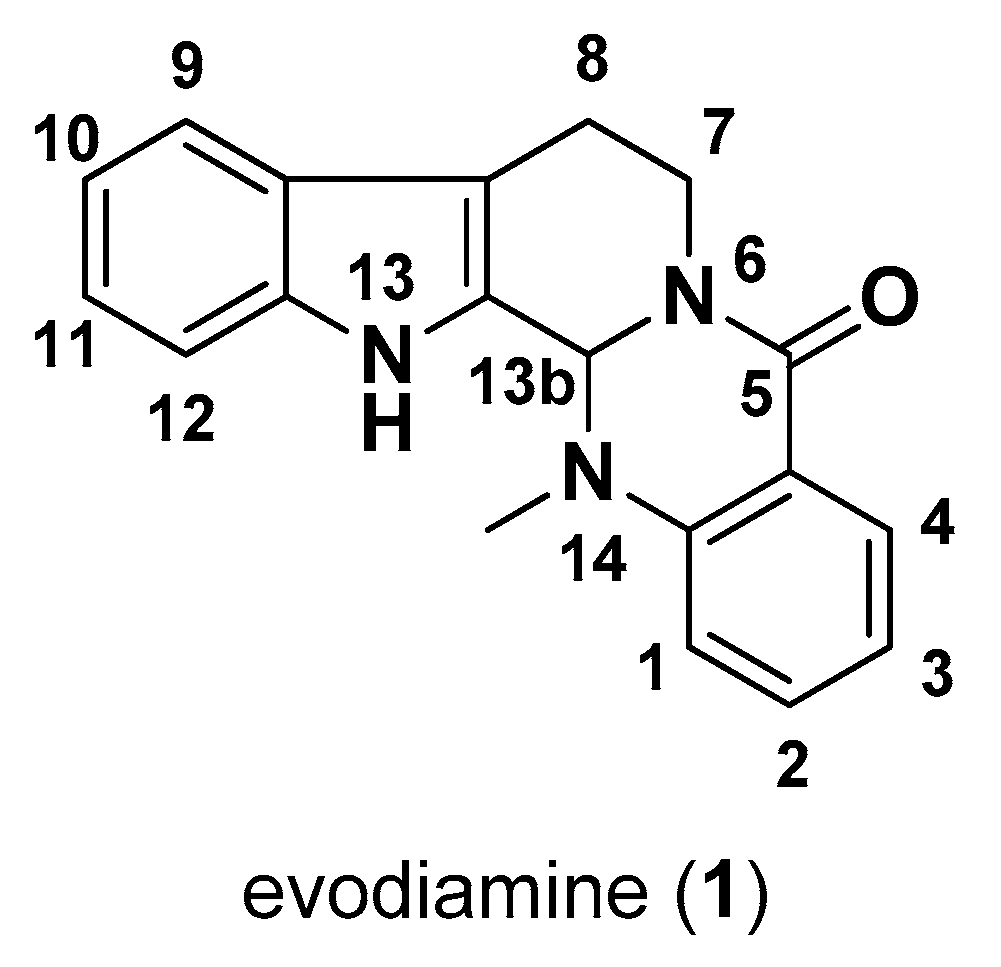
Antiproliferative Effects of Alkaloid Evodiamine and Its Derivatives
Abstract
1. Introduction
2. Pharmacological Activity
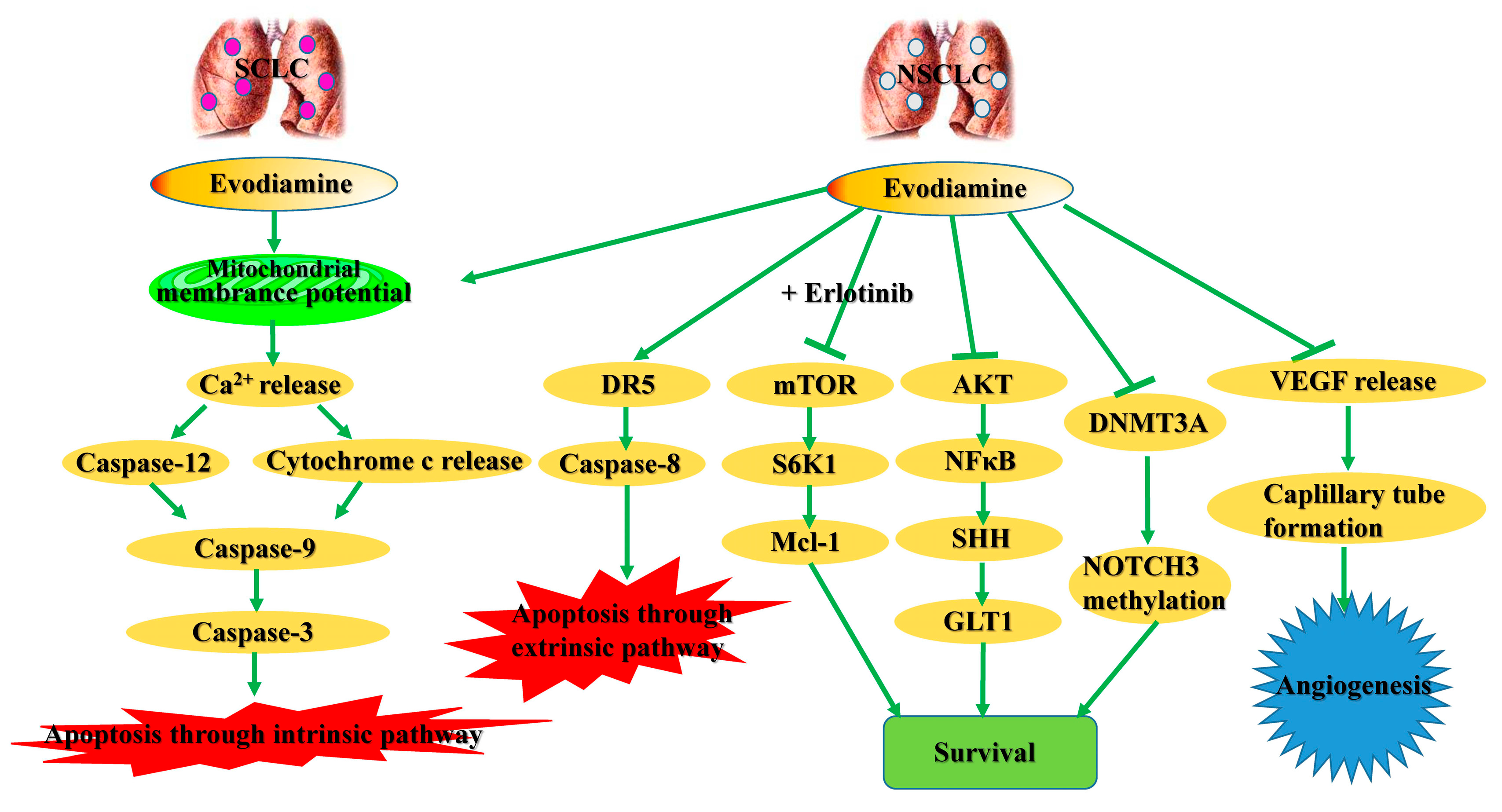
2.1. Cytotoxic Effects in Lung Cancer Cells
2.2. Cytotoxic Effects in Liver Cancer Cells
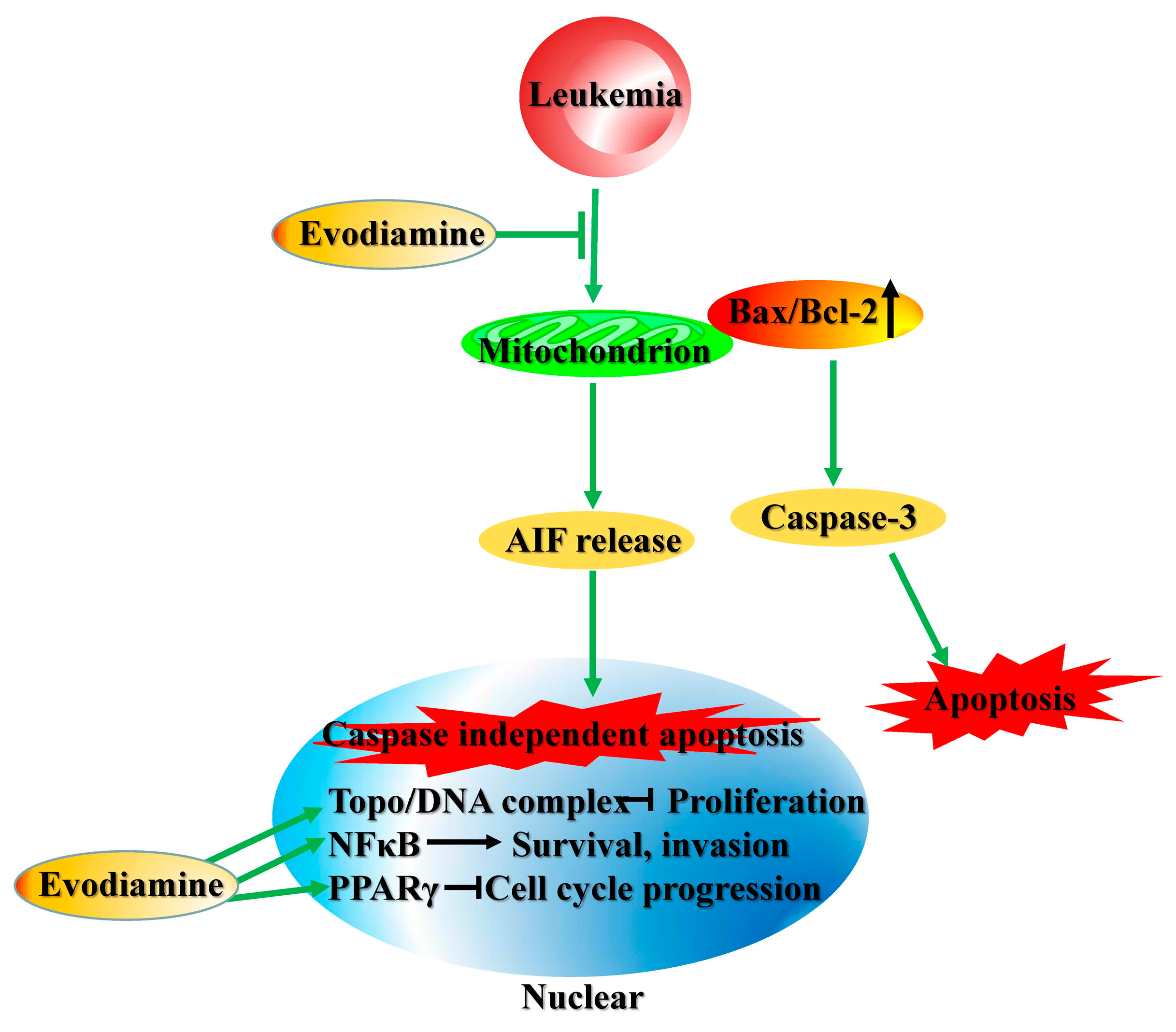
2.3. Cytotoxic Effects in Leukemia Cells
2.4. Cytotoxic Effects in Breast Cancer Cells
2.5. Cytotoxic Effects in Ovarian Cancer Cells
2.6. Cytotoxic Effects in Cervical Cancer Cells
2.7. Cytotoxic Effects in Glioma Cells
2.8. Cytotoxic Effects in Colorectal Carcinoma Cells
2.9. Cytotoxic Effects in Gastric Cancer Cells
2.10. Cytotoxic Effects in Melanoma Cells
2.11. Cytotoxic Effects in Thyroid Cancer Cells
2.12. Cytotoxic Effects in Nasopharyngeal Carcinoma Cells
2.13. Cytotoxic Effects in Renal Cancer Cells
2.14. Cytotoxic Effects in Bladder Cancer Cells
2.15. Cytotoxic Effects in Pancreatic Cancer Cells
2.16. Cytotoxic Effects in Prostate Cancer Cells
2.17. Cytotoxic Effects in Osteosarcoma Cells
2.18. Cytotoxic Effects in Oral Cancer Cells
2.19. Cytotoxic Effects in Salivary Adenoid Cystic Carcinoma Cells
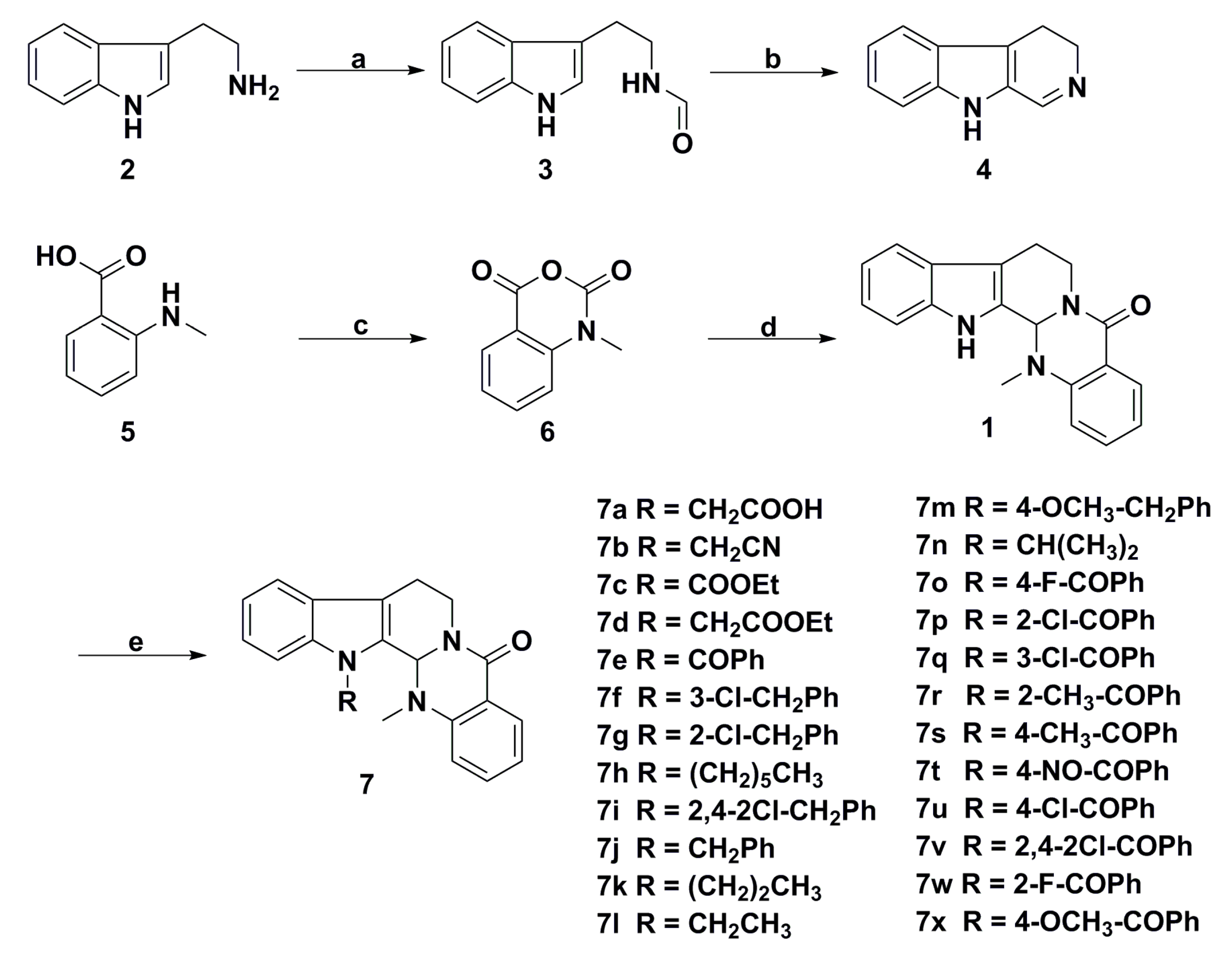
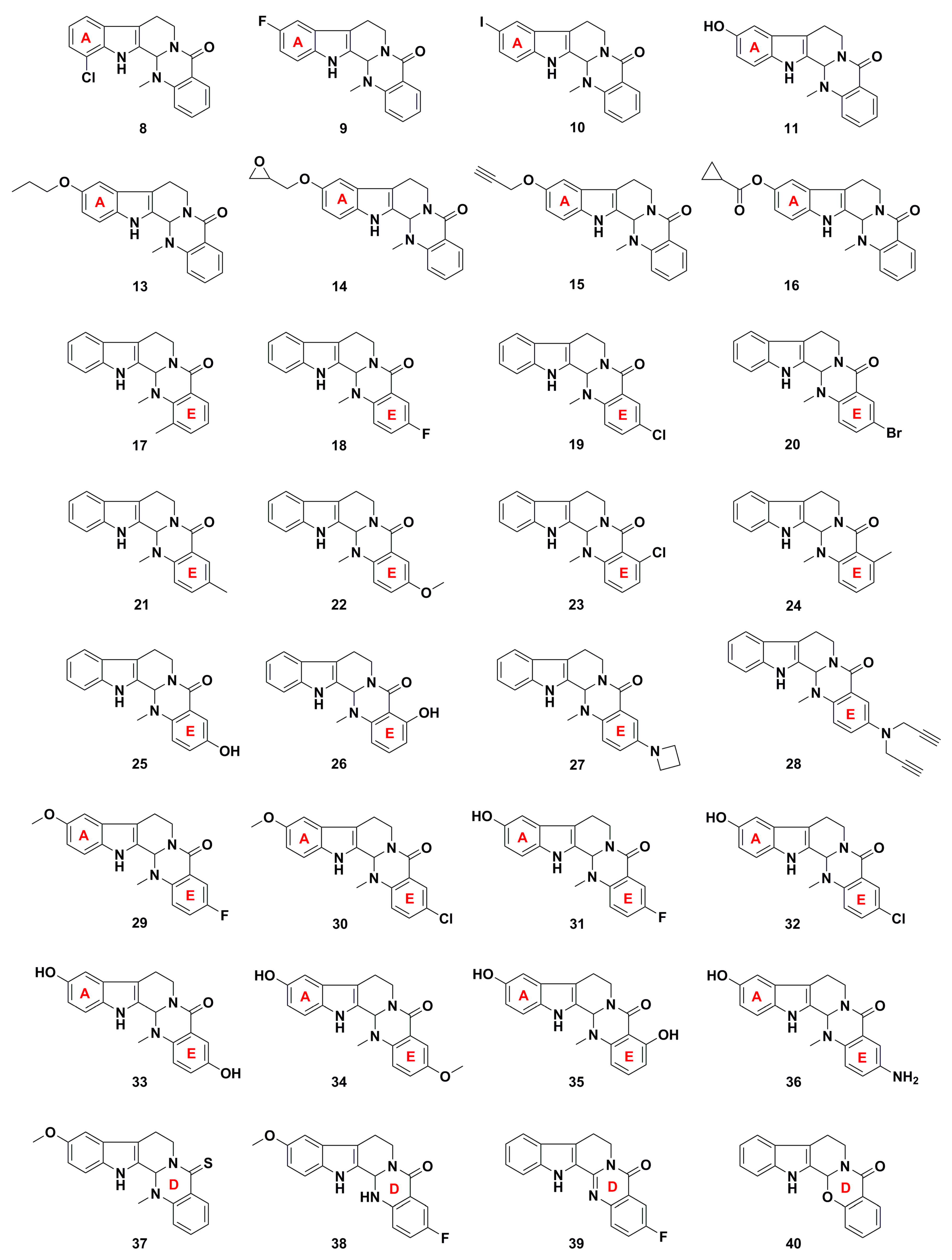
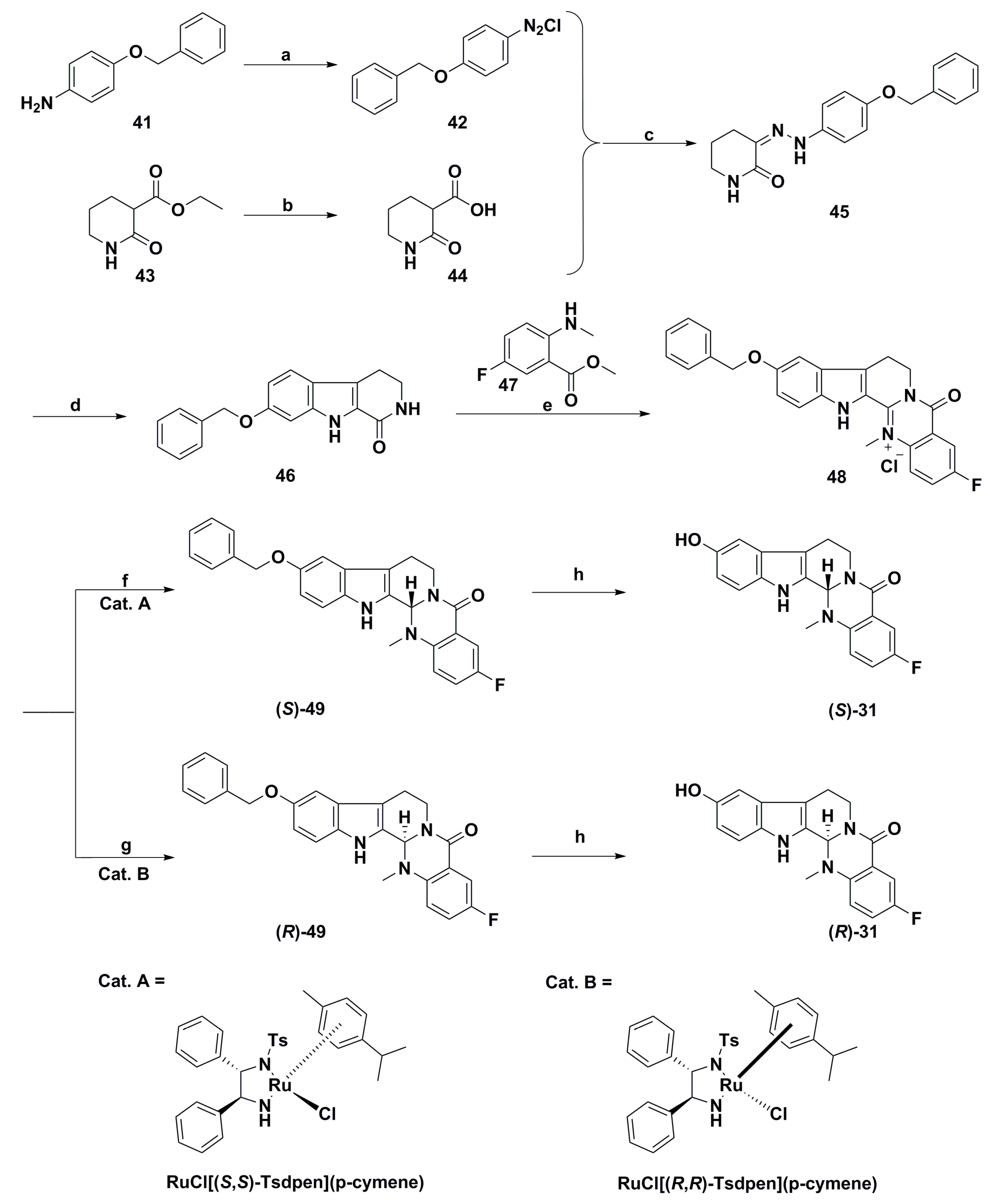
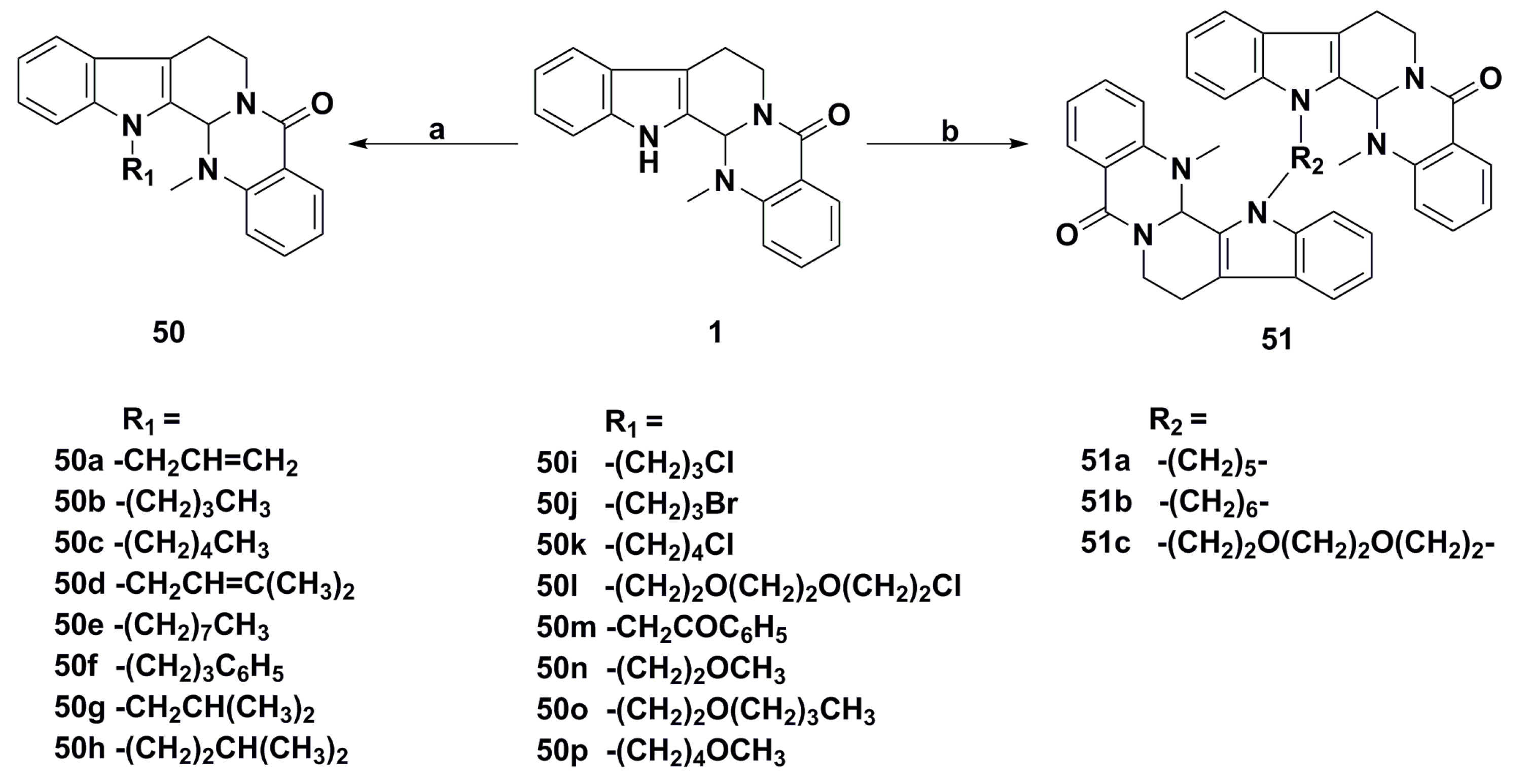
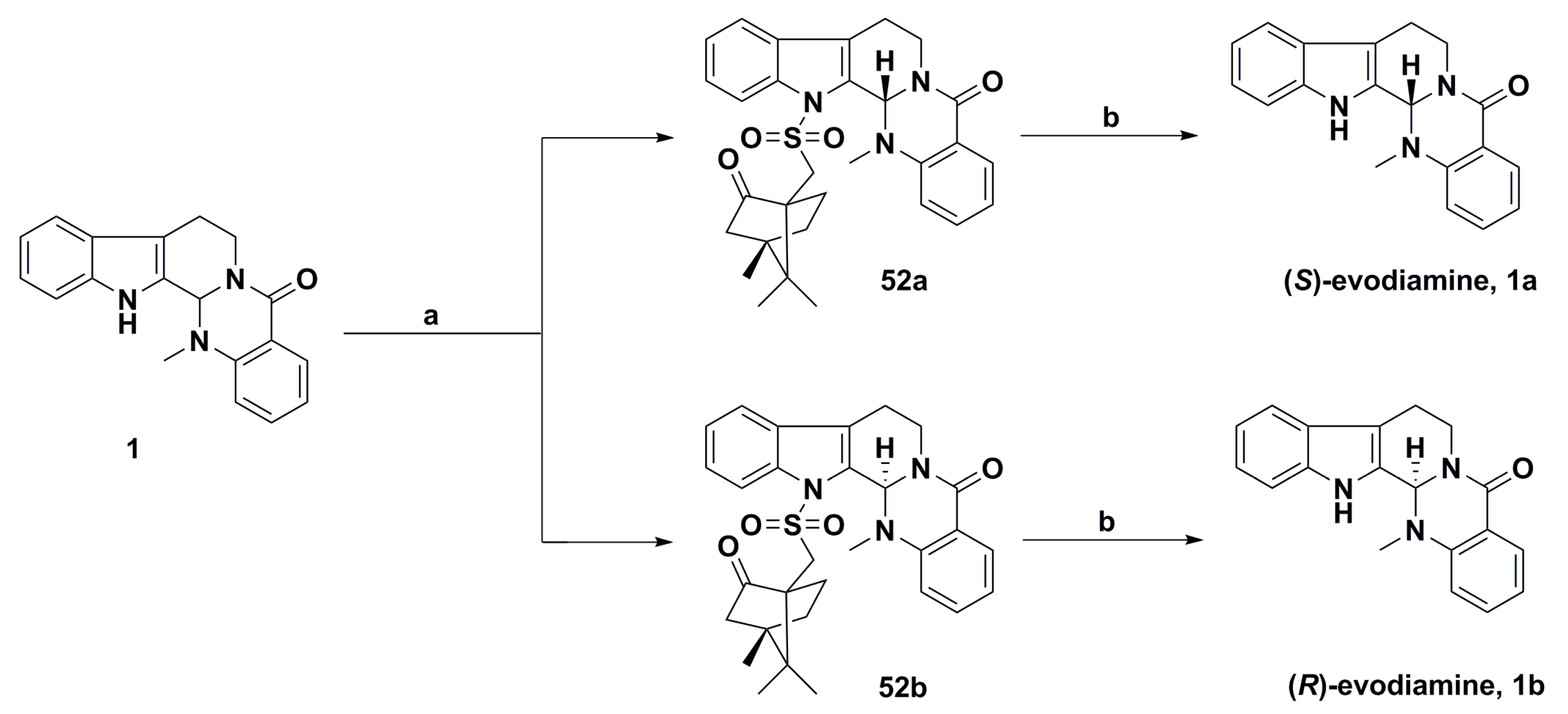
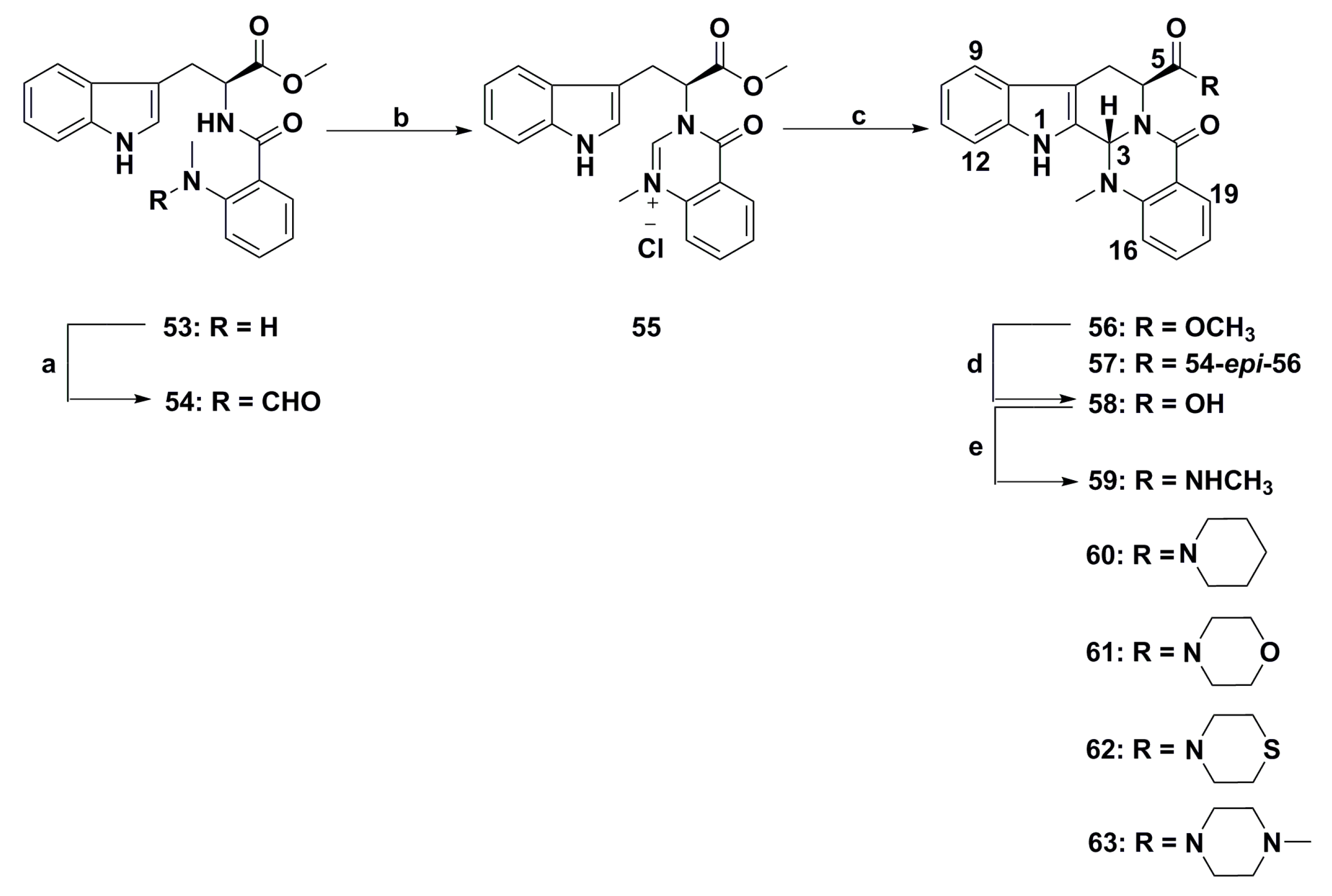
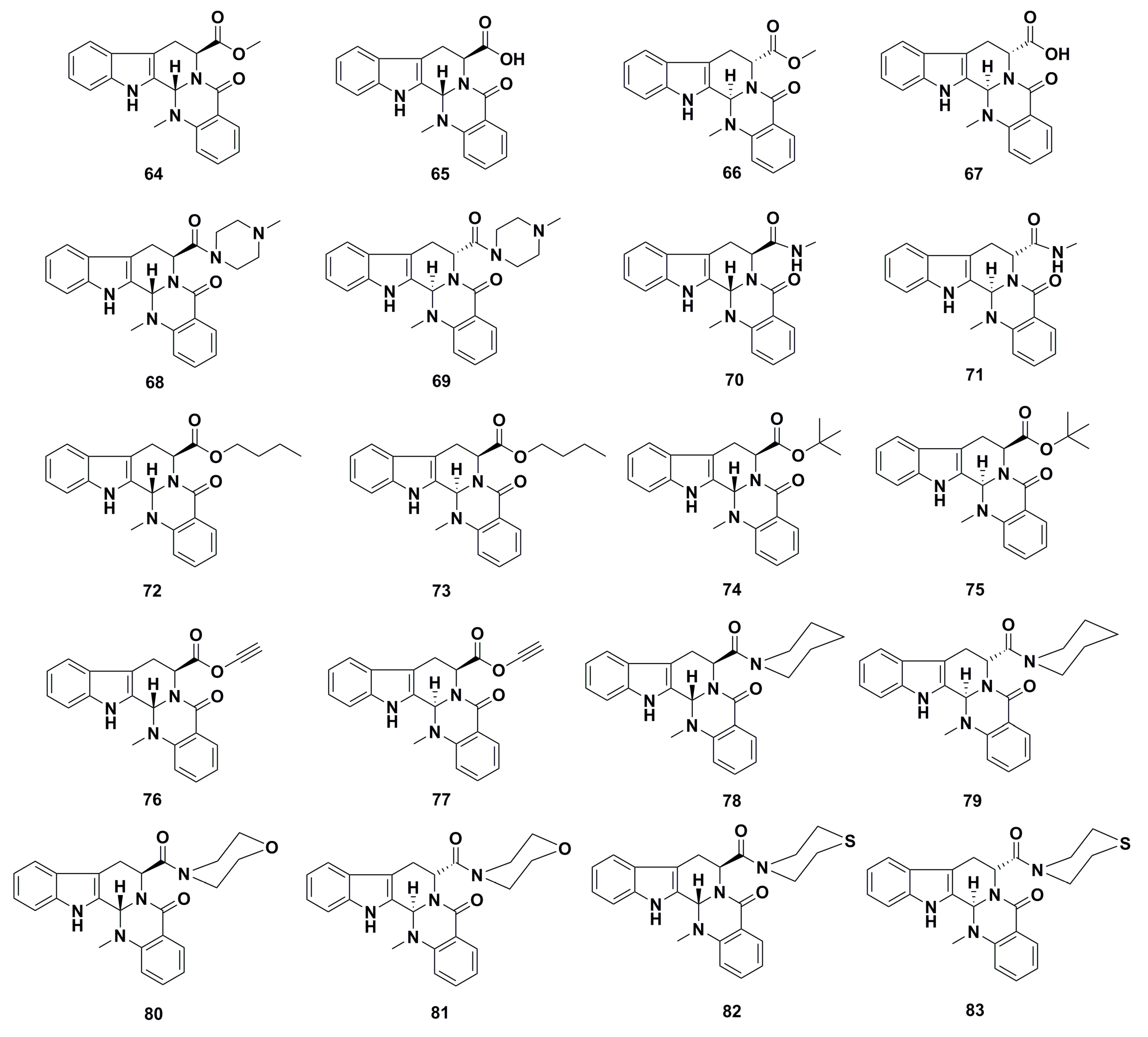
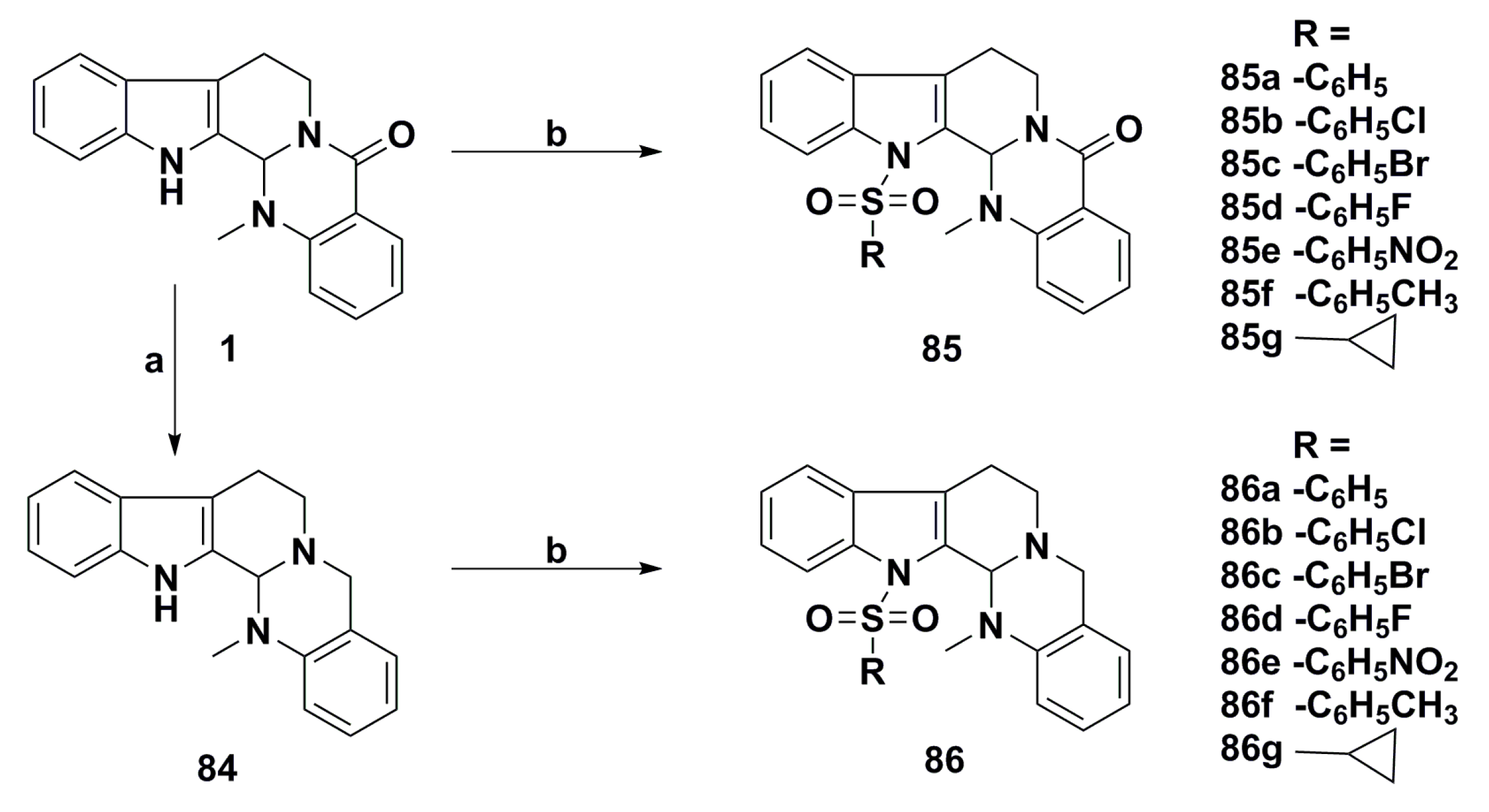
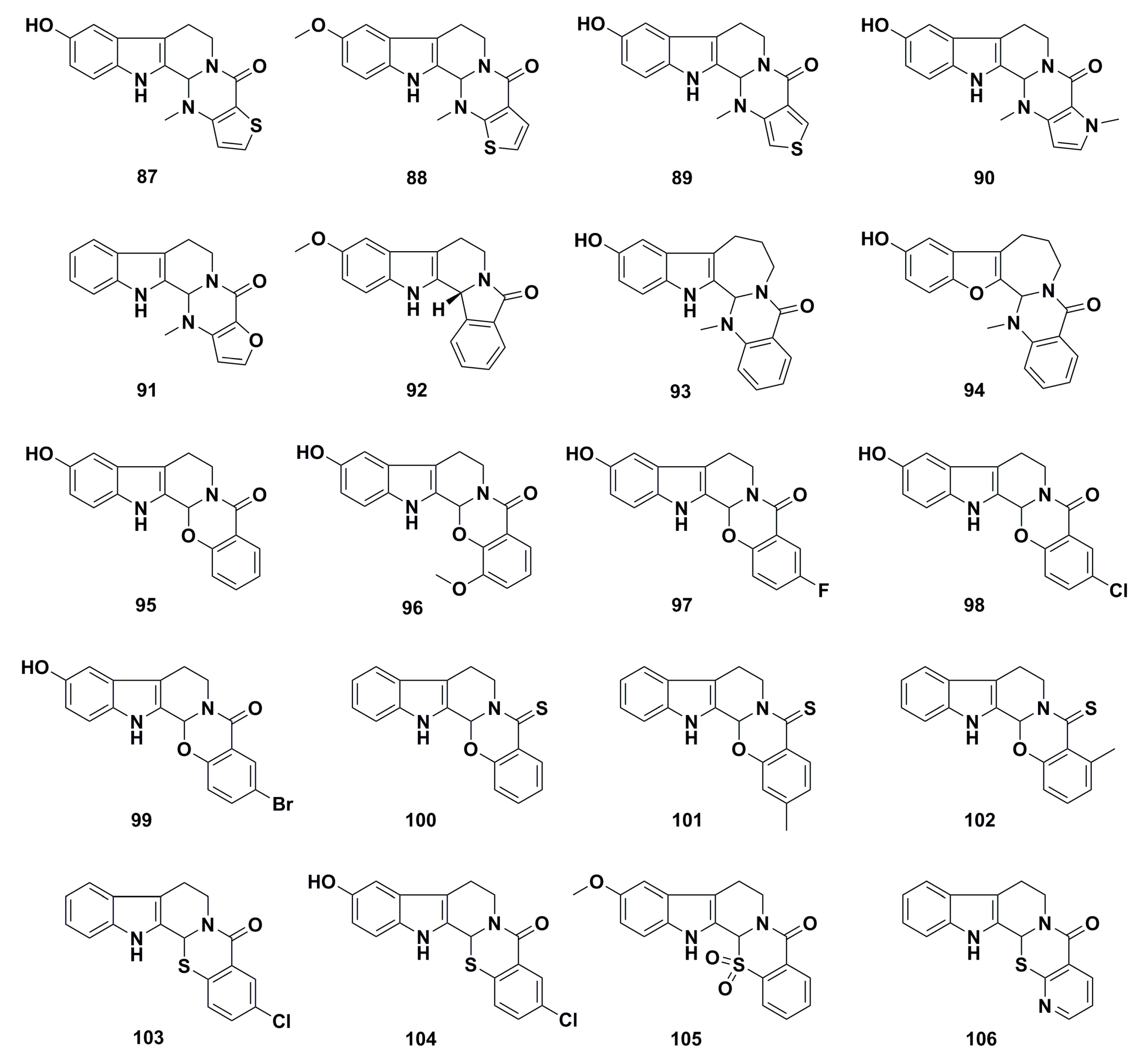
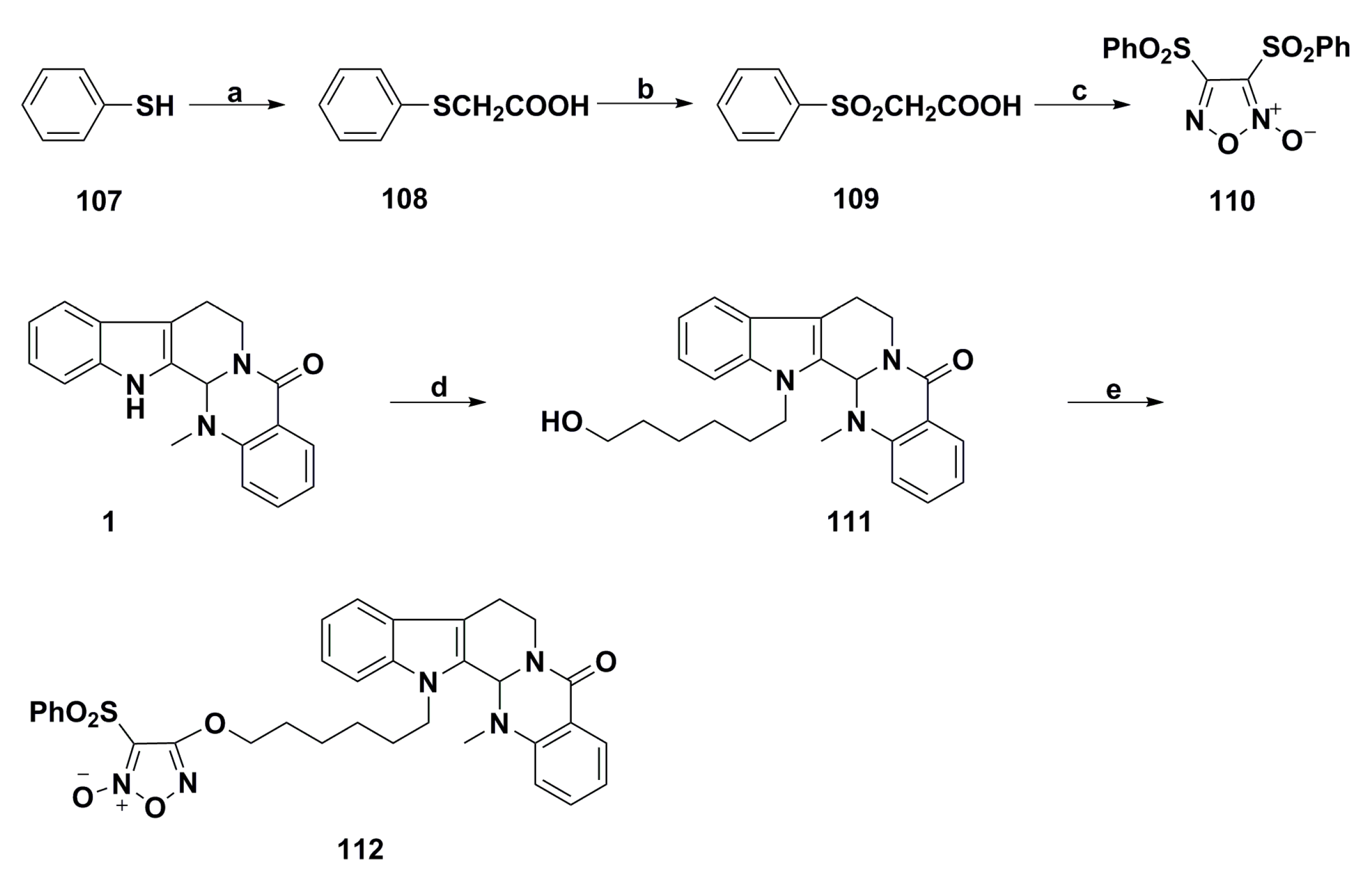
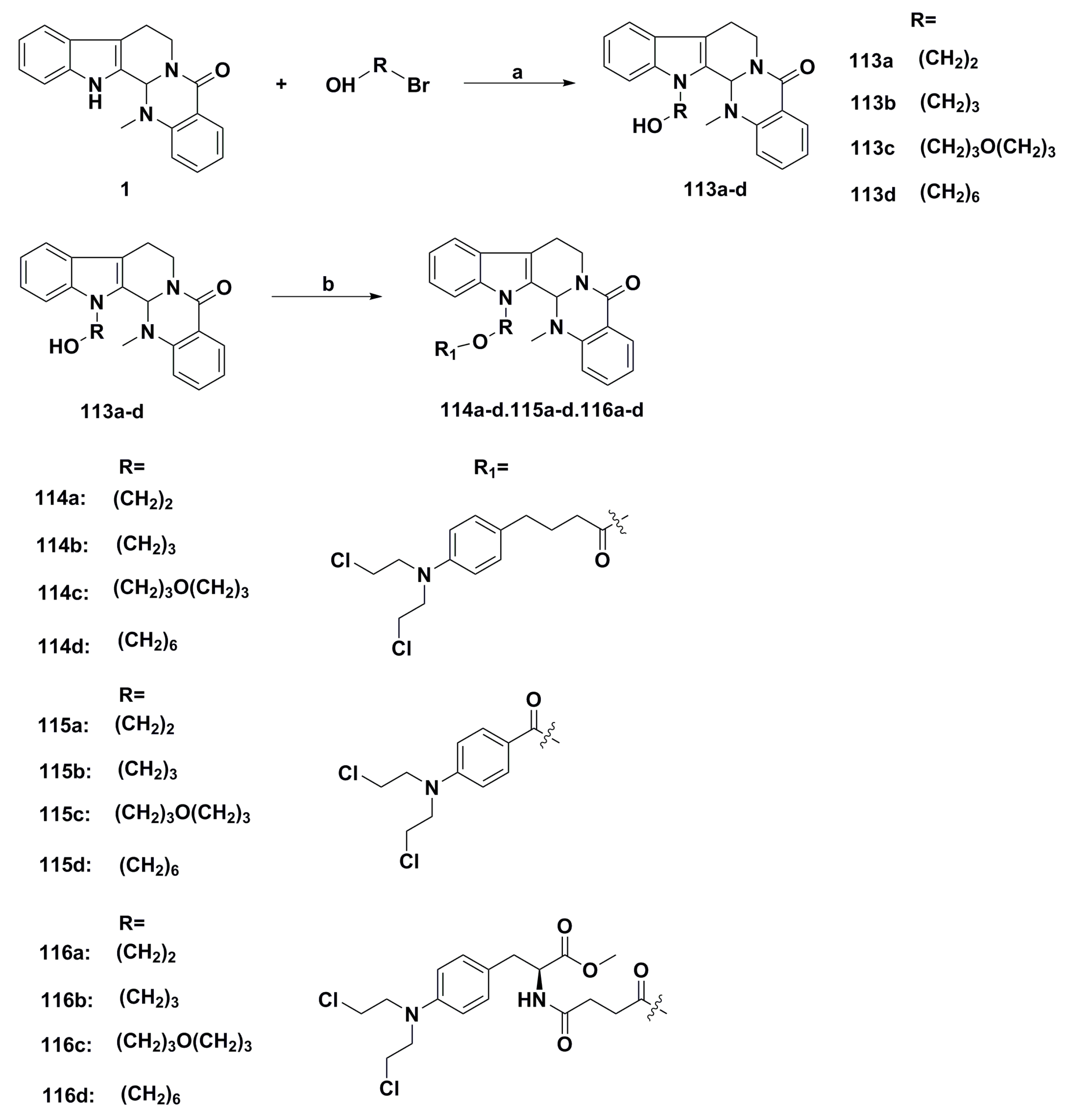
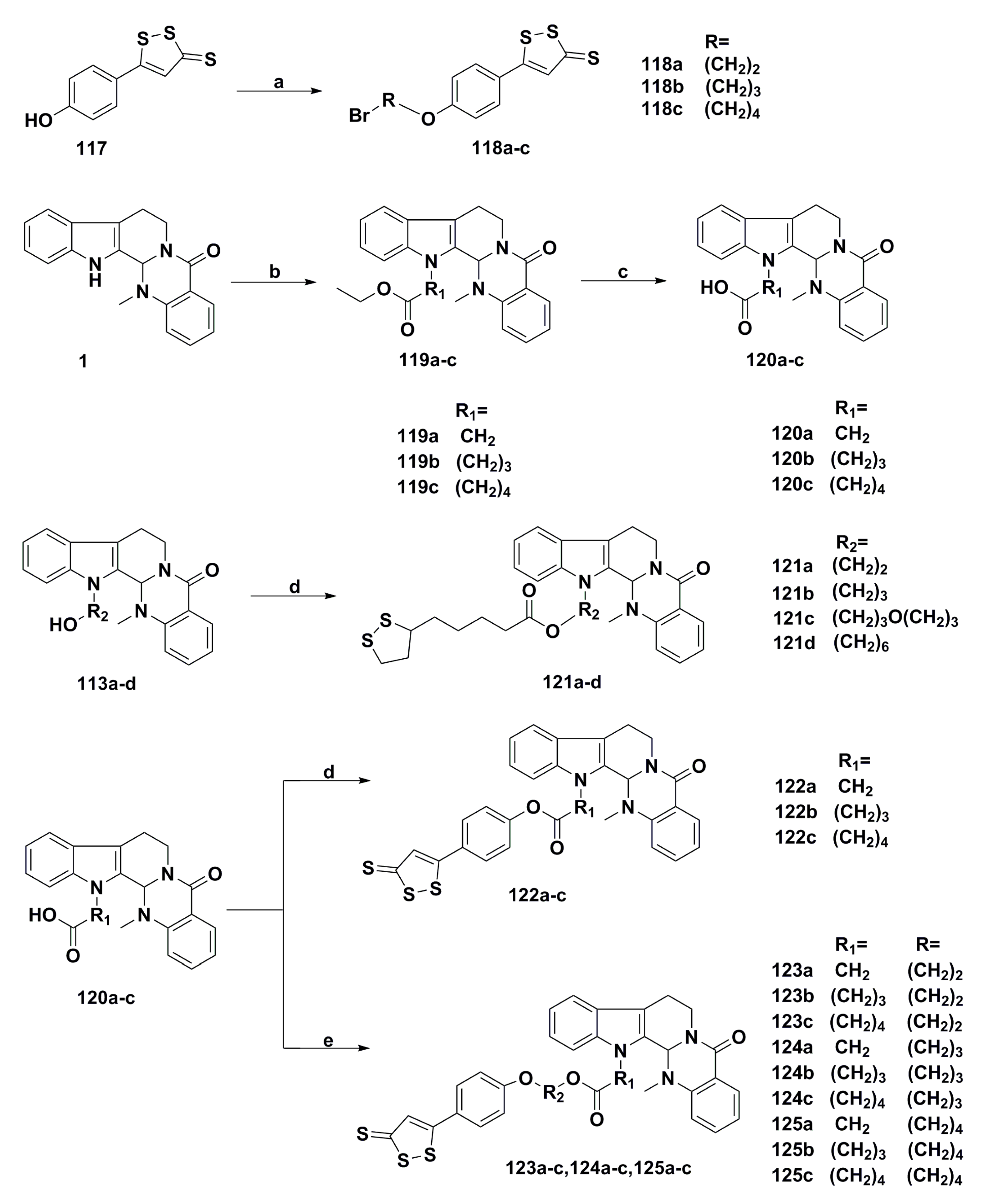
3. Advances in Antiproliferative Evodiamine Derivatives
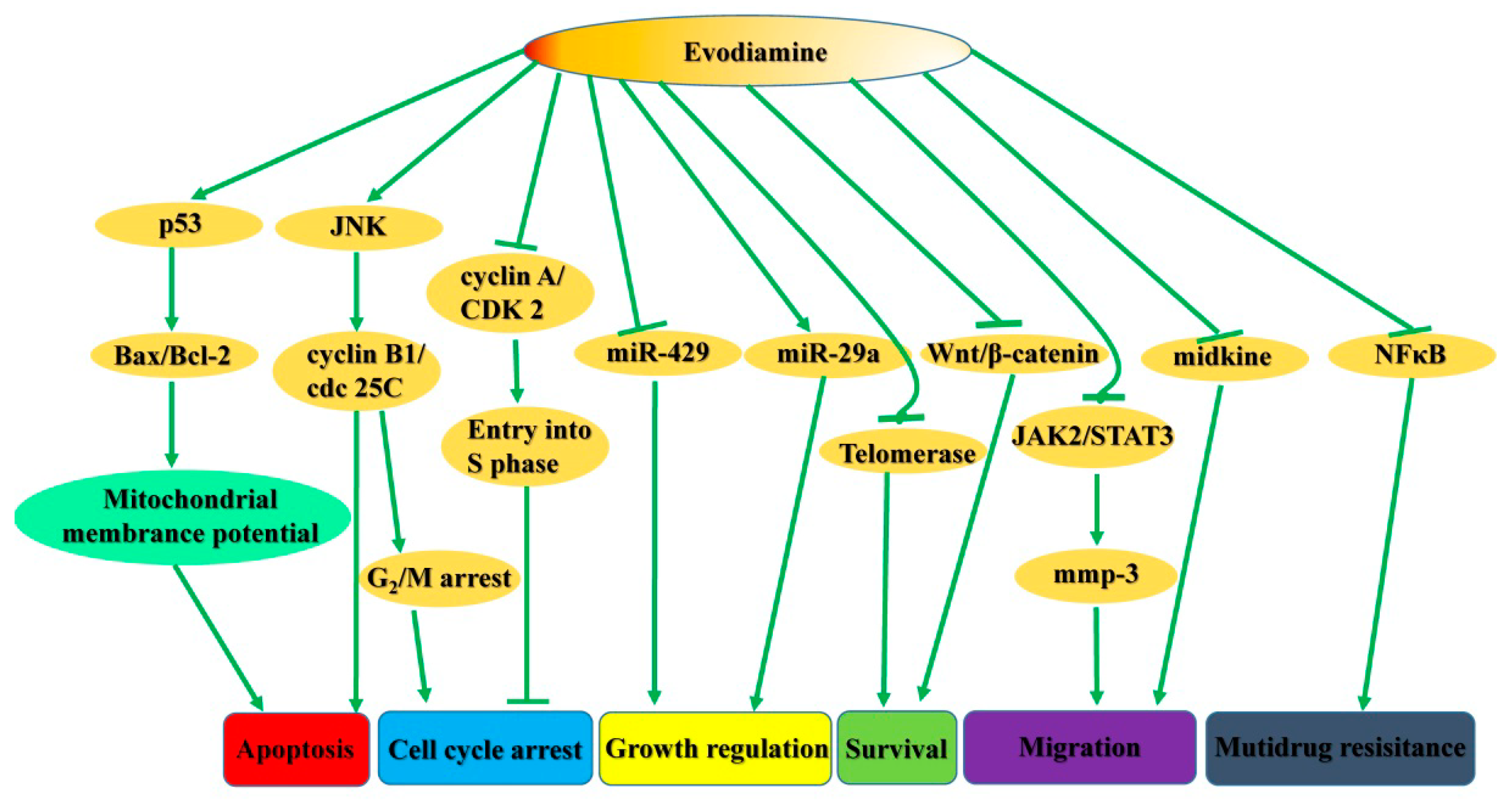
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | Activator protein 1 |
| AIF | Apoptosis-inducing factor |
| AKT | Protein kinase B |
| aSMase | Acid sphingomyelinase |
| CSLCs | Cancer stem-like cells |
| DR | Death receptor |
| DNMT | DNA methyltransferase |
| EGFR | Epidermal growth factor receptor |
| EVO/HP-β-CD | Evodiamine into hydroxypropyl-β-cyclodextrin |
| ERK | Extracellular signal-regulated kinase |
| ER | Endoplasmic reticulum |
| ERCC1 | Excision repair cross-complementing 1 |
| Fas-L | Fas-ligand |
| HUVECs | Human umbilical vein endothelial cells |
| HCC | Hepatocellular carcinoma |
| HIF-1α | Hypoxia-inducible factor 1α |
| IL-1 | Interleukin 1 |
| JNK | c-Jun N-terminal kinase |
| JAK2 | Janus kinase 2 |
| LLC | Lewis lung carcinoma |
| L-OHP | Oxaliplatin |
| mTOR/S6K1 | Mammalian target of rapamycin/p70 ribosomal S6 kinase 1 |
| MAPK | Mitogen-activated protein kinase |
| miRNAs | microRNAs |
| MMP3 | Matrix metalloproteinase 3 |
| MDR | Multidrug resistance |
| Mcl-1 | Myeloid cell leukemia 1 |
| NF-κB | Nuclear factor-κB |
| NPs | Natural products |
| NSCLC | Non-small cell lung cancer |
| NO | Nitric oxide |
| NOTCH3 | Neurogenic locus notch homolog protein 3 |
| PARP | Poly (ADP-ribose) polymerase |
| PBMC | Peripheral blood mononuclear cell |
| PERK | Double-stranded RNA-activated protein kinase-like ER kinase |
| PTK | Protein tyrosine kinase |
| PLK1 | Polo-like kinase 1 |
| PKC | Protein kinase C |
| PPAR-γ | Peroxisome proliferator-activated receptor γ |
| ROS | Reactive oxygen species |
| SCLC | Small cell lung cancer |
| SHH/GLI1 | Sonic hedgehog/GLI family zinc finger 1 |
| STAT3 | Signal transducer and activator of transcription signaling 3 |
| SHP-1 | Shatterproof 1 |
| SARs | Structure-activity relationships |
| SIRT1 | Silent information regulator of transcription |
| Topo | Topoisomerase |
| TRAIL | Tumor necrosis factor-α-related apoptosis‑inducing ligand |
| TGF-β1 | Transforming growth factor-β1 |
| TS | Thymidylate synthase |
| TRPV1 | Transient receptor potential vanilloid type-1 |
| VEGF | Vascular endothelial growth factor |
| WWOX | WWdomain-containing oxidoreductase |
| z-VAD-fmk | Benzyloxycarbonyl-Val-Ala-Aspfluoromethyl ketone |
References
- Rodrigues, T.; Reker, D.; Schneider, P. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Bracing for the biosimilar wave. Nat. Rev. Drug Discov. 2017, 16, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Plowright, A.T.; Valeur, E. Directing evolution: The next revolution in drug discovery? Nat. Rev. Drug Discov. 2017, 16, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Sashidhara, K.V. Lipid lowering agents of natural origin: An account of some promising chemotypes. Eur. J. Med. Chem. 2017, 140, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Opportunities for natural products in 21st century antibiotic discovery. Nat. Prod. Rep. 2017, 34, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bai, L.; He, J.; Zhong, L.; Duan, X.; Ouyang, L.; Zhu, Y.; Wang, T.; Zhang, Y.; Shi, J. Recent advances in discovery and development of natural products as source for anti-Parkinson’s disease lead compounds. Eur. J. Med. Chem. 2017, 141, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Sieglitz, F.; Bernardes, G.J. Natural product modulators of transient receptor potential (TRP) channels as potential anti-cancer agents. Chem. Soc. Rev. 2016, 45, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Koide, K. Discoveries, target identifications, and biological applications of natural products that inhibit splicing factor 3B subunit 1. Nat. Prod. Rep. 2016, 33, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Kontani, Y.; Kobayashi, Y.; Sato, Y.; Mori, N.; Yamashita, H. Evodiamine improves diet-induced obesity in a uncoupling protein-1-independent manner: Involvement of antiadipogenic mechanism and extracellularly regulated kinase/mitogen-activated protein kinase signaling. Endocrinology 2008, 149, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Yamashita, H. Evodiamine inhibits adipogenesis via the EGFR-PKCα-ERK signaling pathway. FEBS Lett. 2009, 583, 3655–3659. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.F.; Sung, Y.J.; Liao, J.F.; Shum, A.Y.; Chen, C.F. Inhibitory effect of dehydroevodiamine and evodiamine on nitric oxide production in cultured murine macrophages. J. Nat. Prod. 1997, 60, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhu, J.Y.; Sun, Y.; Luo, L.; Yan, J.; Yang, X.; Yu, J.; Tang, W.Q.; Ma, W.; Liang, H.P. Evodiamine inhibits zymosan-induced inflammation in vitro and in vivo: Inactivation of NF-κB by inhibiting IκBα phosphorylation. Inflammation 2017, 40, 1012–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Eftekhari, P.; Schachner, D.; Ignatova, I.D.; Palme, V.; Schilcher, N.; Ladurner, A.; Heiss, E.H.; Stangl, H.; Dirsch, V.M.; et al. Novel interactomics approach identifies abca1 as direct target of evodiamine, which increases macrophage cholesterol efflux. Sci. Rep. 2018, 8, 11061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Yang, Y.F.; Yang, X.W. Blood-brain barrier permeability and neuroprotective effects of three main alkaloids from the fruits of Euodia rutaecarpa with MDCK-pHaMDR cell monolayer and PC12 cell line. Biomed. Pharmacother. 2018, 98, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Wang, C.; Li, Z.; Liu, X.; Zhang, J.; Lu, J.; Wang, D. Pharmacological basis for the use of evodiamine in Alzheimer’s disease: Antioxidation and antiapoptosis. Int. J. Mol. Sci. 2018, 19, 1527. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.X.; Tang, Q.C.; Yan, L.; Xia, H.; Luo, H.S. Evodiamine inhibits gastrointestinal motility via CCK and CCK1 receptor in water-avoidence stress rat model. Life Sci. 2018, 209, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, J. Evodiamine and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 315–328. [Google Scholar] [PubMed]
- Jiang, J.; Hu, C. Evodiamine: A novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules 2009, 14, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, H.; Gong, W.; Wang, Z.; Liang, H. Pharmacological actions of multi-target-directed evodiamine. Molecules 2013, 18, 1826–1843. [Google Scholar] [CrossRef] [PubMed]
- Gavaraskar, K.; Dhulap, S.; Hirwani, R.R. Therapeutic and cosmetic applications of evodiamine and its derivatives—A patent review. Fitoterapia 2015, 106, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yao, L.D. Strategies to improve outcomes of patients with egrf-mutant non-small cell lung cancer: Review of the literature. J. Thorac. Oncol. 2016, 11, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, J.; Qi, D.; Fan, X.; Luo, J.; Liu, L.; Tan, Q. Evodiamine induces G2/M arrest and apoptosis via mitochondrial and endoplasmic reticulum pathways in H446 and H1688 human small-cell lung cancer cells. PLoS ONE 2014, 9, e115204. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.; Agarwal, R.; Singh, R.P. A novel alkaloid, evodiamine causes nuclear localization of cytochrome-c and induces apoptosis independent of p53 in human lung cancer cells. Biochem. Biophys. Res. Commun. 2016, 477, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Park, S.H.; Min, H.Y.; Park, H.J.; Lee, S.K. Anti-proliferative effects of evodiamine in human lung cancer cells. J. Cancer Prev. 2014, 19, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Qin, X.; Xiong, H.; Zhu, F.; Chen, T.; Wu, H. Apoptosis of human non-small-cell lung cancer A549 cells triggered by evodiamine through MTDH-dependent signaling pathway. Tumour Biol. 2015, 36, 5187–5193. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Ren, L.; Wen, L.; Wang, Y.; Qi, J. Effect of evodiamine on the proliferation and apoptosis of A549 human lung cancer cells. Mol. Med. Rep. 2016, 14, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yang, X.; Deng, J.H.; Huang, Q.J.; Huang, S.C.; Zhang, Y.M.; Zheng, H.M.; Wang, Y.; Lu, L.L.; Liu, Z.Q. Evodiamine, a novel NOTCH3 methylation stimulator, significantly suppresses lung carcinogenesis in vitro and in vivo. Front. Pharmacol. 2018, 9, 434. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.J.; Fan, X.; Yang, X.; Zhang, C.; Liang, H.P. Evodiamine activates autophagy as a cytoprotective response in murine lewis lung carcinoma cells. Oncol. Rep. 2013, 29, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Matsunaga, T.; Takahashi, S.; Saiki, I.; Suzuki, H. Anti-invasive and metastatic activities of evodiamine. Biol. Pharm. Bull. 2002, 25, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Suzuki, H. Inhibition by evodiamine of hepatocyte growth factor-induced invasion and migration of tumor cells. Biol. Pharm. Bull. 2004, 27, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Sozzi, G.; Conte, D.; Leon, M.; Ciricione, R.; Roz, L.; Ratcliffe, C.; Roz, E.; Cirenei, N.; Bellomi, M.; Pelosi, G.; et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J. Clin. Oncol. 2003, 21, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.G.; Lin, S.; Lee, C.C.; Chen, E.; Lin, L.C.; Wang, B.W.; Tsai, S.C. Evodiamine inhibits in vitro angiogenesis: Implication for antitumorgenicity. Life Sci. 2006, 78, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Bianco, R.; Troiani, T.; Tortora, G.; Ciardiello, F. Intrinsic and acquired resistance to EGFR inhibitors in human cancer therapy. Endocr. Relat. Cancer 2005, 12 (Suppl. 