Screening the Marker Components in Psoralea corylifolia L. with the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS2
Abstract
1. Introduction
2. Results and Discussion
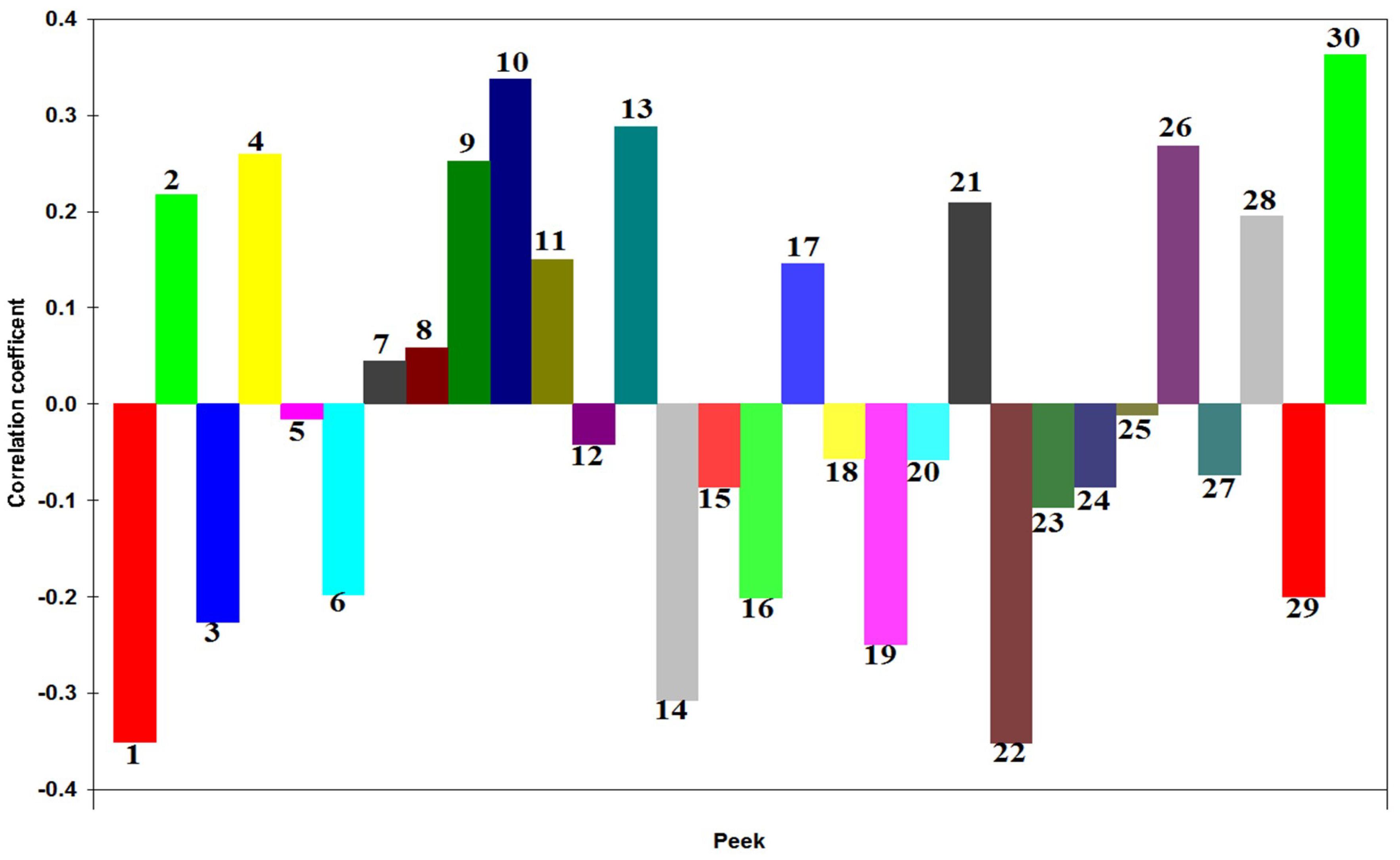
2.1. Spectrum-Effect Relationships
2.1.1. Quantitative Determination of Chromatographic Peaks
2.1.2. Determination of Activation Effect of PEs on Tyrosinase Activity In Vitro
2.1.3. Regression Equation of Partial Least Squares Analysis
2.2. Component Knock-Out Methods
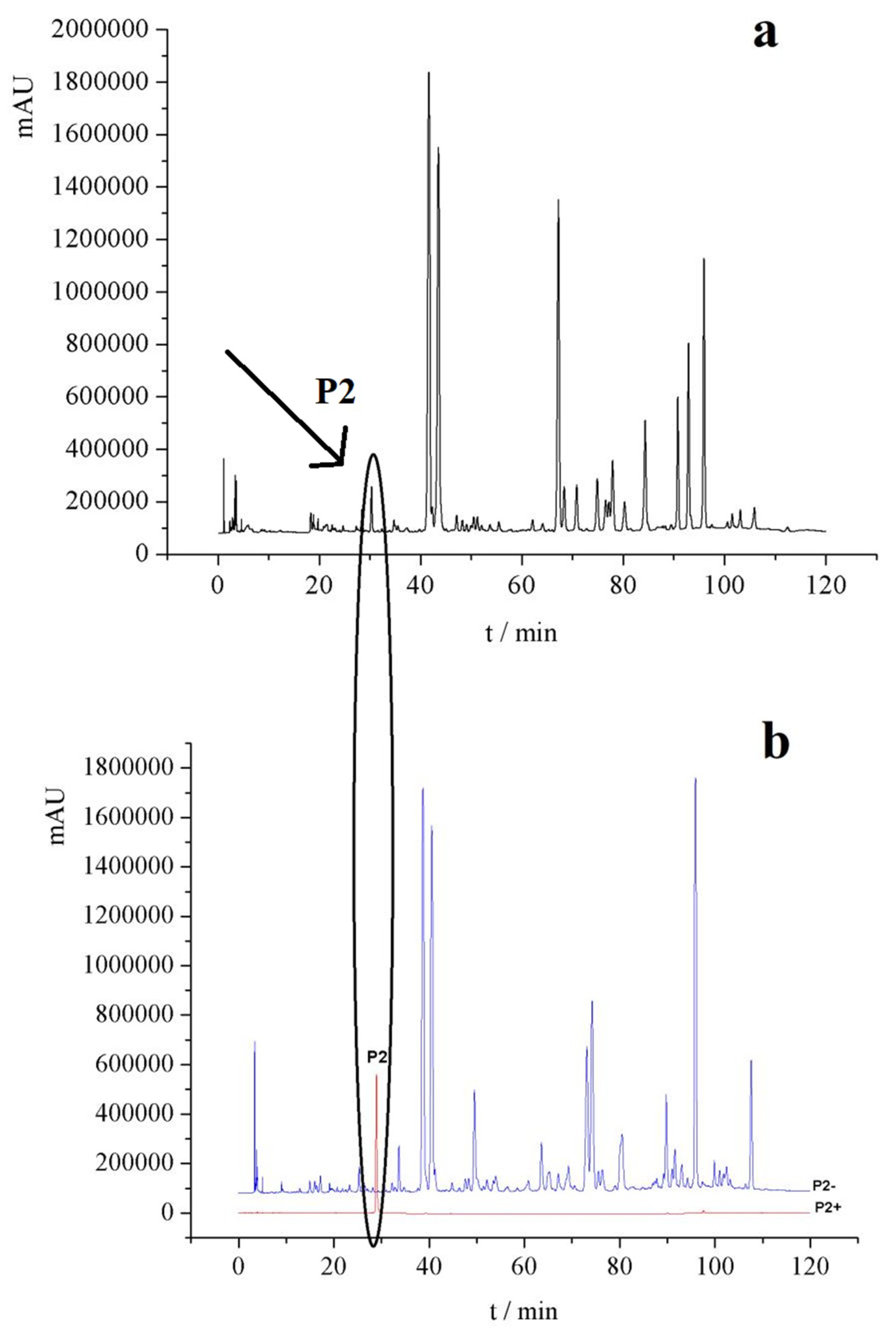
2.2.1. HPLC Chromatogram of Knock-Out Components and Negative Samples
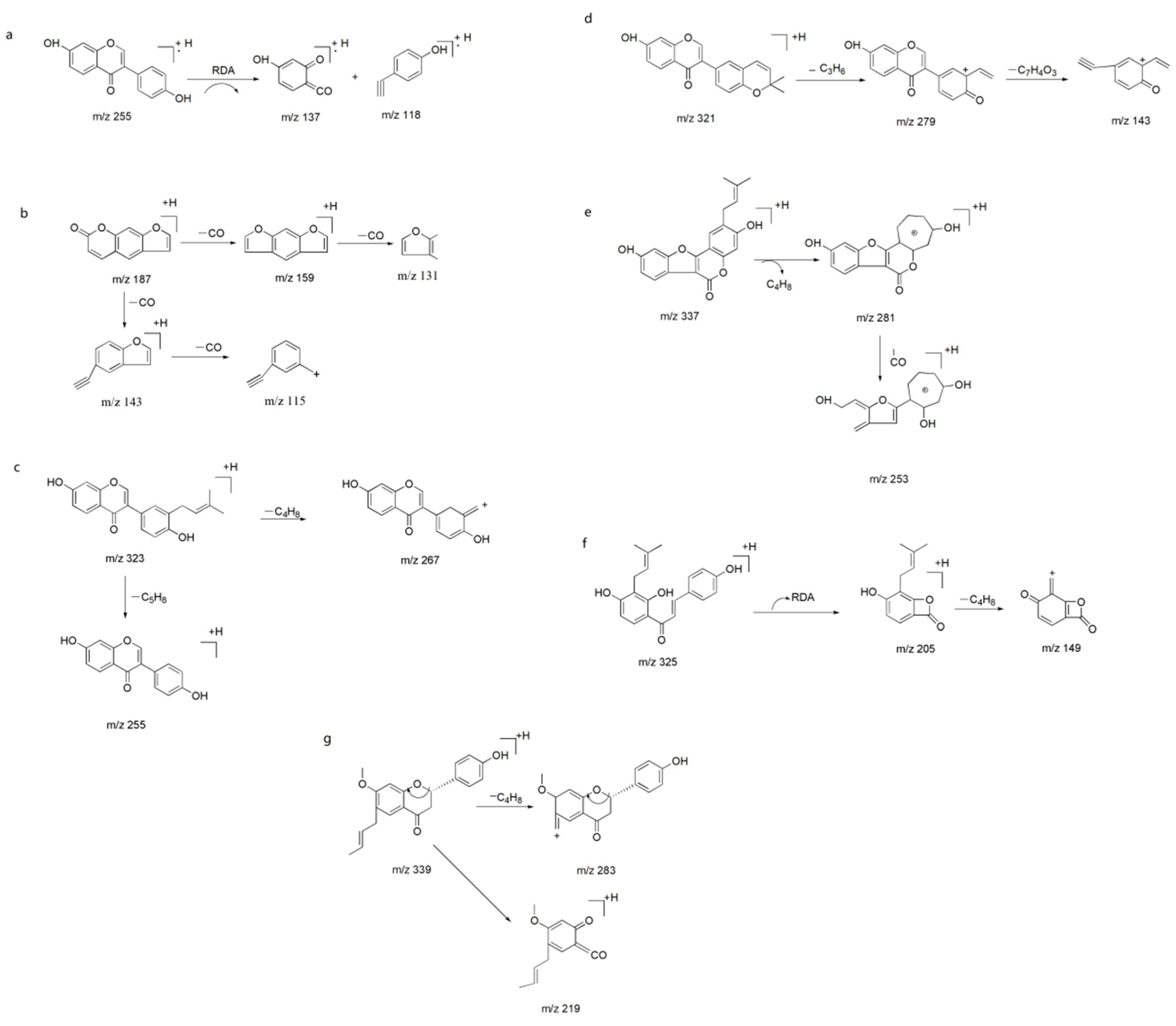
2.2.2. Component Identification
2.3. Effect of Knocked-Out Components and Negative Samples on Tyrosinase Activity In Vitro
3. Materials and Methods
3.1. Materials
3.2. Plant Materials
3.3. Extraction
3.4. HPLC Analysis
3.5. Tyrosinase Inhibition Assay In Vitro
3.6. Partial Least Squares Analysis
3.7. Knock-Out Method
3.8. Mass Spectrometry Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CCA | canonical correlation analysis |
| DOPA | dihydroxyphenylalanine |
| GRDA | grey relational analysis |
| HPLC | high performance liquid chromatography |
| MS | mass spectrum |
| PE | total ethanol extract of P. corylifolia |
| PCA | principal component analysis |
| PLSR | partial least squares analysis |
| UPLC | ultra performance liquid chromatography |
| TYP | tyrosinase |
| TCM | traditional Chinese medicine |
References
- Zhou, L.; Shi, Y.L.; Li, K.; Hamzavi, I.; Gao, T.W.; Huggins, R.H.; Lim, H.W.; Mi, Q.S. Increased circulating Th17 cells and elevated serum levels of TGF-β and IL-21 are correlated with human non-segmental vitiligo development. Pigment. Cell Melanoma Res. 2015, 28, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.I.; Ai, X.U. Modern Research of Traditional Chinese Medicine Treatment of Vitiligo. Med. Recapitul. 2015, 21, 1. [Google Scholar]
- Committee, N.P. People’s Republic of China Pharmacopoeia; China Medical Science Press: Beijing, China, 2015. [Google Scholar]
