Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis
Abstract
:1. Introduction
2. Results
2.1. Adenosine and Cordycepin Contents in GRC-ON89A
2.2. Total Polyphenol Contents (TPC) and Total Flavonoid Contents (TFC) in GRC-ON89A
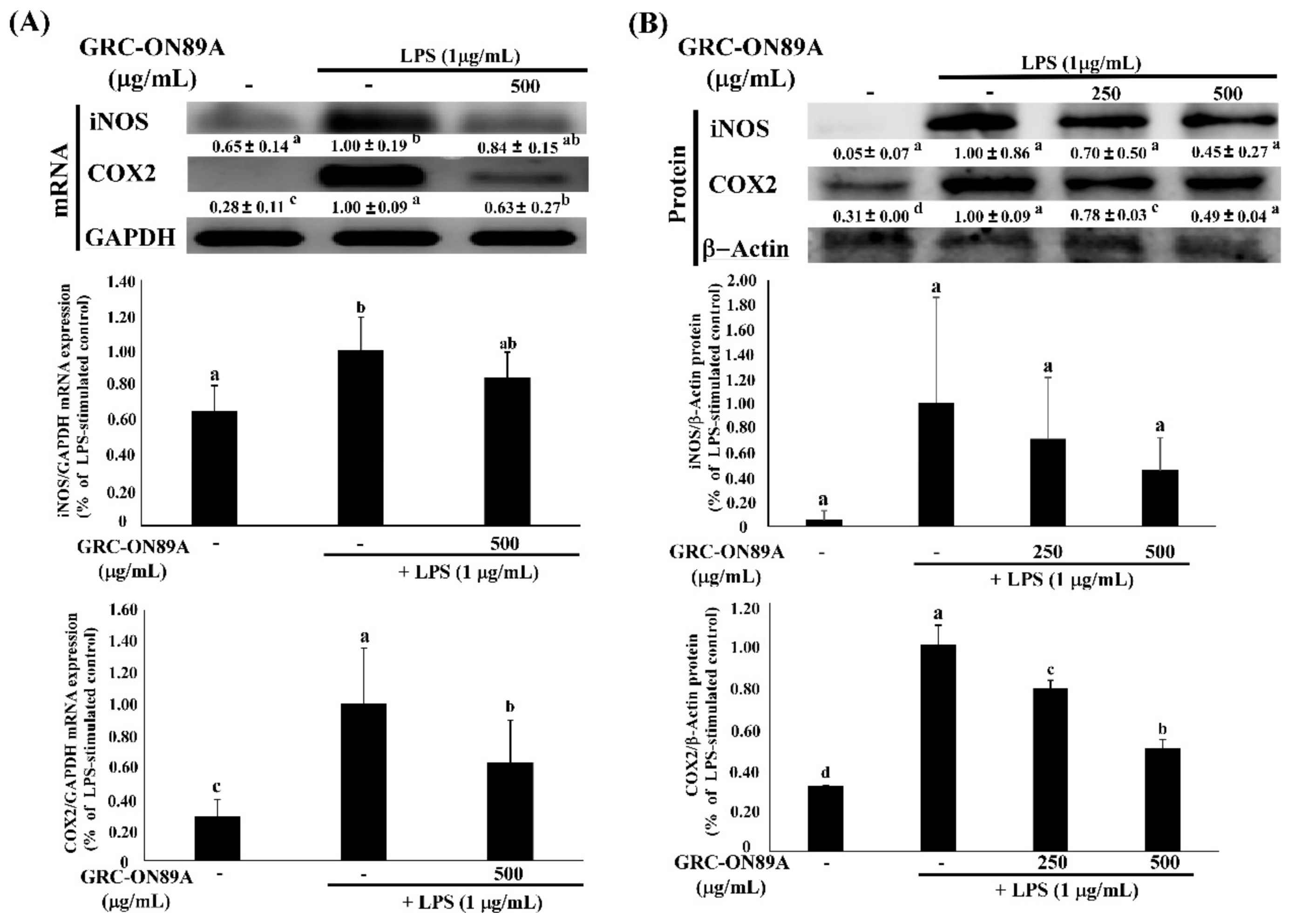
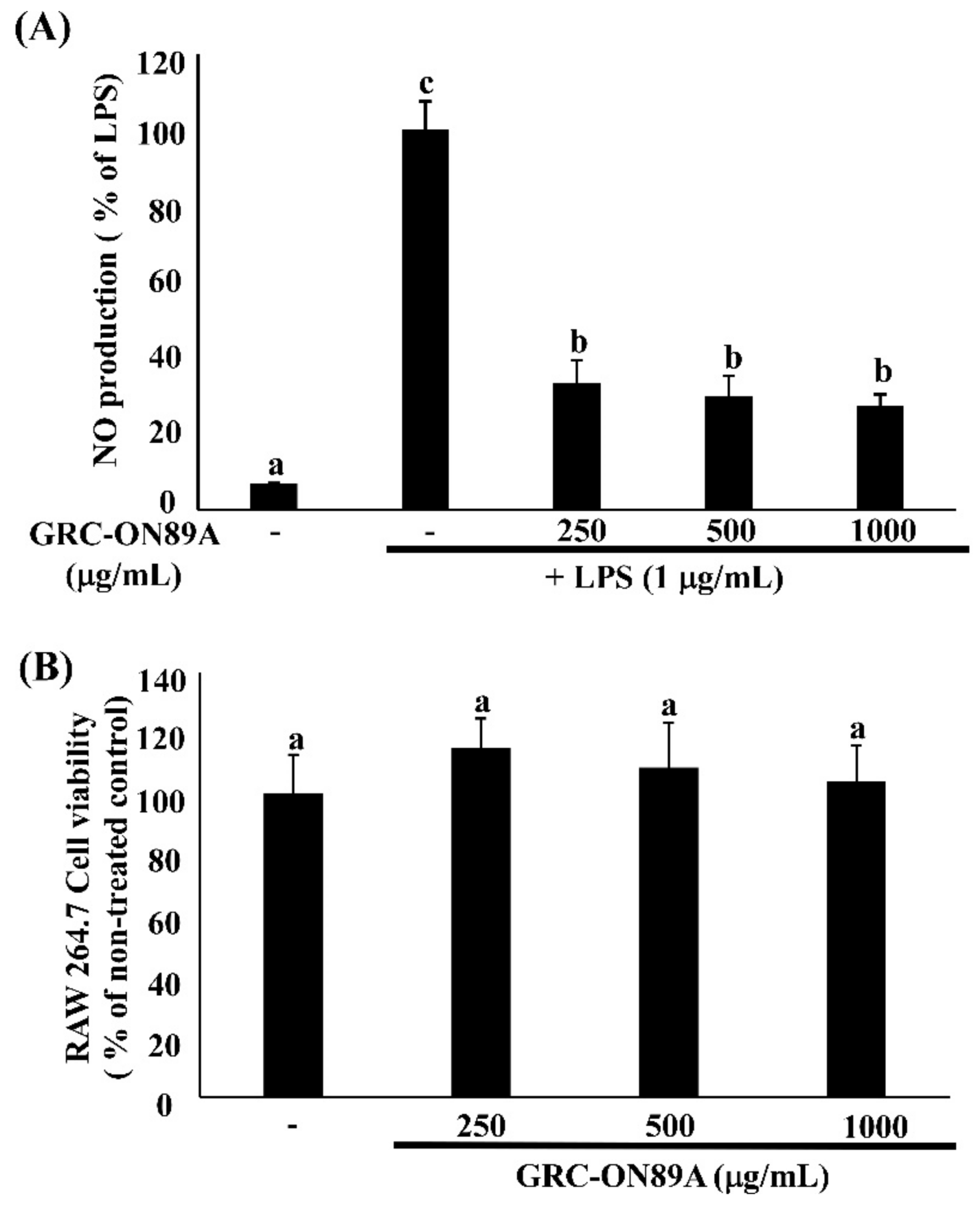
2.3. GRC-ON89A Downregulates Nitric Oxide (NO) Secretion and Inducible NO Synthase (iNOS) mRNA and Protein Expression in LPS-Stimulated RAW 264.7 Macrophages
2.4. GRC-ON89A-Hex Inhibits mRNA and Protein Expression of Inflammatory Mediators
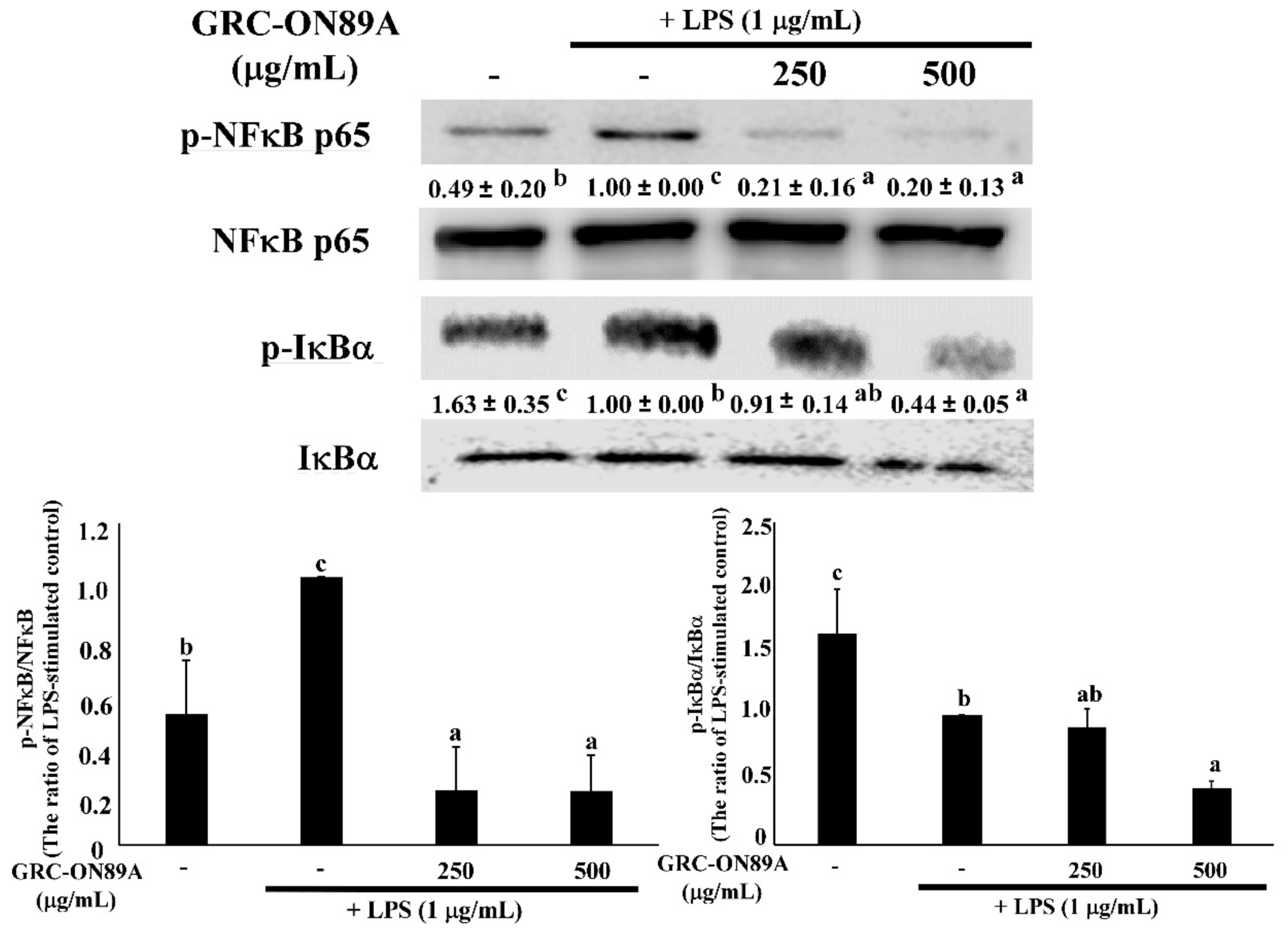
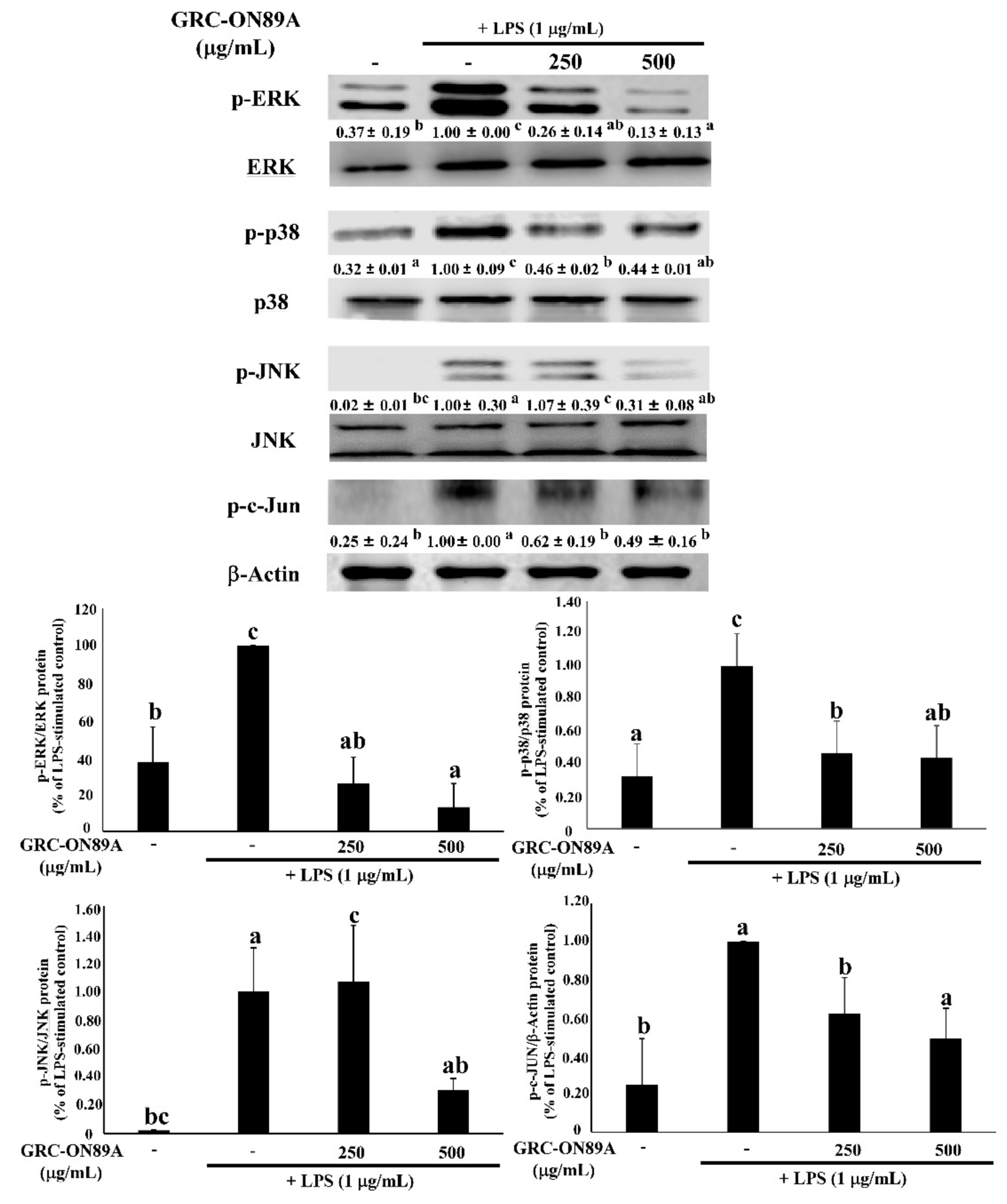
2.5. GRC-ON89A-Hex Suppresses Phosphorylation of Mitogen-Activated Protein Kinases (MAPKs) and Nuclear Factor (NF)-κB p65 in LPS-Stimulated RAW 264.7 Macrophages
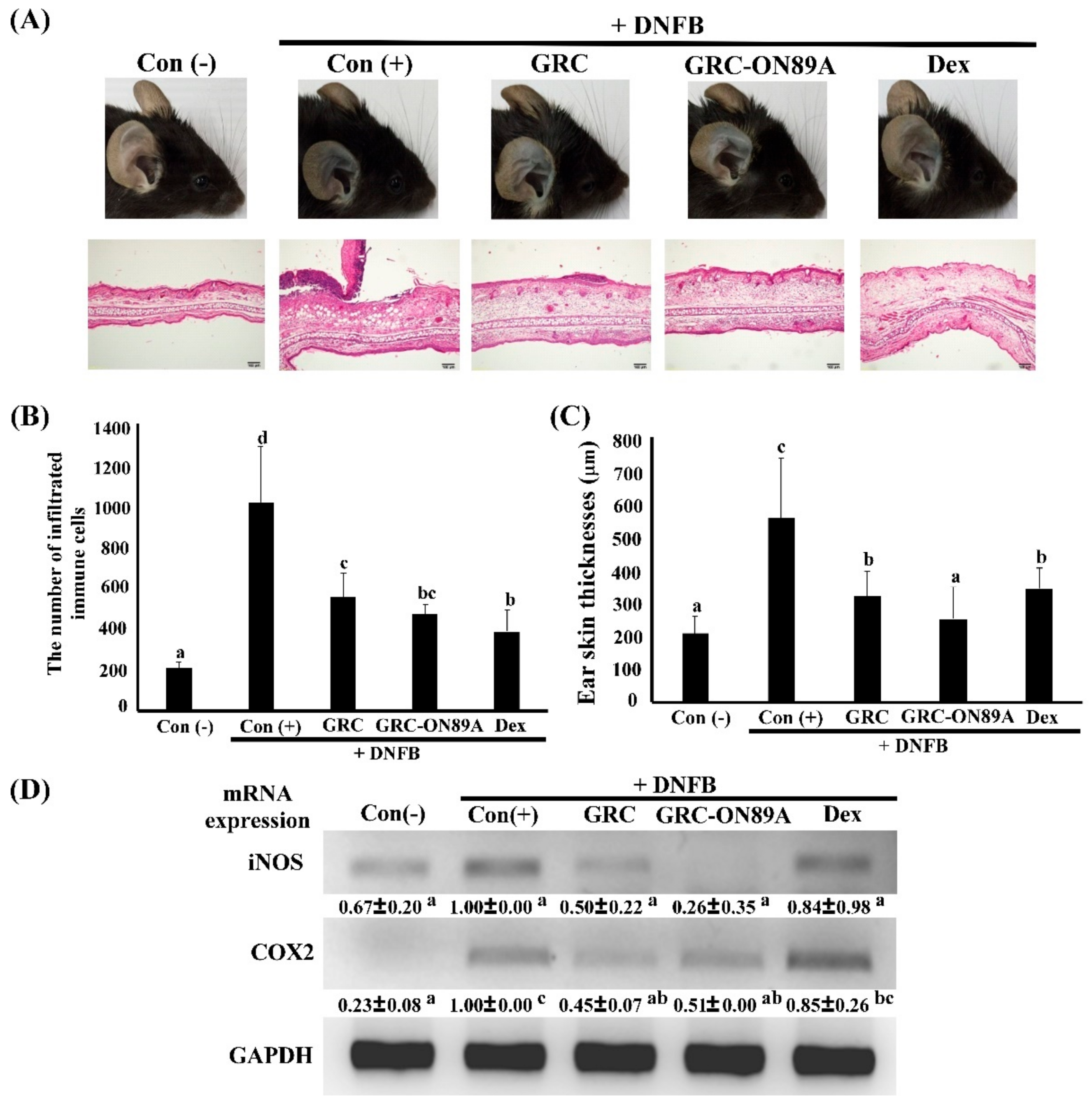
2.6. GRC-ON89A Reduces DNFB-Induced ACD in BALB/c Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of GRC Fermented with Probiotic Strains
4.2. Preparation of GRC-ON89A-Hex
4.3. Quantitative Analysis of GRC and GRC-ON89A Extracts Using GC-TOF MS
4.4. Determination of TPC and TFC
4.5. Cell Culture
4.6. RAW 264.7 Macrophages Viability
4.7. Measurement of NO Production
4.8. RNA Isolation and RT-PCR
4.9. Western Blot Analysis
4.10. Experimental Design
4.11. Histopathological Examination of Animal Tissue
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Lee, M.R.; San Lee, G.; An, W.G.; Cho, S.I. Effect of Sophora flavescens Aiton extract on degranulation of mast cells and contact dermatitis induced by dinitrofluorobenzene in mice. J. Ethnopharmacol. 2012, 142, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Park, D.K.; Lee, Y.G.; Park, H.-J. Extract of Rhus verniciflua bark suppresses 2,4-dinitrofluorobenzene-induced allergic contact dermatitis. Evid.