1), S159–S171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Pan, Y.N.; Wu, W.J.; Mao, S.Y.; Sun, J.; Zhao, Y.M.; Dong, J.Y.; Zhang, D.Y.; Pan, J.P.; Zhang, C.; et al. Evodiamine induces apoptosis and enhances apoptotic effects of erlotinib in wild-type EGFR NSCLC cells via S6K1-mediated Mcl-1 inhibition. Med. Oncol. 2016, 33, 16. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Shan, L.; Chen, X.; Wu, M.; Hong, W.; Yin, H.; Dan, H.; Xiong, H.; Zhang, J. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS PharmSciTech 2012, 13, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Aboalnaja, K.O.; Yaghmoor, S.; Kumosani, T.A.; Mcclements, D.J. Utilization of nanoemulsions to enhance bioactivity of pharmaceuticals, supplements, and nutraceuticals: Nanoemulsion delivery systems and nanoemulsion excipient systems. Expert Opin. Drug Deliv. 2016, 13, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, S.; Hu, X.; Zhang, Y.; Yan, S.; Zhao, H.; Zeng, M.; Li, Y.; Yang, L.; Zhang, J. Improved delivery of natural alkaloids into lung cancer through woody oil-based emulsive nanosystems. Drug Deliv. 2018, 25, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Shi, L.; Liang, T.; Ji, L.; Zhang, G.; Shen, Y.; Zhu, F.; Xu, L. Anti-tumor effect of evodiamine by inducing Akt-mediated apoptosis in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2017, 485, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cai, X.; Lu, W.; Hu, C.; Xu, X.; Yu, Q.; Cao, P. Evodiamine inhibits STAT3 signaling by inducing phosphatase shatterproof 1 in hepatocellular carcinoma cells. Cancer Lett. 2013, 328, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Wu, H.T.; Su, Y.C.; Lin, C.H.; Chang, C.J.; Wu, C.L. Evodiamine exerts an anti-hepatocellular carcinoma activity through a wwox-dependent pathway. Molecules 2017, 22, 1175. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Wu, S.L.; Ho, T.Y. Activation of activator protein 1 and extracellular signal-regulated kinases in human hepatocellular transformation. Tumour Biol. 2004, 25, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.C.; Lin, L.J.; Hsiang, C.Y.; Li, C.C.; Lo, H.Y.; Liang, J.A.; Kao, S.T.; Wu, S.L.; Ho, T.Y. Evodiamine inhibits 12-o-tetradecanoylphorbol-13-acetate-induced activator protein 1 transactivation and cell transformation in human hepatocytes. Phytother. Res. 2011, 25, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zhang, N.Y.; Hu, X.; Chen, J.L.; Rao, M.J.; Wu, L.W.; Li, Q.Y.; Zhang, B.; Yan, W.; Zhang, C. Evodiamine induces apoptosis and promotes hepatocellular carcinoma cell death induced by vorinostat via downregulating HIF-1α under hypoxia. Biochem. Biophys. Res. Commun. 2018, 498, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.N.; Han, X.; Xu, L.N.; Yin, L.H.; Xu, Y.W.; Qi, Y.; Peng, J.Y. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine 2008, 15, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yang, F.; Luo, F.; Liu, Y.; Zhang, F.; Zou, M.; Liu, Q. Evodiamine exerts anti-tumor effects against hepatocellular carcinoma through inhibiting β-catenin-mediated angiogenesis. Tumour Biol. 2016, 37, 12791–12803. [Google Scholar] [CrossRef] [PubMed]
- Nejak-Bowen, K.N.; Monga, S.P.S. Beta-catenin signaling, liver regeneration and hepatocellular cancer: Sorting the good from the bad. Semin. Cancer Biol. 2011, 21, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Gao, L.N.; Yan, K.; Cui, Y.L.; Zhang, Y. A promising antitumor activity of evodiamine incorporated in hydroxypropyl-β-cyclodextrin: Pro-apoptotic activity in human hepatoma HepG2 cells. Chem. Cent. J. 2016, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.E.; Kien, N.D.; Elmaraezy, A.; Abdelaziz, O.A.M.; Elsayed, A.L.; Halhouli, O.; Montasr, A.M.; Vu, T.L.; Ho, C.; Foly, A.S.; et al. Early vaccination protects against childhood leukemia: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 15986. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hartley, J.M.; Hartley, J.A.; White, K.N.; Wang, Z.; Bligh, S.W.A. Evodiamine, a dual catalytic inhibitor of type I and II topoisomerases, exhibits enhanced inhibition against camptothecin resistant cells. Phytomedicine 2012, 19, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Guh, J.H.; Teng, C.M. Induction of mitotic arrest and apoptosis by evodiamine in human leukemic T-lymphocytes. Life Sci. 2004, 75, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Kim, E.J.; Kim, S.; Jung, E.M.; Park, J.W.; Jeong, S.H.; Park, S.E.; Yoo, Y.H.; Kwon, T.K. Caspase-dependent and caspase-independent apoptosis induced by evodiamine in human leukemic U937 cells. Mol. Cancer Ther. 2006, 5, 2398–2407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Gillespie, S.K.; Hersey, P. Staurosporine induces apoptosis of melanoma by both caspase-dependent and -independent apoptotic pathways. Mol. Cancer Ther. 2004, 3, 187–197. [Google Scholar] [PubMed]
