Characterization of Hydroxyproline-Containing Hairpin-Like Antimicrobial Peptide EcAMP1-Hyp from Barnyard Grass (Echinochloa crusgalli L.) Seeds: Structural Identification and Comparative Analysis of Antifungal Activity
Abstract
1. Introduction
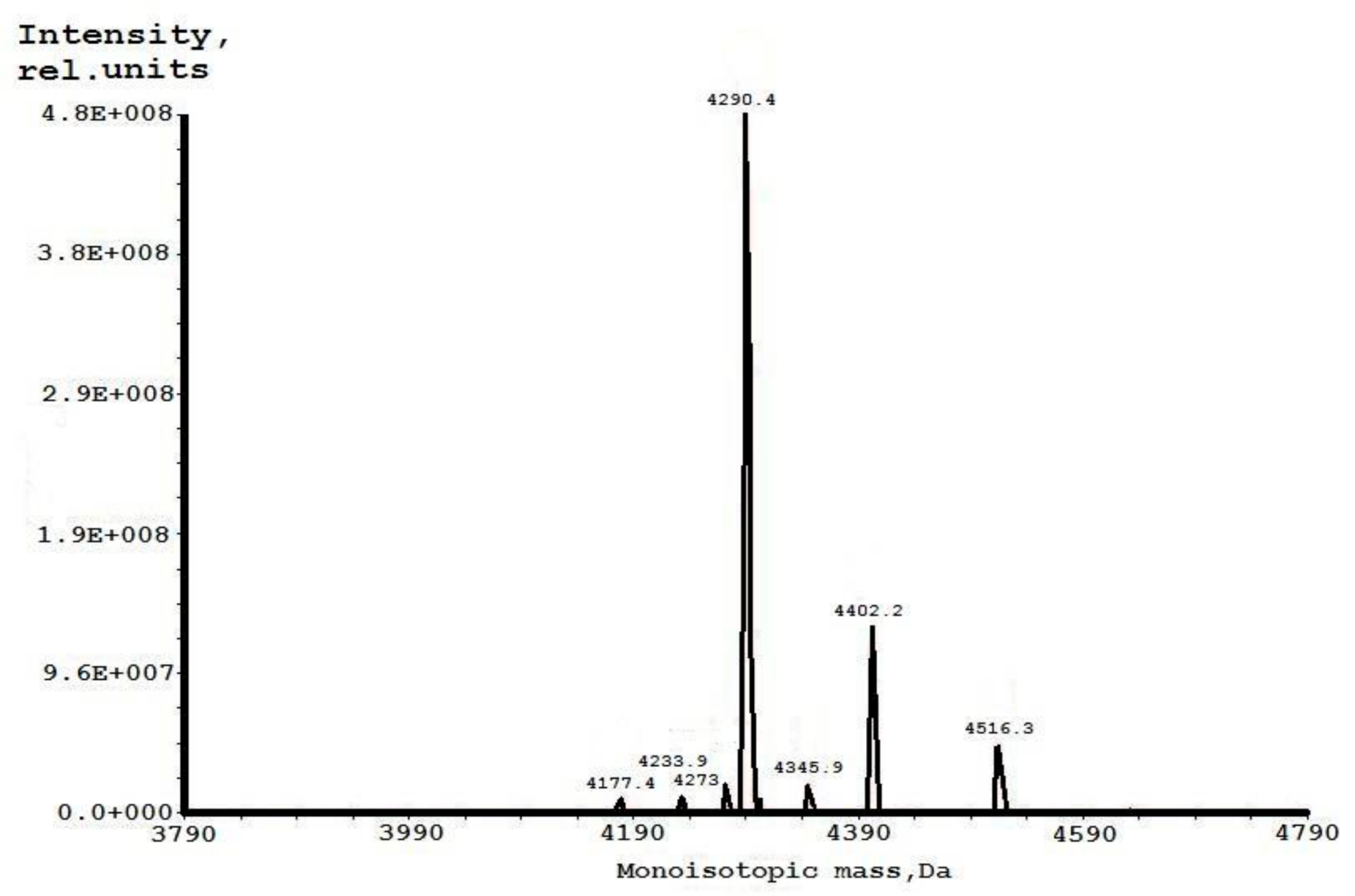
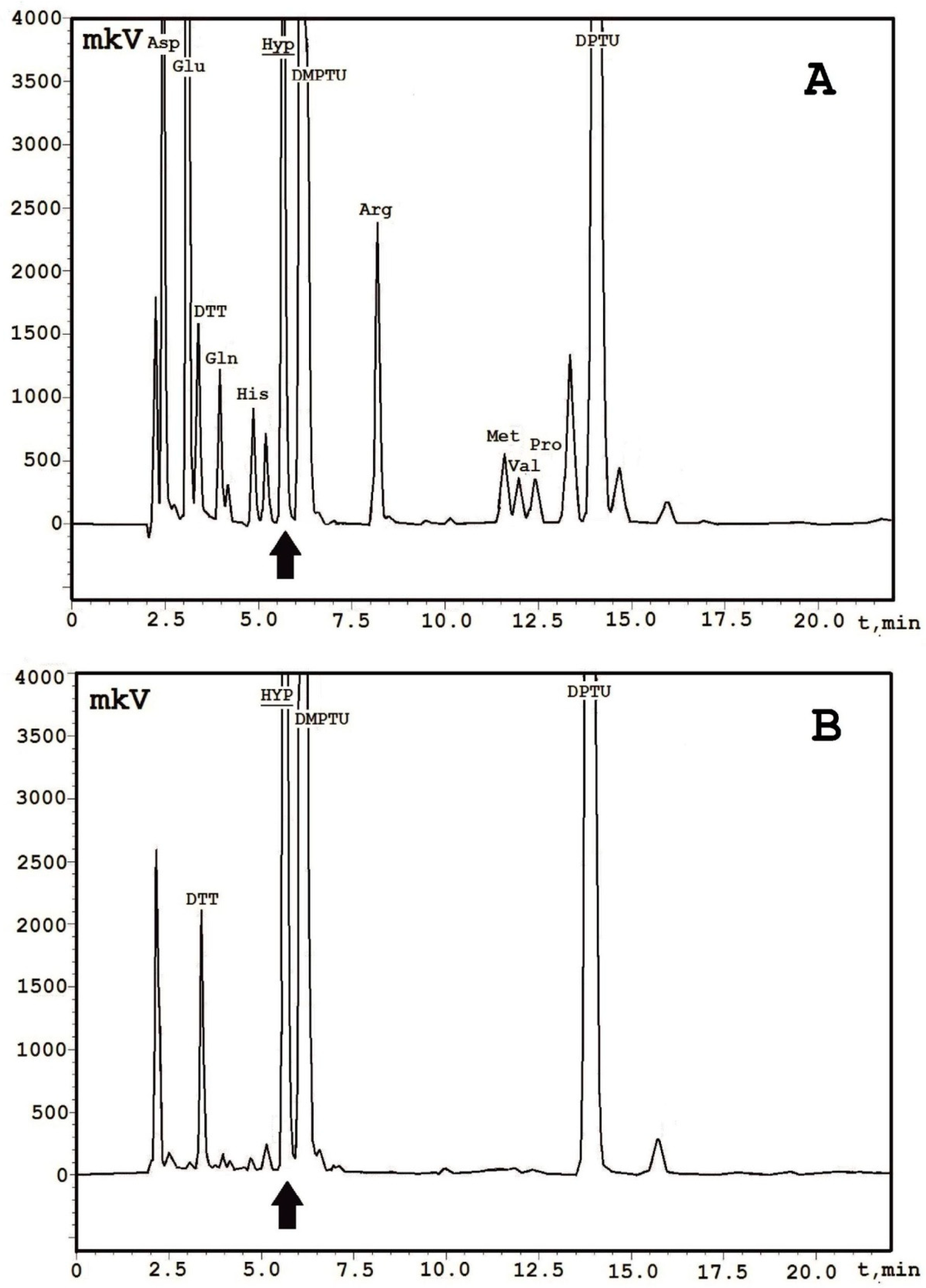
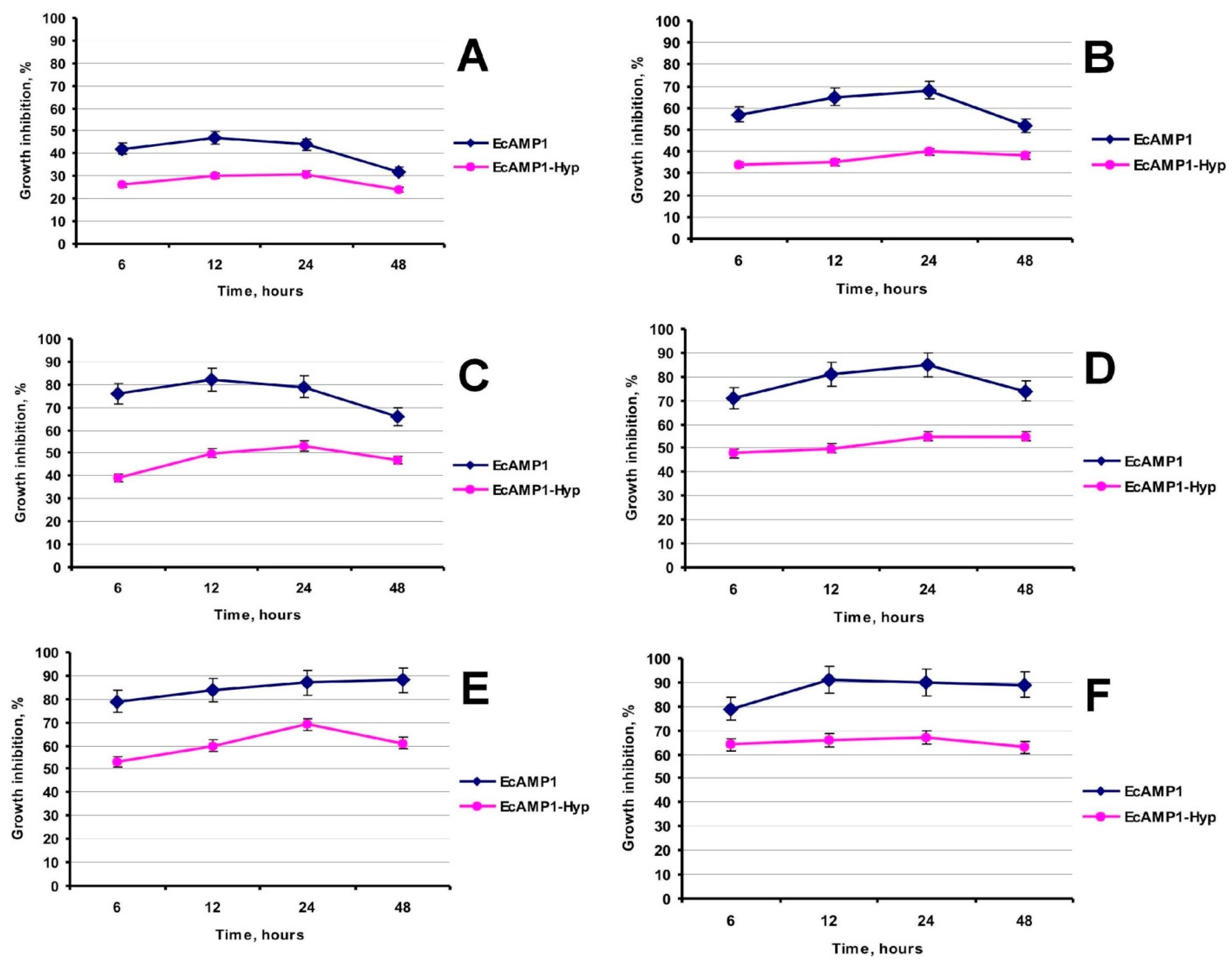
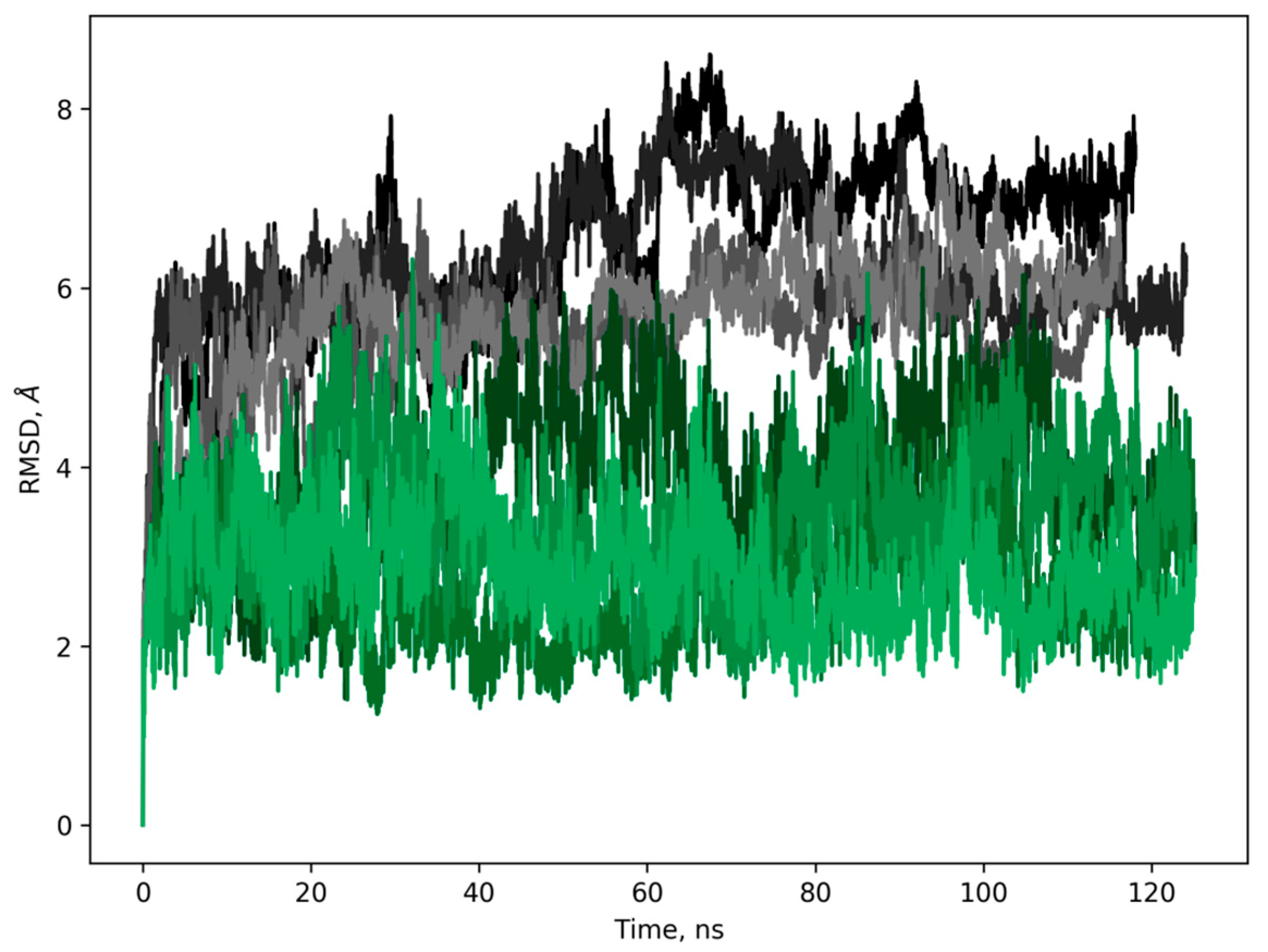
2. Results
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Isolation of Hyp-Containing EcAMP1 from E. crusgalli Seeds
4.3. Mass Spectrometry
4.4. N-Terminal Sequencing
4.5. Peptide Synthesis
4.6. Obtainment of the Recombinant EcAMP1 in the E. coli System
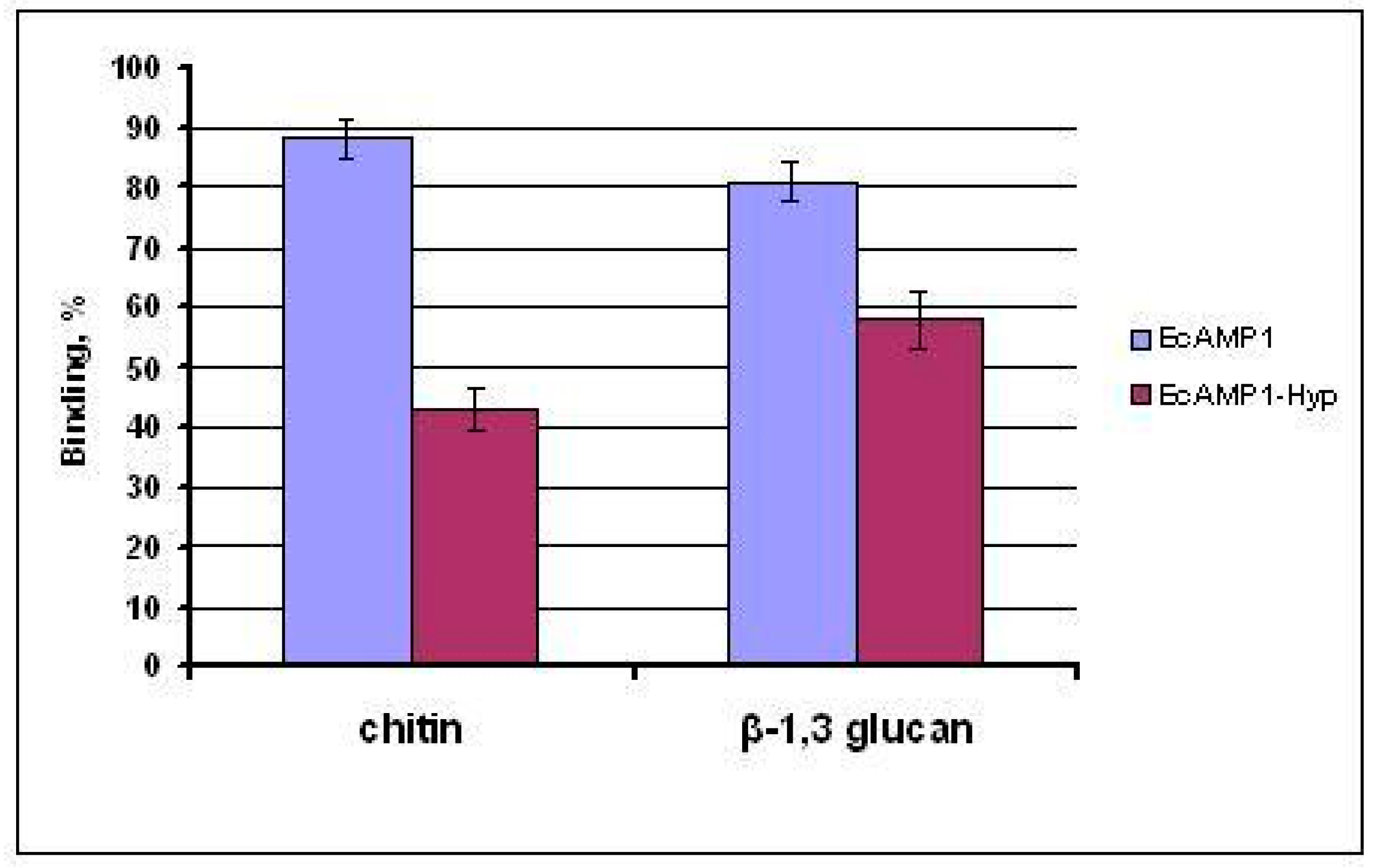
4.7. Chitin- and β-1.3-Glucan-Binding Assay In Vitro
4.8. Molecular Modeling
4.9. Antifungal Activity