- Zhang, X.; Zhao, W.; Wang, Y.; Lu, J.; Chen, X. The Chemical Constituents and Bioactivities of Psoralea corylifolia Linn.: A Review. Am. J. Chin. Med. 2016, 44, 35. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, P.N.; Anandi, C.; Balasubramanian, S.; Pugalendi, K.V. Antidermatophytic activity of extracts from Psoralea corylifolia (Fabaceae) correlated with the presence of a flavonoid compound. J. Ethnopharmacol. 2004, 91, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yingjun, Y.U.; Yao, Y.; Jiang, Y.; Zhang, N.; Zhang, M.; Gao, H.; Jianmin, L.I. Effect of bavachinin on melanin synthesis of A375 cells and the signal pathway of ER/MAPK. China Med. Herald 2015, 36, 003. [Google Scholar]
- Wang, S.; Geng, F.; Zhang, M.; Chen, C.; Wang, X.; Wang, J.; Zhang, N. Regulatory Effect of Psoralen on A375 Cells Melanogenesis and Its Related Cell Signaling Pathways. Tradit. Chin. Drug Res. Clin. Pharmacol. 2014, 25, 704–708. [Google Scholar]
- Na, L.I.; Wang, Y.J.; Zhu, G.M.; Wang, T.T.; Zhou, H.; Tian, Q.Y.; Zhang, Z. Kinetics and Effect of Bacuchiol on Tyrosinase Activity. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 30–33. [Google Scholar]
- Li, A.; Ou, R.; Zhang, B. Quantitative Comparison of Effective Ingredients Extracted from Various Parts of Fructus Psoraleae. J. Guangzhou Univ. Tradit. Chin. Med. 1999, 16, 52–54. [Google Scholar]
- Zhang, W.; Yin, Z.H.; Peng, T.; Kang, W.Y. Effect of High-temperature Heat Treatment of Psoralea corylifolia on the Activity of Tyrosinase. Chin. J. Exp. Tradit. Med. Formulae 2014, 4, 71–73. [Google Scholar]
- Yi, X.Z.; Hong, Z.Q. The proliferation of human epidermal melanocytes by rhubarb Bi-directional regulation of tyrosinase. Chin. J. Dermatol. 1997, 30, 404–405. [Google Scholar]
- Zeng, L.J.; Lin, B.; Song, H.T. Progress in study of spectrum-effect relationship of traditional Chinese medicine and discussions. Zhongguo Zhong Yao Za Zhi 2015, 40, 1425–1432. [Google Scholar] [PubMed]