- Sun, C.; Zhang, G.; Luan, S.; Luan, C.; Shao, H.; Dong, F.; Liu, X. Evodiamine inhibits the proliferation of leukemia cell line K562 by regulating peroxisome proliferators-activated receptor gamma (PPARγ) pathway. J. Recept. Signal Transduct. Res. 2016, 36, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Takada, Y.; Kobayashi, Y.; Aggarwal, B.B. Evodiamine abolishes constitutive and inducible NF-κB activation by inhibiting IκBα kinase activation, thereby suppressing NF-κB-regulated antiapoptotic and metastatic gene expression, up-regulating apoptosis, and inhibiting invasion. J. Biol. Chem. 2005, 280, 17203–17212. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Kaur, H.; Gautam, N.; Mantha, A.K. Biosensors for breast cancer diagnosis: A review of bioreceptors, biotransducers and signal amplification strategies. Biosens. Bioelectron. 2017, 88, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, X.F.; Zhou, Q.M.; Zhang, T.L.; Lu, Y.Y.; Zhang, H.; Su, S.B. Evodiamine induces apoptosis and inhibits metastasis in MDA-MB-231 human breast cancer cells in vitro and in vivo. Oncol. Rep. 2013, 30, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, Y.; Lu, Y.Y.; Lau, E.; Zhao, M.; Zhou, Q.M.; Su, S.B. Berberine and evodiamine act synergistically against human breast cancer MCF-7 cells by inducing cell cycle arrest and apoptosis. Anticancer Res. 2017, 37, 6141–6151. [Google Scholar] [PubMed]
- Wang, K.L.; Hsia, S.M.; Yeh, J.Y.; Cheng, S.C.; Wang, P.S.; Wang, S.W. Anti-proliferative effects of evodiamine on human breast cancer cells. PLoS ONE 2013, 8, e67297. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, M.; Wang, Y. Tumor suppressive activity of evodiamine in breast cancer cells via inhibition of Ras/MEK/ERK pathway and activation of PPARγ. Planta Med. 2012, 78, P_79. [Google Scholar] [CrossRef]
- Chan, A.L.; Chang, W.S.; Chen, L.M.; Lee, C.M.; Chen, C.E.; Lin, C.M.; Hwang, J.L. Evodiamine stabilizes topoisomerase I-DNA cleavable complex to inhibit topoisomerase I activity. Molecules 2009, 14, 1342–1352. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, J.K.; Jung, Y.; Jeong, D.; Kang, M.; Yoo, Y.J.; Lee, H.; Oh, S.H.; Ryu, J.H.; Kim, W.Y. Evodiamine selectively targets cancer stem-like cells through the p53-p21-Rb pathway. Biochem. Biophys. Res. Commun. 2016, 469, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Pan, S.L.; Guh, J.H.; Chang, Y.L.; Pai, H.C.; Lin, C.H.; Teng, C.M. Antitumor mechanism of evodiamine, a constituent from chinese herb Evodiae fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis 2005, 26, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Shi, Z.; Zhong, Z.; Chen, M.; Wang, Y. Evodiamine synergizes with doxorubicin in the treatment of chemoresistant human breast cancer without inhibiting p-glycoprotein. PLoS ONE 2014, 9, e97512. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Chen, F.; Bao, J.; Wang, S.; Wang, L.; Chen, M.; He, C.; Wang, Y. Preparation, characterization, and anticancer efficacy of evodiamine-loaded PLGA nanoparticles. Drug Deliv. 2016, 23, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Ramser, M.; Eichelberger, S.; Däster, S.; Weixler, B.; Kraljević, M.; Mechera, R.; Tampakis, A.; Delko, T.; Güth, U.; Stadlmann, S.; et al. High OX40 expression in recurrent ovarian carcinoma is indicative for response to repeated chemotherapy. BMC Cancer 2018, 18, 425. [Google Scholar] [CrossRef] [PubMed]
- Rauh-Hain, J.A.; Krivak, T.C.; Carmen, M.G.D.; Olawaiye, A.B. Ovarian cancer screening and early detection in the general population. Rev. Obstet. Gynecol. 2011, 4, 15–21. [Google Scholar] [PubMed]
- Chen, T.C.; Chien, C.C.; Wu, M.S.; Chen, Y.C. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine 2016, 23, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Jin, X.; Cao, Z.; Li, W. Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase/phosphatidylinositol-3-kinase/protein kinase B signaling pathways. J. Tradit. Chin. Med. 2016, 36, 353–359. [Google Scholar] [PubMed]
- Zhong, Z.F.; Tan, W.; Wang, S.P.; Qiang, W.A.; Wang, Y.T. Anti-proliferative activity and cell cycle arrest induced by evodiamine on paclitaxel-sensitive and -resistant human ovarian cancer cells. Sci. Rep. 2015, 5, 16415. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Lin, R.-C.; Wang, P.S. Anti-proliferative effects of evodiamine and rutaecarpine on human ovarian cancer cell line SKOV3. Biol. Reprod. 2010, 83 (Suppl. 1), 134. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Evodiamine induces tumor cell death through different pathways: Apoptosis and necrosis. Acta Pharmacol. Sin. 2004, 25, 83–89. [Google Scholar] [PubMed]
- Fei, X.F.; Wang, B.X.; Li, T.J.; Tashiro, S.I.; Minami, M.; Xing, D.J.; Ikejima, T. Evodiamine, a constituent of Evodiae fructus, induces anti-proliferating effects in tumor cells. Cancer Sci. 2003, 94, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.; Onodera, S.; Ikejima, T. Reactive oxygen species and nitric oxide regulate mitochondria-dependent apoptosis and autophagy in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic. Res. 2008, 42, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.I.; Onodera, S.; Ikejima, T. Protein tyrosine kinase pathway-derived ROS/NO productions contribute to G2/M cell cycle arrest in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic. Res. 2010, 44, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, C.H.; Tsai, H.P.; An, H.W.; Lee, C.M.; Wu, J.C.; Chen, C.S.; Huang, S.H.; Hwang, J.; Cheng, K.T.; et al. Targeting of topoisomerase I for prognoses and therapeutics of camptothecin-resistant ovarian cancer. PLoS ONE 2015, 10, e0132579. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.V.; Conroy, S.; Pavlov, K.; Sontakke, P.; Tomar, T.; Eggens-Meijer, E.; Balasubramaniyan, V.; Wagemakers, M.; den Dunnen, W.F.; Kruyt, F.A. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis. Cancer Lett. 2015, 359, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.J.; Wang, S.H.; Chen, K.C.; Kuei, H.P.; Shih, Y.L.; Hou, S.Y.; Chiu, W.T.; Hsiao, S.H.; Shih, C.M. Evodiamine, a plant alkaloid, induces calcium/JNK-mediated autophagy and calcium/mitochondria-mediated apoptosis in human glioblastoma cells. Chem. Biol. Interact. 