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarethy, I.P. Plant Peptides: Bioactivity, Opportunities and Challenges. Protein Pept. Lett. 2017, 24, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.M.B.; Dias, S.C.; Franco, O.L. Antimicrobial Peptides and Nanotechnology, Recent Advances and Challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.-F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Koramutla, M.K.; Negi, M.; Pearce, G.; Ryan, C.A. Hydroxyproline-rich glycopeptide signals in potato elicit signalling associated with defense against insects and pathogens. Plant Sci. 2013, 207, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Bhattacharya, R.; Chen, Y.-C.; Barona, G.; Yamaguchi, Y.; Ryan, C.A. Isolation and characterization of hydroxyproline-rich glycopeptide signals in black nightshade leaves. Plant Physiol. 2009, 150, 1422–1433. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M. A novel hydroxyproline rich glycopeptide from pericarp of Datura stramonium: proficiently eradicate the biofilm of antifungals resistant Candida albicans. Biopolymers 2012, 98, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Wan, W.-L.; Lin, J.-S.; Kuo, Y.-W.; King, Y.-C.; Chen, Y.-C.; Jeng, S.-T. Signal transduction and regulation of IbpreproHypSys in sweet potato. Plant Cell Environ. 2016, 39, 1576–1587. [Google Scholar] [CrossRef] [PubMed]
- Astafieva, A.A.; Enyenihi, A.A.; Rogozhin, E.A.; Kozlov, S.A.; Grishin, E.V.; Odintsova, T.I.; Zubarev, R.A.; Egorov, T.A. Novel proline-hydroxyproline glycopeptides from the dandelion (Taraxacum officinale Wigg.) flowers: de novo sequencing and biological activity. Plant Sci. 2015, 238, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, E.A. Studies of the Specificity of the Antifungal Activity of Glycopeptides of Flowers of Dandelion (Taraxacum officinale Wigg.). Achiev. Life Sci. 2016, 10, S42. [Google Scholar] [CrossRef]