- Fu, J.; Zhang, B.; Liu, H.Y.; Li, M.J.; Gu, L.; Li, J.; Wang, F.Q.; Zhang, Z.Y. A consideration on screening of allelochemicals by using “knock-out/in” of targeting ingredients based on identification model of medicinal effect. Zhongguo Zhong Yao Za Zhi 2017, 42, 805–808. [Google Scholar] [PubMed]
- Wang, L.Y.; Tang, Y.P.; Liu, X.; Ge, Y.H.; Li, S.J.; Shang, E.X.; Duan, J.A. Study on material base of Carthamus tinctorius with antioxidant effect based on selective knock-out. China J. Chin. Mater. Med. 2014, 39, 1285. [Google Scholar]
- Gong, A.G.; Li, N.; Lau, K.M.; Lee, P.S.; Yan, L.; Xu, M.L.; Lam, C.T.; Kong, A.Y.; Lin, H.Q.; Dong, T.T. Calycosin orchestrates the functions of Danggui Buxue Tang, a Chinese herbal decoction composing of Astragali Radix and Angelica Sinensis Radix: An evaluation by using calycosin-knock out herbal extract. J. Ethnopharmacol. 2015, 168, 150. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, R.P.; Pope, A.J. High-throughput screening: New technology for the 21st century. Curr. Opin. Chem. Boil. 2000, 4, 445. [Google Scholar] [CrossRef]
- Fan, Y.-F.; Cheng, S.-L.; Zhang, J.-Y. Structure-activity Relationship Model for Predicting the Hallucinogenic Activity of Phenylalkylamines. Chin. J. Struct. Chem. 2010, 29, 1201–1208. [Google Scholar]
- Liu, M.; Liu, X.; Wan, P.; Wu, Q.; Hu, W. Determination of structure-activity relationships between fentanyl analogs and human p-opioid receptors based on active binding site models. Neural Regen. Res. 2011, 6, 267–276. [Google Scholar]
- Xu, G.L.; Xie, M.; Yang, X.Y.; Song, Y.; Yan, C.; Yang, Y.; Zhang, X.; Liu, Z.Z.; Tian, Y.X.; Wang, Y. Spectrum-Effect Relationships as a Systematic Approach to Traditional Chinese Medicine Research: Current Status and Future Perspectives. Molecules 2014, 19, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Yan, H.U.; Liu, M.H.; Qin, S.; Zhang, S.J. Spectrum-effect relationship of antibacterial extracts from Isatidis Radix. Chin. Tradit. Herb. Drugs 2013, 44, 1615–1620. [Google Scholar]