2013, 205, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.J.; Wang, S.H.; Hou, S.Y.; Lin, C.J.; Chiu, W.T.; Hsiao, S.H.; Chen, T.H.; Shih, C.M. Evodiamine induces transient receptor potential vanilloid-1-mediated protective autophagy in U87-MG astrocytes. Evid. Based Complement. Altern. Med. 2013, 2013, 354840. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S.; Chien, C.C.; Liu, K.H.; Chen, Y.C.; Chiu, W.T. Evodiamine prevents glioma growth, induces glioblastoma cell apoptosis and cell cycle arrest through JNK activation. Am. J. Chin. Med. 2017, 45, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Deng, D.; Shao, N.; Xu, Y.; Xue, L.; Peng, Y.; Liu, Y.; Zhi, F. Evodiamine activates cellular apoptosis through suppressing PI3K/AKT and activating MAPK in glioma. OncoTargets Ther. 2018, 11, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Intracellular mechanisms of TRAIL: Apoptosis through mitochondrial-dependent and -independent pathways. Oncogene 2001, 20, 2122–2133. [Google Scholar]
- Khan, M.; Bi, Y.; Qazi, J.I.; Fan, L.; Gao, H. Evodiamine sensitizes U87 glioblastoma cells to trail via the death receptor pathway. Mol. Med. Rep. 2015, 11, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Chen, A.; Liu, B. Antiproliferation and apoptosis induced by evodiamine in human colorectal carcinoma cells (COLO-205). Chem. Biodivers. 2010, 6, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, H.; Gong, X.L.; Wu, L.Y.; Wen, B. Effect of evodiamine and berberine on the interaction between dnmts and target micrornas during malignant transformation of the colon by tgf-beta1. Oncol. Rep. 2017, 37, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, C.; Wu, L.; Wen, B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. OncoTargets Ther. 2016, 9, 4121–4127. [Google Scholar]
- Chang, J.; Wen, B.; Wang, R.; Chen, W.; Wang, J. Effects of evodiamine and berberine hydrochloride on cell apoptosis and expression of hTERT-mRNA of human colorectal cancer cell line HT-29. Pharmacol. Clin. Chin. Mater. Med. 2008, 19, 191–194. [Google Scholar]
- Chang, J.R.; Chen, W.W.; Wang, J.H. Effects of evodiamine and berberine hydrochloride on telomerase activity of human colorectal cancer cell line HT29. Liaoning J. Tradit. Chin. Med. 2011, 38, 1326–1329. [Google Scholar]
- Wang, Y.H.; Zhou, Z.H. Regulatory effect of evodiamine on the malignant biological behaviors and wnt/β-catenin signaling pathway of colorectal cancer cell lines HT29. J. Hainan Med. Univ. 2016, 22, 729–731. [Google Scholar]
- Chien, C.C.; Wu, M.S.; Shen, S.C.; Ko, C.H.; Chen, C.H.; Yang, L.L.; Chen, Y.C. Activation of JNK contributes to evodiamine-induced apoptosis and G2/M arrest in human colorectal carcinoma cells: A structure-activity study of evodiamine. PLoS ONE 2014, 9, e99729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, X.; Xu, X.; Yang, X.; Wang, X.; Liang, H.P. Evodiamine induces caspase-dependent apoptosis and S phase arrest in human colon lovo cells. Anti-Cancer Drugs 2010, 21, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Han, S.; Fan, X.; Wang, X.; Zhang, C.; Liang, H.P.; Yang, W.J. The effects of evodiamine on autophagy in human colon adenocarcinoma lovo cells. Chin. J. Gen. Surg. 2011, 26, 41–44. [Google Scholar]
- Ogasawara, M.; Matsubara, T.; Suzuki, H. Inhibitory effects of evodiamine on in vitro invasion and experimental lung metastasis of murine colon cancer cells. Biol. Pharm. Bull. 2001, 24, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liao, K.; Li, K.; Li, J. Modulatory effect of evodiamine on JAK2/STAT3 signal pathway in HCT-116 cells. Chin. Pharm. Bull. 2015, 1398, 1394–1397. [Google Scholar]
- Zhao, L.C.; Li, J.; Liao, K.; Luo, N.; Shi, Q.Q.; Feng, Z.Q.; Chen, D.L. Evodiamine induces apoptosis and inhibits migration of HCT-116 human colorectal cancer cells. Int. J. Mol. Sci. 2015, 16, 27411–27421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, Y.; Zhang, Y. Effects of evodiamine on invasion and midkine expression of human colon cancer cell. Int. J. Surg. 2009, 36, 742–744. [Google Scholar]
- Sui, H.; Zhou, L.H.; Zhang, Y.L.; Huang, J.P.; Liu, X.; Ji, Q.; Fu, X.L.; Wen, H.T.; Chen, Z.S.; Deng, W.L.; et al. Evodiamine suppresses ABCG2 mediated drug resistance by inhibiting p50/p65 NF-κB pathway in colorectal cancer. J. Cell. Biochem. 2016, 117, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.P.; Peng, L.X.; Xiong, W.; Xing, L.H.; Guo, P.; Wang, F.; Chen, D.L.; Jing, L.I. Evodiamine suppresses proliferation of colon cancer HCT-116 cells in mice. Basic Clin. Med. 2017, 37, 1373–1377. [Google Scholar]
- Rugge, M.; Fassan, M.; Graham, D.Y. Epidemiology of gastric cancer. In Gastric Cancer; Springer: Berlin, Germany, 2015; pp. 23–34. [Google Scholar]
- Yue, G.; Wei, J.; Qian, X.; Yu, L.; Zou, Z.; Guan, W.; Wang, H.; Shen, J.; Liu, B. Synergistic anticancer effects of polyphyllin I and evodiamine on freshly-removed human gastric tumors. PLoS ONE 2013, 8, e65164. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Gao, X.; Han, Y.; Guo, Q.I.; Zhang, K.; Liu, M.; Wang, Y.; Wang, J. Evodiamine sensitizes BGC-823 gastric cancer cells to radiotherapy in vitro and in vivo. Mol. Med. Rep. 2016, 14, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Yu, B.; Zhong, L.; Khan, M.; Yang, H.; Ma, T. Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol. Rep. 2012, 27, 1481–1487. [Google Scholar] [PubMed]
- Liu, S.P.; Qian, W.G.; Mao, W.D. Effect of evodiamine on inducing apoptosis of human gastric cancer cell line SGC-7901 in vitro. J. Int. Transl. Med. 2014, 2, 380–384. [Google Scholar]