- Duvick, J.P.; Rood, T.; Rao, A.G.; Marshak, D.R. Purification and characterization of a novel antimicrobial peptide from maize (Zea mays L.) kernels. J. Biol. Chem. 1992, 267, 18814–18820. [Google Scholar] [PubMed]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Nolde, S.B.; Vassilevski, A.A.; Rogozhin, E.A.; Barinov, N.A.; Balashova, T.A.; Samsonova, O.V.; Baranov, Y.V.; Feofanov, A.V.; Egorov, T.A.; Arseniev, A.S.; et al. Disulfide-stabilized helical hairpin structure and activity of a novel antifungal peptide EcAMP1 from seeds of barnyard grass (Echinochloa crus-galli). J. Biol. Chem. 2011, 286, 25145–25153. [Google Scholar] [CrossRef] [PubMed]
- Utkina, L.L.; Andreev, Y.A.; Rogozhin, E.A.; Korostyleva, T.V.; Slavokhotova, A.A.; Oparin, P.B.; Vassilevski, A.A.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. Genes encoding 4-Cys antimicrobial peptides in wheat Triticum kiharae Dorof. et Migush.: multimodular structural organization, instraspecific variability, distribution and role in defence. FEBS J. 2013, 280, 3594–3608. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Rogozhin, E.A.; Musolyamov, A.K.; Andreev, Y.A.; Oparin, P.B.; Berkut, A.A.; Vassilevski, A.A.; Egorov, T.A.; Grishin, E.V.; Odintsova, T.I. Novel antifungal α-hairpinin peptide from Stellaria media seeds: structure, biosynthesis, gene structure and evolution. Plant Mol. Biol. 2014, 84, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Conners, R.; Konarev, A.V.; Forsyth, J.; Lovegrove, A.; Marsh, J.; Joseph-Horne, T.; Shewry, P.; Brady, R.L. An unusual helix-turn-helix protease inhibitory motif in a novel trypsin inhibitor from seeds of Veronica (Veronica hederifolia L.). J. Biol. Chem. 2007, 282, 27760–27768. [Google Scholar] [CrossRef] [PubMed]
- Oparin, P.B.; Mineev, K.S.; Dunaevsky, Y.E.; Arseniev, A.S.; Belozersky, M.A.; Grishin, E.V.; Egorov, T.A.; Vassilevski, A.A. Buckwheat trypsin inhibitor with helical hairpin structure belongs to a new family of plant defence peptides. Biochem. J. 2012, 446, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, X.; Xia, H.; Zeng, R.; Hu, W.; Li, Z.; Zhang, Z. Purification and characterization of Luffin P1, a ribosome-inactivating peptide from the seeds of Luffa cylindrica. Peptides 2003, 24, 799–805. [Google Scholar] [CrossRef]
- Sousa, D.A.; Porto, W.F.; Silva, M.Z.; da Silva, T.R.; Franco, O.L. Influence of Cysteine and Tryptophan Substitution on DNA-Binding Activity on Maize α-Hairpinin Antimicrobial Peptide. Molecules 2016, 21, 1062. [Google Scholar] [CrossRef] [PubMed]
- Crankshaw, M.W.; Grant, G.A. Identification of Modified PTH-Amino Acids in Protein Sequence Analysis; Applied Biosystems: Foster City, CA, USA, 1993; Available online: https://www.protein.iastate.edu/docs/IdentificationOfModifiedPTH-AminoAcids.pdf (accessed on 1 November 2018).