- Peng, L.I.; Xiang, L.I.; Chen, J.W.; Lu-Ling, W.U. Spectrum-effect Relationship of Active Components from Taohong Siwutang tang in Dysmenorrheal Model Mice. Chin. J. Exp. Tradit. Med. Formulae 2010, 9, 045. [Google Scholar]
- Song, Y.C.; Hai-Bo, M.A.; Zhang, Q.; Cui, X.R.; Lian, C.J.; Qiang, L.I. Spectrum-effect relationship between LC-MS fingerprint chromatogram and cell inhibitory rate of Paeoniae Radix Rubra and Paeoniae Radix Alba. Drugs Clin. 2012, 27, 103–106. [Google Scholar]
- Sun, X.J.; Liu, T.T.; Zhao, Y.L.; Zou, W.J.; Wang, J.B.; Liu, S.X.; Sun, Z.Y. Toxicity of Five Herbs in Aconitum L. on Tetrahymena thermophila Based on Spectrum-effect Relationship. Chin. Herb. Med. 2014, 6, 29–35. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhao, Y.; Wang, J.; Liu, T.; Zhang, B.; Gong, M.; Li, J.; Liu, H.; Han, B.; Zhang, Y. Spectrum-effect relationships between UPLC fingerprints and bioactivities of crude secondary roots of Aconitum carmichaelii Debeaux (Fuzi) and its three processed products on mitochondrial growth coupled with canonical correlation analysis. J. Ethnopharmacol. 2014, 153, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Han, Z.H.; Liu, X.H.; Yang, Y.F.; Lan, Z.H.; Feng, S.L.; Pharmacy, S.O.; University, L. Spectrum-effect relationship of active fraction from Hedysari Radix on improving immunity. Chin. Tradit. Herbal Drugs 2016, 47, 101–105. [Google Scholar]
- Xiao, S. A Method for Research of Antibacterial Constituent Recognition of Traditional Chinese Medicine (Acalypha australis Linn.) by Spectrum-Effect Relationship. Ph.D. Thesis, Chinese Academic Agriculture Science, Beijing, China, 2013. [Google Scholar]
- Zhao, X.M.; Shi-Biao, P.U.; Zhao, Q.G. Preliminary study on effective components of Tripterygium wilfordii for liver toxicity based on spectrum-effect correlation analysis. China J. Chin. Mater. Med. 2016, 41, 2915–2921. [Google Scholar]