-Based Complement. Altern. Med. 2013, 2013, 879696. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yoon, S.H.; Ahn, H.S.; Lee, M.W. Inhibitory Activity of Allergic Contact Dermatitis and Atopic Dermatitis-Like Skin in BALB/c Mouse through Oral Administration of Fermented Barks of Alnus sibirica. Molecules 2018, 23, 450. [Google Scholar] [CrossRef] [PubMed]
- Poveda-Montoyo, I.; Álvarez-Chinchilla, P.; Silvestre, J. Allergic Contact Dermatitis: Therapeutic Management. Curr. Treat. Options Allergy 2018, 5, 140–153. [Google Scholar] [CrossRef]
- Uter, W.; Werfel, T.; White, I.R.; Johansen, J.D. Contact Allergy: A Review of Current Problems from a Clinical Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Asati, D.P. Pediatric contact dermatitis. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.; Goldenberg, A.; Welsh, O.; Jacob, S. Contact dermatitis: Therapeutics when avoidance fails. J. Allergy Ther. 2014, 5, 1–4. [Google Scholar] [CrossRef]
- Park, H.-J.; Han, E.S.; Park, D.K.; Lee, C.; Lee, K.W. An extract of Phellinus linteus grown on germinated brown rice inhibits inflammation markers in RAW264. 7 macrophages by suppressing inflammatory cytokines, chemokines, and mediators and up-regulating antioxidant activity. J. Med. Food 2010, 13, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. DARU J. Pharm. Sci. 2015, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.; Son, M.; Lee, M.; Lee, K.; Cho, J.Y.; Cho, S.; Lee, S.K.; Lee, Y.M.; Cho, H.; Sung, G.-H. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience 2014, 1, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Pochard, P.; Gosset, P.; Grangette, C.; Andre, C.; Tonnel, A.