- Tian, X.L.; Zhang, J.; Wang, X.L.; Wang, S.; Shi, Q.Y.; Zhao, G.H.; Quan, L.Q.; Song, Y. Effect of evodiamine on human gastric adenocarcinoma cell line (SGC-7901). J. Tradit. Chin. Med. 2011, 34, 115–118. [Google Scholar]
- Yang, L.; Liu, X.; Wu, D.; Zhang, M.; Ran, G.; Bi, Y.; Huang, H. Growth inhibition and induction of apoptosis in SGC7901 human gastric cancer cells by evodiamine. Mol. Med. Rep. 2014, 9, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Ya-Kun, G.E. Study on synergetic anti-tumor effect of evodiamine and paclitaxel in SGC-7901 cells. J. Jilin Agric. Univ. 2014, 36, 314–318. [Google Scholar]
- Shen, H.; Zhao, S.; Xu, Z.; Zhu, L.; Han, Y.; Ye, J. Evodiamine inhibits proliferation and induces apoptosis in gastric cancer cells. Oncol. Lett. 2015, 10, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Huang, H.; Zhang, Y.Y.; Liu, X.; Bi, Y.; Liu, L. Study of evodiamine on proliferation inhibition and apoptosis induction in human gastric cancer cell line SGC-7901. Lab. Med. 2010, 25, 952–955. [Google Scholar]
- Huang, H.; Zhang, Y.; Liu, X.; Li, Z.; Xu, W.; He, S.; Huang, Y.; Zhang, H. Acid sphingomyelinase contributes to evodiamine-induced apoptosis in human gastric cancer SGC-7901 cells. DNA Cell Biol. 2011, 30, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.M.; Zhou, Y.J.; Zhang, Y.L.; Qi, W.D.; Fan, Y. Effects of evodiamine on invasion and polo-like kinase 1 expression of human gastric cancer cell. J. Jiangsu Univ. 2011, 21, 69–72. [Google Scholar]
- Qi, W.D.; Zhou, Y.J.; Fan, Y. Effects of evodiamine on invasion and proliferation in human gastric cancer cell. Shandong Med. J. 2010, 34, 016. [Google Scholar]
- Singh, S.R. Gastric cancer stem cells: A novel therapeutic target. Cancer Lett. 2013, 338, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Feng, S.; Wei, L.; Wang, Z.; Hong, D.; Wang, Q. Evodiamine, a novel inhibitor of the wnt pathway, inhibits the self-renewal of gastric cancer stem cells. Int. J. Mol. Med. 2015, 36, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.L.; Wu, X.J.; Liu, Y.; Xie, J.Q. Berberine counteracts enhanced IL-8 expression of AGS cells induced by evodiamine. Life Sci. 2013, 93, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.; Day, C.P.; Yang, H.; Michalowski, A.; Lee, M.; Merlino, G.; Michael, H.; Day, C.P.; Yang, H.; Michalowski, A. Abstract 1037: Progression from melanocytic nevi to melanoma is associated with increased genomic mutations in a uv-induced mouse model of human melanoma. Cancer Res. 2017, 77, 1037. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Effect of protein kinase C on human melanoma A375-S2 cell death induced by evodiamine. Acta Pharm. Sin. 2005, 40, 1033–1036. [Google Scholar]
- Wang, C.; Wang, M.S.; Onodera, S.; Ikejima, T. Roles of SIRT1 and phosphoinositide 3-OH kinase/protein kinase C pathways in evodiamine-induced human melanoma A375-S2 cell death. J. Pharmacol. Sci. 2005, 97, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Evodiamine induces A375-S2 cell death through two different pathways. Acta Pharm. Sin. 2003, 38, 650–653. [Google Scholar]
- Zhang, Y.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Intracellular regulation of evodiamine-induced A375-S2 cell death. Biol. Pharm. Bull. 2003, 26, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashino, S.; Onodera, S.; Ikejima, T. Critical roles of reactive oxygen species in mitochondrial permeability transition in mediating evodiamine-induced human melanoma A375-S2 cell apoptosis. Free Radic. Res. 2007, 41, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, L.J.; Tashiro, S.; Onodera, S.; Ikejima, T. Nitric oxide activated by p38 and NF-κB facilitates apoptosis and cell cycle arrest under oxidative stress in evodiamine-treated human melanoma A375-S2 cells. Free Radic. Res. 2008, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, M.S.; Onodera, S.; Ikejima, T. Evodiamine induced human melanoma A375-S2 cell death partially through interleukin 1 mediated pathway. Chin. Pharmacol. Bull. 2005, 28, 984–989. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Wang, M.W. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-κB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol. In Vitro 2010, 24, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yin, L.; Chen, J.; Chen, J. The apoptotic effect of shikonin on human papillary thyroid carcinoma cells through mitochondrial pathway. Tumor Biol. 2014, 35, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhao, D.; Liu, R.; Guo, J.; Lin, Y.; Zhang, M. Evodiamine inhibits proliferation of human papillary thyroid cancer cell line k1 by regulating of PI3K/Akt signaling pathway. Int. J. Clin. Exp. Med. 2016, 9, 15216–15225. [Google Scholar]
- Chen, M.C.; Yu, C.H.; Wang, S.W.; Pu, H.F.; Kan, S.F.; Lin, L.C.; Chi, C.W.; Ho, L.L.; Lee, C.H.; Wang, P.S. Anti-proliferative effects of evodiamine on human thyroid cancer cell line ARO. J. Cell Biochem. 2010, 110, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Lu, C.H.; Chang, Y.S.; Chen, S.H.; Liu, Y.W. Evodiamine inhibits human thyroid cancer cells in vitro and in vivo. Endocrine 2015, 37, EP902. [Google Scholar] [CrossRef]