- Xu, J.J. Studies in Quality Control and Evaluation Method of Menthae Haplocalycis Herba Based on the Correlation between Its Chromatographic Fingerprint and Antioxidant Activity. Master’s Thesis, Chinese Medicine, Beijing University, Beijing, China, 2014. [Google Scholar]
- Wang, Q.S. Study on the Spectrum-Effect Relationship of Bupluerum Chinense and GC-MS Analysis of Supercritical-CO2 Fluid Extraction. Master’s Thesis, Henan College of Traditional Chinese Medicine, Zhengzhou, China, 2011. [Google Scholar]
- Wang, J.; Peng, L.; Shi, M.; Li, C.; Zhang, Y.; Kang, W. Spectrum Effect Relationship and Component Knock-Out in Angelica Dahurica Radix by High Performance Liquid Chromatography-Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Qi, L.; Li, P. Quality control of Chinese herbal medicines with chromatographic fingerprints. Chin. J. Chromatogr. 2008, 26, 153–159. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Liu, X.; Ye, Z.; Yang, X.; Meyboom, R.; Chan, K.; Shaw, D.; Duez, P. Pharmacovigilance practice and risk control of Traditional Chinese Medicine drugs in China: Current status and future perspective. J. Ethnopharmacol. 2012, 140, 519. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.P.; Fan, X.H.; Yu, J.; Cheng, Y.Y. A method for predicting activity of traditional Chinese medicine based on quantitative composition-activity relationship of neural network model. China J. Chin. Mater. Med. 2004, 29, 1082. [Google Scholar]
- Alciaturi, C.E.; Escobar, M.E.; Cruz, C.D.L.; Rincón, C.; Rincón, C. Partial least squares (PLS) regression and its application to coal analysis. Revista Técnica de la Facultad de Ingeniería Universidad del Zulia 2003, 26, 197–204. [Google Scholar]
- Zhao, W.; Shang, Z.; Li, Q.; Huang, M.; He, W.; Wang, Z.; Zhang, J. Rapid Screening and Identification of Daidzein Metabolites in Rats Based on UHPLC-LTQ-Orbitrap Mass Spectrometry Coupled with Data-Mining Technologies. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hick, L.A.; Price, W.E. A Fragmentation Study of Isoflavones in Negative Electrospray Ionization by MS n Ion Trap Mass Spectrometry and Triple Quadrupole Mass Spectrometry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 857–868. [Google Scholar]