-B.; Pestel, J.; Mercenier, A. Lactic acid bacteria inhibit TH2 cytokine production by mononuclear cells from allergic patients. J. Allergy Clin. Immunol. 2002, 110, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Chapat, L.; Chemin, K.; Dubois, B.; Bourdet-Sicard, R.; Kaiserlian, D. Lactobacillus casei reduces CD8+ T cell-mediated skin inflammation. Eur. J. Immunol. 2004, 34, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J. Ethanol extract of Cordyceps militaris grown on germinated soybeans inhibits 2,4-dinitrophenolfluorobenzene-induced allergic contact dermatitis. J. Funct. Foods 2015, 17, 938–947. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Jo, W.-R.; Park, H.-J. Immune-enhancing activity of C. militaris fermented with Pediococcus pentosaceus (GRC-ON89A) in CY-induced immunosuppressed model. BMC Complement. Altern. Med. 2018, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Ando, T.; Maeda, O.; Ohmiya, N.; Niwa, Y.; Goto, H. Acetate inhibits NFAT activation in T cells via importin β1 interference. Eur. J. Immunol. 2007, 37, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Moon, S.; Park, Y.; Kwon, J.; Lee, S.; Lee, C.K.; Cho, K.; Ha, N.J.; Kim, K. Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines. Immune Netw. 2009, 9, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, G.-Y.; Lee, H.H. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through Toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-κB signaling pathways. Drug Des. Dev. Ther. 2014, 8, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.B.; Reddanna, P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-κB and MAPK activation in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2009, 381, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Man, M.