- Yu, H.I.; Chou, H.C.; Su, Y.C.; Lin, L.H.; Lu, C.H.; Chuang, H.H.; Tsai, Y.T.; Liao, E.C.; Wei, Y.S.; Yang, Y.T.; et al. Proteomic analysis of evodiamine-induced cytotoxicity in thyroid cancer cells. J. Pharm. Biomed. Anal. 2018, 160, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Q.; Zeng, Y.; Li, J.; Wang, L.; Ai, P. Evodiamine inhibits the migration and invasion of nasopharyngeal carcinoma cells in vitro via repressing MMP-2 expression. Cancer Chemother. Pharmacol. 2015, 76, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Krause, M.; Samoylenko, A.; Vainio, S. Wnt signaling in renal cell carcinoma. Cancers 2016, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S.; Chien, C.C.; Chen, Y.C.; Chiu, W.T. Protein kinase RNA-like endoplasmic reticulum kinase-mediated Bcl-2 protein phosphorylation contributes to evodiamine-induced apoptosis of human renal cell carcinoma cells. PLoS ONE 2016, 11, e0160484. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Zhang, P.; Liu, X.M.; Du, Y.M.; Hou, X.D.; Cheng, S.; Zhang, Z.F. Cytological assessments and transcriptome profiling demonstrate that evodiamine inhibits growth and induces apoptosis in a renal carcinoma cell line. Sci. Rep. 2017, 7, 12572. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A. Bladder cancer: Clinical and pathological profile. Scand. J. Urol. Nephrol. Suppl. 2008, 42, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Qu, S.; Shi, Q.; He, D.; Jin, X. Evodiamine induces apoptosis and enhances TRAIL-induced apoptosis in human bladder cancer cells through mTOR/S6K1-mediated downregulation of Mcl-1. Int. J. Mol. Sci. 2014, 15, 3154–3171. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Li, J.M.; Chin, C.C.; Kuo, Y.H.; Lee, Y.R.; Huang, Y.C. Evodiamine induces cell growth arrest, apoptosis and suppresses tumorigenesis in human urothelial cell carcinoma cells. Anticancer Res. 2017, 37, 1149–1159. [Google Scholar] [PubMed]
- Maisonneuve, P.; Lowenfels, A. Epidemiology and Prospects for Prevention of Pancreatic Cancer, 2nd ed.; Springer: New York, NY, USA, 2016; pp. 3–25. [Google Scholar]
- Khan, M.; Qazi, J.I.; Rasul, A.; Zheng, Y.; Ma, T. Evodiamine induces apoptosis in pancreatic carcinoma PANC-1 cells via NFκB inhibition. Bangladesh J. Pharmacol. 2013, 8. [Google Scholar] [CrossRef]
- Wei, W.T.; Chen, H.; Wang, Z.H.; Ni, Z.L.; Liu, H.B.; Tong, H.F.; Guo, H.C.; Liu, D.L.; Lin, S.Z. Enhanced antitumor efficacy of gemcitabine by evodiamine on pancreatic cancer via regulating PI3K/Akt pathway. Int. J. Biol. Sci. 2012, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Varambally, S.; Dhanasekaran, S.M.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.; Otte, A.P. The polycomb group protein ezh2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.M.; Guh, J.H.; Huang, Y.T.; Chueh, S.C.; Chiang, P.C.; Teng, C.M. Induction of mitotic arrest and apoptosis in human prostate cancer PC-3 cells by evodiamine. J. Urol. 2005, 173, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.F.; Yu, C.H.; Pu, H.F.; Hsu, J.M.; Chen, M.J.; Wang, P.S. Anti-proliferative effects of evodiamine on human prostate cancer cell lines DU145 and PC-3. J. Cell Biochem. 2007, 101, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.F.; Huang, W.J.; Lin, L.C.; Wang, P.S. Inhibitory effects of evodiamine on the growth of human prostate cancer cell line LNCaP. Int. J. Cancer 2004, 110, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Poon, V.I.; Roth, M.; Piperdi, S.; Geller, D.; Gill, J.; Rudzinski, E.R.; Hawkins, D.S.; Gorlick, R. Ganglioside GD2 expression is maintained upon recurrence in patients with osteosarcoma. Clin. Sarcoma Res. 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.J.; Wu, N.; Liu, Y.; Shu, K.J.; Zou, X.; Zhang, R.X.; Pi, C.J.; He, B.C.; Ke, Z.Y.; Chen, L. Evodiamine inhibits the proliferation of human osteosarcoma cells by blocking PI3K/Akt signaling. Oncol. Rep. 2015, 34, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Meng, H.; Ma, L.; Guo, A. Inhibitory effects of evodiamine on human osteosarcoma cell proliferation and apoptosis. Oncol. Lett. 2015, 9, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, J. Evodiamine induces apoptosis, G2/M cell cycle arrest, and inhibition of cell migration and invasion in human osteosarcoma cells via Raf/MEK/ERK signalling pathway. Med. Sci. Monit. 2018, 24, 5874–5880. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, S.; Wang, Z.; Shen, H.; Li, W.; Wang, W. Evodiamine inhibits apoptosis of human osteosarcoma MG-63 cells by blocking wnt/β-catenin signaling. Int. J. Oncol. 2017, 44, 86–90. [Google Scholar]
- Sankaranarayanan, R.; Ramadas, K.; Thomas, G.; Muwonge, R.; Thara, S.; Mathew, B.; Rajan, B. Effect of screening on oral cancer mortality in kerala, india: A cluster-randomised controlled trial. Lancet 2005, 365, 1927–1933. [Google Scholar] [CrossRef]
- Sachita, K.; Kim, Y.; Yu, H.J.; Cho, S.D.; Lee, J.S. In vitro assessment of the anticancer potential of evodiamine in human oral cancer cell lines. Phytother. Res. 2015, 29, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mitani, Y.; Rao, X.; Zafereo, M.; Zhang, J.; Zhang, J.; Futreal, P.A.; Lozano, G.; El-Naggar, A.K. Spatio-temporal genomic heterogeneity, phylogeny, and metastatic evolution in salivary adenoid cystic carcinoma. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Di, Y.U.; Zeng, D.S.; Hong, H.P. Regulatory impact of evodiamine on salivary adenoid cystic carcinoma SACC-M cell growth. Chin. Arch. Tradit. Chin. Med. 2012, 5, 1127–1129. [Google Scholar]