- Chen, Q.; Li, Y.; Chen, Z. Separation, identification, and quantification of active constituents in Fructus Psoraleae by high-performance liquid chromatography with UV, ion trap mass spectrometry, and electrochemical detection. J. Pharm. Anal. 2012, 2, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Shen, X.; Liu, X.; Wu, Y.; Tan, M. Qualitative analysis of Psoraleae Fructus by HPLC-DAD/TOF-MS fingerprint and quantitative analysis of multiple components by single marker. Biomed. Chromatogr. BMC 2017, 32, e4059. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Liu, M.; Zhang, M.; Zhang, K.; Liu, S.; Qiao, S.; Shi, R.; Jiang, X.; Wang, Q. Identification of in vitro and in vivo metabolites of isoimperatorin using liquid chromatography/mass spectrometry. Food Chem. 2013, 141, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.M.; Xue, X.; Liu, H.; Tian, Y.W.; Lei, G.; Jia-Kun, S.U.; Cai, J.B.; Luo, G.A. Analysis on chemical constitutes in Psoraleae Fructus by combination of HPLC/TOF-MS and HPLC/IT-MS~n. Chin. Tradit. Herb. Drugs 2014, 45, 924–928. [Google Scholar]
- Tan, G.; Yang, T.; Miao, H.; Chen, H.; Chai, Y.; Wu, H. Characterization of Compounds in Psoralea corylifolia Using High-Performance Liquid Chromatography Diode Array Detection, Time-of-Flight Mass Spectrometry and Quadrupole Ion Trap Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Ohno, O.; Watabe, T.; Nakamura, K.; Kawagoshi, M.; Uotsu, N.; Chiba, T.; Yamada, M.; Yamaguchi, K.; Yamada, K.; Miyamoto, K. Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from Piper longum on melanin production in B16 mouse melanoma cells. J. Agric. Chem. Soc. Jpn. 2010, 74, 1504–1506. [Google Scholar] [CrossRef]