-Q.; Hupe, M.; Sun, R.; Man, G.; Mauro, T.M.; Elias, P.M. Topical apigenin alleviates cutaneous inflammation in murine models. Evid.-Based Complement. Altern. Med. 2012, 2012, 912028. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, L.; Sung, G.H.; Kim, T.W.; Byeon, S.E.; Cho, J.Y.; Park, C.W.; Park, H.J. Inhibition of 2,4-dinitrofluorobenzene-induced atopic dermatitis by topical application of the butanol extract of Cordyceps bassiana in NC/Nga mice. J. Ethnopharmacol. 2011, 134, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, V.; A Jhaveri, K.; Xie, X.; Jajoo, S.; A Toth, L. Nuclear Factor κB and Adenosine Receptors: Biochemical and Behavioral Profiling. Curr. Neuropharmacol. 2011, 9, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Scoka, B.; Nemeth, Z.; Virag, L.; Gergely, P.; Leibovich, S.; Pacher, P.; Sun, C.; Blackburn, M.; Vizi, E.; Deitch, E. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to E. coli. Blood 2007, 110, 2685–2695. [Google Scholar]
- Ryzhov, S.; Zaynagetdinov, R.; Goldstein, A.E.; Novitskiy, S.V.; Blackburn, M.R.; Biaggioni, I.; Feoktistov, I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J. Pharmacol. Exp. Ther. 2008, 324, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, F.G.; Takabayashi, K.; Foster, A.C.; Domingo, R.C.; Firestein, G.S. Inhibition of TNF-alpha expression by adenosine: Role of A3 adenosine receptors. J. Immunol. 1996, 156, 3435–3442. [Google Scholar] [PubMed]
- Cronstein, B.N.; Haskó, G. Regulation of inflammation by adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the interaction of tannins with co-existing substances. VI.: Effects of tannins and related polyphenols on superoxide anion radical, and on 1,1-Diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Dick, C.A.; Brown, D.M.; Donaldson, K.; Stone, V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal. Toxicol. 