- Showalter, A.M.; Keppler, B.; Lichtenberg, J.; Gu, D.; Welch, L.R. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Physiol. 2010, 153, 485–513. [Google Scholar] [CrossRef] [PubMed]
- Bann, J.G.; Bächinger, H.P. Glycosylation/Hydroxylation-induced stabilization of the collagen triple helix. 4-trans-hydroxyproline in the Xaa position can stabilize the triple helix. J. Biol. Chem. 2000, 275, 24466–24469. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Yuryev, M.; Ryazantsev, D.Y.; Zavriev, S.K.; Feofanov, A.V.; Grishin, E.V.; Rogozhin, E.A. Studying of cellular interaction of hairpin-like peptide EcAMP1 from barnyard grass (Echinochloa crusgalli L.) seeds with plant pathogenic fungus Fusarium solani using microscopy techniques. Scanning 2016, 38, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Ryazantsev, D.Y.; Rogozhin, E.A.; Dimitrieva, T.V.; Drobyazina, P.E.; Khadeeva, N.V.; Egorov, T.A.; Grishin, E.V.; Zavriev, S.K. A novel hairpin-like antimicrobial peptide from barnyard grass (Echinochloa crusgalli L.) seeds: Structure-functional and molecular-genetics characterization. Biochimie 2014, 99, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, E.A.; Ryazantsev, D.Y.; Grishin, E.V.; Egorov, T.A.; Zavriev, S.K. Defense peptides from barnyard grass (Echinochloa crusgalli L.) seeds. Peptides 2012, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 267–292. [Google Scholar] [CrossRef]
- van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell. Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef] [PubMed]
- Breen, S.; Solomon, P.S.; Bedon, F.; Vincent, D. Surveying the potential of secreted antimicrobial peptides to enhance plant disease resistance. Front. Plant Sci. 2015, 6, 900. [Google Scholar] [CrossRef] [PubMed]
- Berkut, A.A.; Usmanova, D.R.; Peigneur, S.; Oparin, P.B.; Mineev, K.S.; Odintsova, T.I.; Tytgat, J.; Arseniev, A.S.; Grishin, E.V.; Vassilevski, A.A. Structural Similarity between Defense Peptide from Wheat and Scorpion Neurotoxin Permits Rational Functional Design. J. Biol. Chem. 2014, 289, 14331–14340. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Du, J.; Li, J.; Wang, Z. Inhibitory site of α-hairpinin peptide from tartary buckwheat has no effect on its antimicrobial activities. Acta Biochim. Biophys. Sin. (Shanghai) 2018, 50, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Edman, P. Method for determination of the amino acid sequence in peptides. Acta Chem. Scand. 1950, 4, 283–293. [Google Scholar] [CrossRef]