- Wu, X.; Hu, X.; Hamblin, M.R. Ultraviolet blood irradiation: Is it time to remember “the cure that time forgot”? J. Photochem. Photobiol. B Boil. 2016, 157, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285. [Google Scholar] [CrossRef] [PubMed]




| Sample | Concentration of Ethanol Extract (Equal to the Amount of Raw Medicinal Herbs) | ||||
|---|---|---|---|---|---|
| 1 g·mL−1 | 0.5 g·mL−1 | 0.25 g·mL−1 | 0.125 g·mL−1 | 0.0625 g·mL−1 | |
| S1 | 454.67 ± 3.85 | 418.99 ± 9.55 ### | 70.91 ± 5.14 &&& | 117.45 ± 1.30 ※※※ | 22.28 ± 0.93 $$$ |
| S2 | 278.83 ± 4.46 *** | 21.58 ± 5.26 ### | 64.59 ± 9.78 &&& | 45.47 ± 9.02 ※※※ | 37.18 ± 7.56 $$$ |
| S3 | 234.17 ± 5.81 *** | 341.8 ± 8.01 ### | 127.43 ± 9.78 &&& | 22.47 ± 2.62 ※※※ | 24.69 ± 1.44 $$$ |
| S4 | 233.08 ± 6.51 *** | 626.31 ± 5.43 | 63.57 ± 6.30 &&& | 143.89 ± 6.49 ※※※ | 42.77 ± 1.96 $$$ |
| S5 | 329.53 ± 1.41 *** | 440.58 ± 2.31 ## | 316.60 ± 0.59 | 69.32 ± 8.33 ※※※ | −106.26 ± 1.60 $$$ |
| S6 | 181.77 ± 8.71 *** | 135.71 ± 8.83 ### | 105.49 ± 5.91 &&& | 170.03 ± 3.29 | 105.84 ± 5.37 |
| S7 | 12.09 ± 8.03 *** | 173.41 ± 7.85 ### | 69.94 ± 6.26 &&& | 119.68 ± 9.18 ※※※ | 120.83 ± 2.19 |
| S8 | −77.81 ± 3.75 *** | 59.77 ± 5.90 ## | 273.46 ± 9.67 &&& | 170.61 ± 8.61 | 60.21 ± 5.47 $$$ |
| S9 | 223.02 ± 9.39 *** | 240.69 ± 2.98 ### | −334.22 ± 9.47 &&& | −317.75 ± 7.6 ※※※ | −233.97 ± 8.21 $$$ |
| S10 | 67.55 ± 3.21 *** | 204.86 ± 9.18 ### | 199.55 ± 0.12 &&& | 166.19 ± 10.51 | 80.28 ± 3.05 $$$ |
| Peek No. | Activation Rate (%) on Tyrosinase Activity | ||
|---|---|---|---|
| Ethanol Extracts | Px+ | Px− | |
| P2 | 188.97 ± 0.13 | 52.19 ± 1.05 | 144.97 ± 1.32 |
| P5 | 188.97 ± 0.13 | −5.62 ± 3.98 | 294.96 ± 3.50 |
| P6 | 188.97 ± 0.13 | 51.83 ± 2.50 | 222.51 ± 1.91 |
| P13 | 188.97 ± 0.13 | 27.01 ± 0.87 | 23.42 ± 1.67 |
| P14 | 188.97 ± 0.13 | 82.15 ± 2.68 | 3.73 ± 1.73 |
| P18 | 188.97 ± 0.13 | 16.48 ± 2.56 | 132.10 ± 0.30 |
| P20 | 188.97 ± 0.13 | −161.24 ± 0.65 | −176.37 ± 1.59 |
| P23 | 188.97 ± 0.13 | 132.72 ± 2.38 | 227.12 ± 0.53 |
| P24 | 188.97 ± 0.13 | 75.47 ± 3.60 | 200.79 ± 0.80 |
| P25 | 188.97 ± 0.13 | 3.71 ± 0.40 | 116.78 ± 0.76 |