2003, 15, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; O’Connell, M.A.; Pawlinski, R.; Hollis, A.; McGovern, P.; Yan, S.-F.; Stern, D.; Mackman, N. Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor α expression by inducing Elk-1 phosphorylation and Egr-1 expression: Presented in abstract form at the 42nd annual meeting of the American Society of Hematology, December 1–5, 2000, San Francisco, CA. Blood 2001, 98, 1429–1439. [Google Scholar] [PubMed]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.-J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-κB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Huang, Q.; Qu, W.; Li, L.; Wang, M.; Li, S.; Chu, F. In vivo and in vitro anti-inflammatory effects of Sophora flavescens residues. J. Ethnopharmacol. 2018, 224, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Higasa, M.; Sagami, S.; Shinka, S. IL-1 and PGE2 Productions by the Regional Lymph Node Cells from DNFB-sensitized Mice. J. Dermatol. 1989, 16, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Tumor necrosis factors: Developments during the last decade. Eur. Cytokine Netw. 1996, 7, 93–124. [Google Scholar] [PubMed]
- Blatteis, C.M.; Li, S.; Li, Z.; Perlik, V.; Feleder, C. Signaling the brain in systemic inflammation: The role of complement. Front. Biosci. 2004, 9, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 10, a001651. [Google Scholar]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Chun, K.-S.; Cha, H.-H.; Han, S.S.; Keum, Y.-S.; Park, K.-K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Takashiba, S.; Van Dyke, T.E.; Amar, S.; Murayama, Y.; Soskolne, A.W.; Shapira, L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor κB. Infect. Immunity 1999, 67, 5573–5578. [Google Scholar]