- Wang, Z.X.; Xiang, J.C.; Wang, M.; Ma, J.T.; Wu, Y.D.; Wu, A.X. One-pot total synthesis of evodiamine and its analogues through a continuous biscyclization reaction. Org. Lett. 2018, 20, 6380–6383. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Xu, F.; Gao, X.; Han, T.; Li, J.; Pan, H.; Zang, L.; Li, D.; Li, Z.; Uchita, T.; et al. Nitric oxide-releasing derivatives of brefeldin A as potent and highly selective anticancer agents. Eur. J. Med. Chem. 2017, 136, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Li, J.; Xue, J.; Li, H.; Xu, F.; Cheng, K.; Li, D.; Li, Z.; Gao, M.; Hua, H. Scutellarin derivatives as apoptosis inducers: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2017, 135, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, J.; Wang, M.; Xu, S.; Liu, W.; Zang, L.; Li, Z.; Hua, H.; Xu, J.; Li, D. Novel enmein-type diterpenoid hybrids coupled with nitrogen mustards: Synthesis of promising candidates for anticancer therapeutics. Eur. J. Med. Chem. 2018, 146, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Tian, K.; Pan, H.; Liu, Y.; Xu, F.; Li, Z.; Uchita, T.; Gao, M.; Hua, H.; Li, D. Novel hybrids of brefeldin A and nitrogen mustards with improved antiproliferative selectivity: Design, synthesis and antitumor biological evaluation. Eur. J. Med. Chem. 2018, 150, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Sheng, C.; Wang, S.; Miao, Z.; Yao, J.; Zhang, W. Selection of evodiamine as a novel topoisomerase I inhibitor by structure-based virtual screening and hit optimization of evodiamine derivatives as antitumor agents. J. Med. Chem. 2010, 53, 7521–7531. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Wang, S.; Miao, Z.; Yao, J.; Zhang, Y.; Guo, Z.; Zhang, W.; Sheng, C. New tricks for an old natural product: Discovery of highly potent evodiamine derivatives as novel antitumor agents by systemic structure-activity relationship analysis and biological evaluations. J. Med. Chem. 2012, 55, 7593–7613. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, Z.; Li, S.; Huang, Y.; Wan, Y.; Song, H. Design, synthesis and evaluation of N13-substituted evodiamine derivatives against human cancer cell lines. Molecules 2013, 18, 15750–15768. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.V.; Lee, K.R.; Lee, Y.J.; Choi, S.; Kang, J.S.; Mar, W.; Kim, K.H. Chiral high-performance liquid chromatographic separation of evodiamine enantiomers and rutaecarpine, isolated from Evodiae fructus. J. Pharm. Biomed. Anal. 2013, 81–82, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.S.; Sacchetti, A.; Ronchetti, V.; Caufin, S.; Silvani, A.; Lesma, G.; Fontana, G.; Minicone, F.; Riva, B.; Ventura, M.; et al. Quinazolinecarboline alkaloid evodiamine as scaffold for targeting topoisomerase I and sirtuins. Bioorg. Med. Chem. 2013, 21, 6920–6928. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Schiano Moriello, A.; Fontana, G.; Sacchetti, A.; Passarella, D.; Appendino, G.; Di Marzo, V. Effect of chirality and lipophilicity in the functional activity of evodiamine and its analogues at TRPV1 channels. Br. J. Pharmacol. 2014, 171, 2608–2620. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, B.; Spiteller, M. Evodiamine and rutaecarpine alkaloids as highly selective transient receptor potential vanilloid 1 agonists. Int. J. Biol. Macromol. 2014, 65, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xia, J.; Song, H.; Zhou, Z.; Xue, Y.; Yao, Q. Synthesis, in vitro and in vivo antitumor activity and docking studies of new evodiamine derivatives. J. Chem. Pharm. Res. 2014, 6, 1161–1171. [Google Scholar]
- Wang, S.; Fang, K.; Dong, G.; Chen, S.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; Sheng, C. Scaffold diversity inspired by the natural product evodiamine: Discovery of highly potent and multitargeting antitumor agents. J. Med. Chem. 2015, 58, 6678–6696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Tian, K.T.; Cheng, K.G.; Han, T.; Hu, X.; Li, D.H.; Li, Z.L.; Hua, H.M. Antiproliferative activity and apoptosis inducing effects of nitric oxide donating derivatives of evodiamine. Bioorg. Med. Chem. 2016, 24, 2971–2978. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Y.; Xue, J.; Han, T.; Jiao, R.; Li, Z.; Liu, W.; Xu, F.; Hua, H.; Li, D. Design and synthesis of novel nitrogen mustard-evodiamine hybrids with selective antiproliferative activity. Bioorg. Med. Chem. Lett. 2017, 27, 4989–4993. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiao, R.; Li, H.; Wang, X.; Lyu, H.; Gao, X.; Xu, F.; Li, Z.; Hua, H.; Li, D. Antiproliferative hydrogen sulfide releasing evodiamine derivatives and their apoptosis inducing properties. Eur. J. Med. Chem. 2018, 151, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Sun, L.; Zhao, D.; Zhang, L.; Ye, M.; Tan, Q.; Fang, C.; Wang, H.; Zhang, J. Supermolecular evodiamine loaded water-in-oil nanoemulsions: Enhanced physicochemical and biological characteristics. Eur. J. Pharm. Biopharm. 2014, 88, 556–564. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Li, D.; Chu, C.; Li, X.; Wang, X.; Jia, Y.; Hua, H.; Xu, F. Antiproliferative Effects of Alkaloid Evodiamine and Its Derivatives. Int. J. Mol. Sci. 2018, 19, 3403. https://doi.org/10.3390/ijms19113403
Hu X, Li D, Chu C, Li X, Wang X, Jia Y, Hua H, Xu F. Antiproliferative Effects of Alkaloid Evodiamine and Its Derivatives. International Journal of Molecular Sciences. 2018; 19(11):3403. https://doi.org/10.3390/ijms19113403
Chicago/Turabian StyleHu, Xu, Dahong Li, Chun Chu, Xu Li, Xianhua Wang, Ying Jia, Huiming Hua, and Fanxing Xu. 2018. "Antiproliferative Effects of Alkaloid Evodiamine and Its Derivatives" International Journal of Molecular Sciences 19, no. 11: 3403. https://doi.org/10.3390/ijms19113403
APA StyleHu, X., Li, D., Chu, C., Li, X., Wang, X., Jia, Y., Hua, H., & Xu, F. (2018). Antiproliferative Effects of Alkaloid Evodiamine and Its Derivatives. International Journal of Molecular Sciences, 19(11), 3403. https://doi.org/10.3390/ijms19113403




