M.K.; Bursill, C.A. The role of cholesterol efflux in mechanisms of endothelial protection by HDL. Curr. Opin. Lipidol. 2012, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Grewal, T.; Laatsch, A.; Rottke, D.; Rinninger, F.; Enrich, C.; Beisiegel, U. Recycling of apoprotein E is associated with cholesterol efflux and high density lipoprotein internalization. J. Biol. Chem. 2003, 278, 14370–14378. [Google Scholar] [CrossRef] [PubMed]
- Heeren, J.; Grewal, T.; Jackle, S.; Beisiegel, U. Recycling of apolipoprotein E and lipoprotein lipase through endosomal compartments in vivo. J. Biol. Chem. 2001, 276, 42333–42338. [Google Scholar] [CrossRef] [PubMed]
- Tuma, P.; Hubbard, A.L. Transcytosis: Crossing cellular barriers. Physiol. Rev. 2003, 83, 871–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Brown, P.S.; Aroeti, B.; Chapin, S.J.; Mostov, K.E.; Dunn, K.W. Apical and basolateral endocytic pathways of MDCK cells meet in acidic common endosomes distinct from a nearly-neutral apical recycling endosome. Traffic 2000, 1, 480–493. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.G.; Sun, G.S.; Marchadier, D.; Maugeais, C.; Glick, J.M.; Rader, D.J. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 2002, 43, 92921–92929. [Google Scholar]
- Robert, J.; Lehner, M.; Frank, S.; Perisa, D.; von Eckardstein, A.; Rohrer, L. Interleukin 6 stimulates endothelial binding and transport of high-density lipoprotein through induction of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Paradis, M.E.; Lamarche, B. Endothelial lipase: Its role in cardiovascular disease. Can. J. Cardiol. 2006, 22 (Suppl. B), 31B–34B. [Google Scholar] [CrossRef]
- Camejo, G.; Hurt-Camejo, E.; Wiklund, O.; Bondjers, G. Association of apo B lipoproteins with arterial proteoglycans: Pathological significance and molecular basis. Atherosclerosis 1998, 139, 205–222. [Google Scholar] [CrossRef]
- Boren, J.; Williams, K.J. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: A triumph of simplicity. Curr. Opin. Lipidol. 2016, 27, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Barter, P.J.; Jones, M.E. Kinetic studies of the transfer of esterified cholesterol between human plasma low and high density lipoproteins. J. Lipid Res. 1980, 21, 238–249. [Google Scholar] [PubMed]
- Miao, L.; Okoro, E.U.; Cao, Z.; Yang, H.; Motley-Johnson, E.; Guo, Z. High-density lipoprotein-mediated transcellular cholesterol transport in mouse aortic endothelial cells. Biochem. Biophys. Res. Commun. 2015, 465, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Yang, H.; Zhou, L.; Okoro, E.U.; Guo, Z. A novel function of apolipoprotein E: Upregulation of ATP-binding cassette transporter A1 expression. PLoS ONE 2011, 6, e21453. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Goldstein, S.; Lagrange, D.; Laplaud, P.M. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 1981, 22, 339–358. [Google Scholar] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, N.; Okoro, E.U.; Guo, Z. Transport of Apolipoprotein B-Containing Lipoproteins through Endothelial Cells Is Associated with Apolipoprotein E-Carrying HDL-Like Particle Formation. Int. J. Mol. Sci. 2018, 19, 3593. https://doi.org/10.3390/ijms19113593
Yang H, Zhang N, Okoro EU, Guo Z. Transport of Apolipoprotein B-Containing Lipoproteins through Endothelial Cells Is Associated with Apolipoprotein E-Carrying HDL-Like Particle Formation. International Journal of Molecular Sciences. 2018; 19(11):3593. https://doi.org/10.3390/ijms19113593
Chicago/Turabian StyleYang, Hong, Ningya Zhang, Emmanuel U. Okoro, and Zhongmao Guo. 2018. "Transport of Apolipoprotein B-Containing Lipoproteins through Endothelial Cells Is Associated with Apolipoprotein E-Carrying HDL-Like Particle Formation" International Journal of Molecular Sciences 19, no. 11: 3593. https://doi.org/10.3390/ijms19113593
APA StyleYang, H., Zhang, N., Okoro, E. U., & Guo, Z. (2018). Transport of Apolipoprotein B-Containing Lipoproteins through Endothelial Cells Is Associated with Apolipoprotein E-Carrying HDL-Like Particle Formation. International Journal of Molecular Sciences, 19(11), 3593. https://doi.org/10.3390/ijms19113593