- Chen, Z.; Hagler, J.; Palombella, V.J.; Melandri, F.; Scherer, D.; Ballard, D.; Maniatis, T. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995, 9, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta BBA Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Adler, V.; Polotskaya, A.; Wagner, F.; Kraft, A.S. Affinity-purified c-Jun amino-terminal protein kinase requires serine/threonine phosphorylation for activity. J. Biol. Chem. 1992, 267, 17001–17005. [Google Scholar] [PubMed]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef] [PubMed]
- Hasko, G.; Szabó, C.; Németh, Z.H.; Kvetan, V.; Pastores, S.; Vizi, E.S. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996, 157, 4634–4640. [Google Scholar] [PubMed]
- Yuan, X.-Y.; Liu, W.; Zhang, P.; Wang, R.-Y.; Guo, J.-Y. Effects and mechanisms of aloperine on 2,4-dinitrofluorobenzene-induced allergic contact dermatitis in BALB/c mice. Eur. J. Pharmacol. 2010, 629, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Schneeberger, E.E.; Siraganian, R.P.; Geha, R.S.; Bhan, A.K. The presence of IgE on macrophages and dendritic cells infiltrating into the skin lesion of atopic dermatitis. Clin. Immunol. Immunopathol. 1987, 42, 328–337. [Google Scholar] [CrossRef]
- Homey, B.; Steinhoff, M.; Ruzicka, T.; Leung, D.Y. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 118, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Zollner, T.; Kaufmann, R.; Podda, M. Redox-modulated pathways in inflammatory skin diseases. Free Radic. Biol. Med. 2001, 30, 337–353. [Google Scholar] [CrossRef]