- Rogozhin, E.A.; Slezina, M.P.; Slavokhotova, A.A.; Istomina, E.A.; Korostyleva, T.V.; Smirnov, A.N.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. A novel antifungal peptide from leaves of the weed Stellaria media L. Biochimie 2015, 116, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Reshetnikov, R.V.; Stolyarova, A.V.; Zalevsky, A.O.; Panteleev, D.Y.; Pavlova, G.V.; Klinov, D.V.; Golovin, A.V.; Protopopova, A.D. A coarse-grained model for DNA origami. Nucleic Acids Res. 2018, 46, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Boller, T. A method for the study of fungal growth inhibition by plant proteins. FEMS Microbiol. Lett. 1990, 69, 61–66. [Google Scholar] [CrossRef]

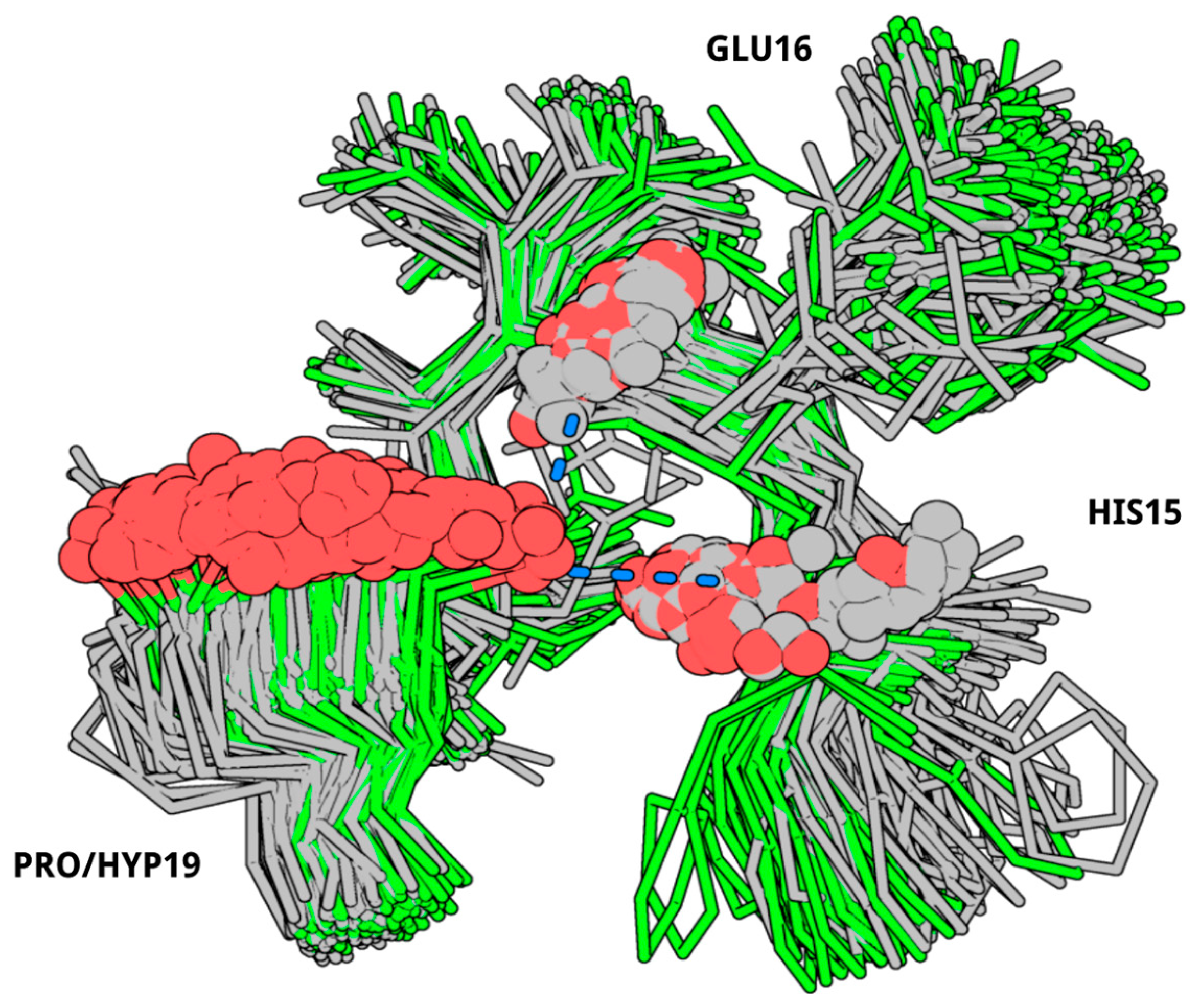
| Peptide | ICmin | IC50 |
|---|---|---|
| EcAMP1-Hyp | 2.9 | 5.4 |
| EcAMP1 | 2.2 | 3.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogozhin, E.; Zalevsky, A.; Mikov, A.; Smirnov, A.; Egorov, T. Characterization of Hydroxyproline-Containing Hairpin-Like Antimicrobial Peptide EcAMP1-Hyp from Barnyard Grass (Echinochloa crusgalli L.) Seeds: Structural Identification and Comparative Analysis of Antifungal Activity. Int. J. Mol. Sci. 2018, 19, 3449. https://doi.org/10.3390/ijms19113449
Rogozhin E, Zalevsky A, Mikov A, Smirnov A, Egorov T. Characterization of Hydroxyproline-Containing Hairpin-Like Antimicrobial Peptide EcAMP1-Hyp from Barnyard Grass (Echinochloa crusgalli L.) Seeds: Structural Identification and Comparative Analysis of Antifungal Activity. International Journal of Molecular Sciences. 2018; 19(11):3449. https://doi.org/10.3390/ijms19113449
Chicago/Turabian StyleRogozhin, Eugene, Artur Zalevsky, Alexander Mikov, Alexey Smirnov, and Tsezi Egorov. 2018. "Characterization of Hydroxyproline-Containing Hairpin-Like Antimicrobial Peptide EcAMP1-Hyp from Barnyard Grass (Echinochloa crusgalli L.) Seeds: Structural Identification and Comparative Analysis of Antifungal Activity" International Journal of Molecular Sciences 19, no. 11: 3449. https://doi.org/10.3390/ijms19113449
APA StyleRogozhin, E., Zalevsky, A., Mikov, A., Smirnov, A., & Egorov, T. (2018). Characterization of Hydroxyproline-Containing Hairpin-Like Antimicrobial Peptide EcAMP1-Hyp from Barnyard Grass (Echinochloa crusgalli L.) Seeds: Structural Identification and Comparative Analysis of Antifungal Activity. International Journal of Molecular Sciences, 19(11), 3449. https://doi.org/10.3390/ijms19113449