| P27 | 188.97 ± 0.13 | 3.66 ± 0.41 | −4.46 ± 0.23 |
| P28 | 188.97 ± 0.13 | 18.03 ± 0.61 | 101.36 ± 0.53 |
| P30 | 188.97 ± 0.13 | −151.24 ± 0.68 | −229.29 ± 0.60 |
| Number | Collecting Land | Collecting Time |
|---|---|---|
| S1 | Bozhou | December 2012 |
| S2 | Hebei | October 2010 |
| S3 | Yunnan | February 2015 |
| S4 | Yunnan | February 2016 |
| S5 | Yunnan | February 2017 |
| S6 | Henan | December 2013 |
| S7 | Imported | November 2013 |
| S8 | Imported | November 2014 |
| S9 | Imported | November 2015 |
| S10 | Imported | November 2016 |
| Time/min | A/% | B/% |
|---|---|---|
| 0–9 min | 15% | 85% |
| 9–11 min | 15–25% | 85–75% |
| 11–35 min | 25–35% | 75–65% |
| 35–75 min | 35–50% | 65–50% |
| 75–95 min | 50–70% | 50–30% |
| 95–120 min | 70–95% | 30–5% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Zhang, Y.; Song, M.; Sun, Y.; Li, C.; Kang, W. Screening the Marker Components in Psoralea corylifolia L. with the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS2. Int. J. Mol. Sci. 2018, 19, 3439. https://doi.org/10.3390/ijms19113439
Shi M, Zhang Y, Song M, Sun Y, Li C, Kang W. Screening the Marker Components in Psoralea corylifolia L. with the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS2. International Journal of Molecular Sciences. 2018; 19(11):3439. https://doi.org/10.3390/ijms19113439
Chicago/Turabian StyleShi, Mengjun, Yan Zhang, Miaomiao Song, Yong Sun, Changqin Li, and Wenyi Kang. 2018. "Screening the Marker Components in Psoralea corylifolia L. with the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS2" International Journal of Molecular Sciences 19, no. 11: 3439. https://doi.org/10.3390/ijms19113439
APA StyleShi, M., Zhang, Y., Song, M., Sun, Y., Li, C., & Kang, W. (2018). Screening the Marker Components in Psoralea corylifolia L. with the Aids of Spectrum-Effect Relationship and Component Knock-Out by UPLC-MS2. International Journal of Molecular Sciences, 19(11), 3439. https://doi.org/10.3390/ijms19113439