- Portugal, M.; Barak, V.; Ginsburg, I.; Kohen, R. Interplay among oxidants, antioxidants, and cytokines in skin disorders: Present status and future considerations. Biomed. Pharmacother. 2007, 61, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Park, H.; Kim, H.P. Inhibition of contact dermatitis in animal models and suppression of proinflammatory gene expression by topically applied flavonoid, wogonin. Arch. Pharmacal. Res. 2004, 27, 442. [Google Scholar] [CrossRef]
- Han, N.-R.; Moon, P.-D.; Kim, H.-M.; Jeong, H.-J. Cordycepin ameliorates skin inflammation in a DNFB-challenged murine model of atopic dermatitis. Immunopharmacol. Immunotoxicol. 2018, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, J.-J.; Barupal, D.K.; Fiehn, O. System response of metabolic networks in Chlamydomonas reinhardtii to total available ammonium. Mol. Cell. Proteom. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Cho, Y.U.; Kim, K.H.; Lee, D.Y. Distinctive metabolomic responses of Chlamydomonas reinhardtii to the chemical elicitation by methyl jasmonate and salicylic acid. Process Biochem. 2016, 51, 1147–1154. [Google Scholar] [CrossRef]
- Lee, J.-E.; Lee, Y.H.; Kim, S.-Y.; Kim, Y.G.; Moon, J.-Y.; Jeong, K.-H.; Lee, T.W.; Ihm, C.-G.; Kim, S.; Kim, K.H. Systematic biomarker discovery and coordinative validation for different primary nephrotic syndromes using gas chromatography–mass spectrometry. J. Chromatogr. A 2016, 1453, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.F.; Hassan, S.A.; Yusoff, U.K.; Abdullah, N.A.P.; Wahab, P.E.M.; Sinniah, U.R. Phenolics, flavonoids, antioxidant activity and cyanogenic glycosides of organic and mineral-base fertilized cassava tubers. Molecules 2012, 17, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yang, H.J.; Kim, K.H.; Kim, S.H. Aqueous extract of Orostachys japonicus A. Berger exerts immunostimulatory activity in RAW 264.7 macrophages. J. Ethnopharmacol. 2015, 170, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Park, H.J. Anti-inflammatory effect of Phellinus linteus grown on germinated brown rice on dextran sodium sulfate-induced acute colitis in mice and LPS-activated macrophages. J. Ethnopharmacol. 2014, 154, 311–318. [Google Scholar] [CrossRef] [PubMed]


| Target Bioactive Compounds | GRC-ON89A | GRC |
|---|---|---|
| Adenosine (mg/g) | 7.03 ± 0.15 | 3.88 ± 0.08 |
| Cordycepin (μg/g) | 1053.33 ± 11.81 | 180.58 ± 1.54 |
| Compounds | GRC-ON89A | GRC |
|---|---|---|
| Total polyphenol contents (mg GAE/g) | 15.92 ± 1.20 *** | 7.65 ± 1.70 |
| Total flavonoid contents (mg QE/g) | 7.47 ± 0.27 *** | 5.37 ± 0.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.-K.; Song, M.-J.; Lee, H.-J.; Park, T.-S.; Kim, M.I.; Park, H.-J. Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. Int. J. Mol. Sci. 2018, 19, 3504. https://doi.org/10.3390/ijms19113504
Kwon H-K, Song M-J, Lee H-J, Park T-S, Kim MI, Park H-J. Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. International Journal of Molecular Sciences. 2018; 19(11):3504. https://doi.org/10.3390/ijms19113504
Chicago/Turabian StyleKwon, Ha-Kyoung, Min-Jung Song, Hye-Ji Lee, Tae-Sik Park, Moon Il Kim, and Hye-Jin Park. 2018. "Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis" International Journal of Molecular Sciences 19, no. 11: 3504. https://doi.org/10.3390/ijms19113504
APA StyleKwon, H.-K., Song, M.-J., Lee, H.-J., Park, T.-S., Kim, M. I., & Park, H.-J. (2018). Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. International Journal of Molecular Sciences, 19(11), 3504. https://doi.org/10.3390/ijms19113504






