mRNA-Expression of KRT5 and KRT20 Defines Distinct Prognostic Subgroups of Muscle-Invasive Urothelial Bladder Cancer Correlating with Histological Variants
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Population, Specimen Collection, and Histopathological Evaluation
2.2. RNA Isolation from FFPE Tissue
2.3. mRNA Quantification by RT-qPCR
2.4. Statistical Analysis
3. Results
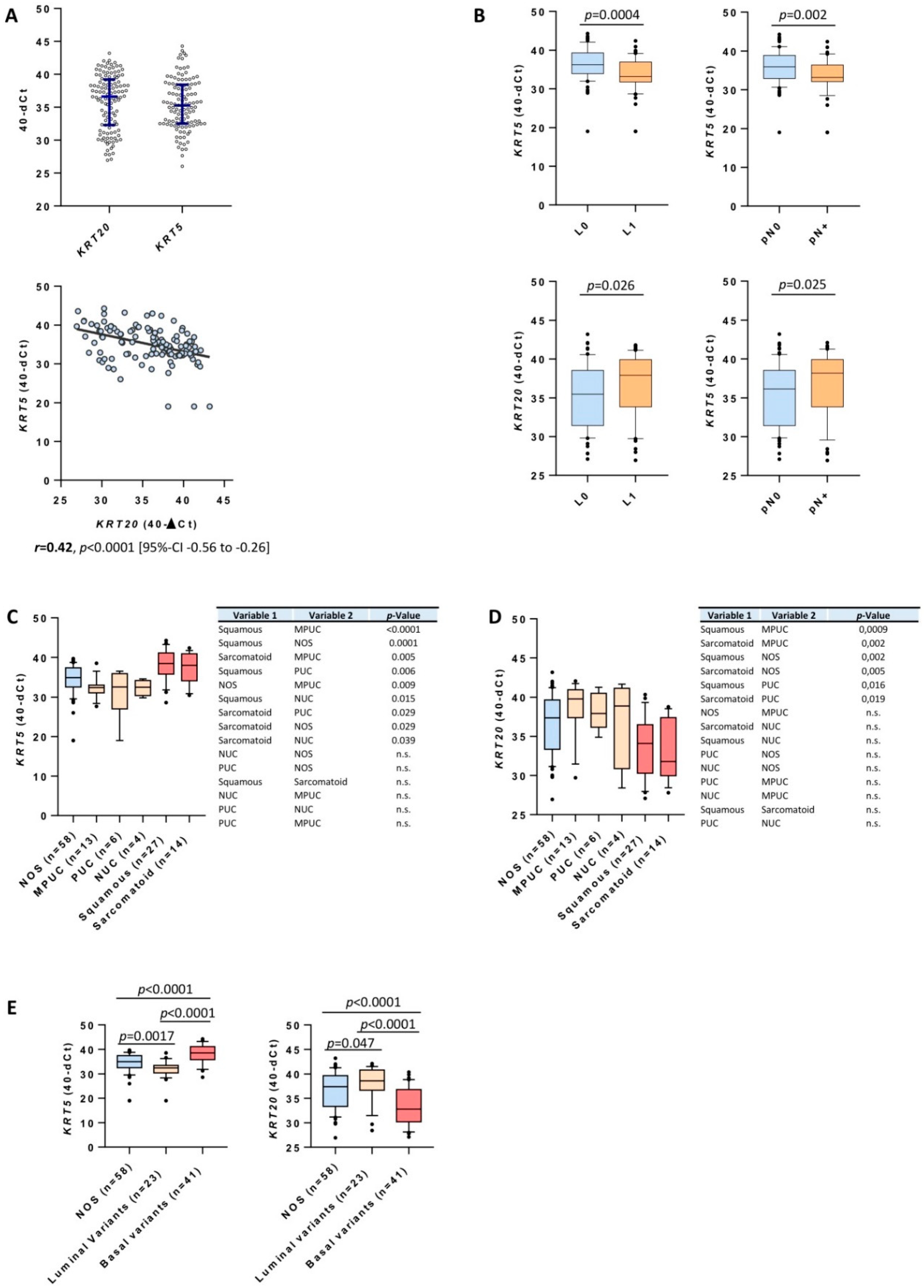
3.1. Clinicopathological Data and Expression of KRT5 and KRT20 mRNA in MIBC
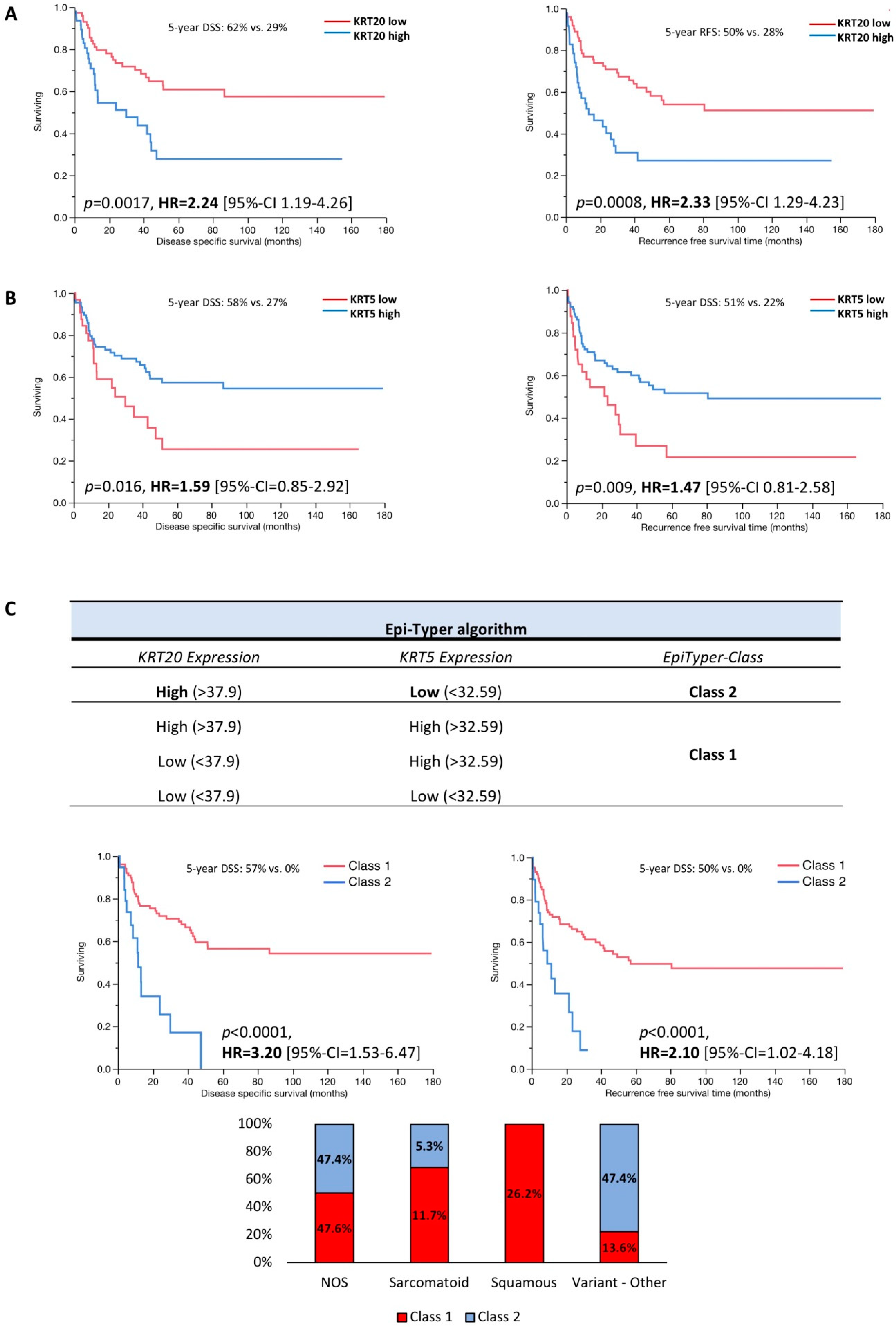
3.2. KRT5 and KRT20 mRNA Expression Defines Highly Prognostic Relevant Subgroups of MIBC
3.3. Multivariate Data Analysis
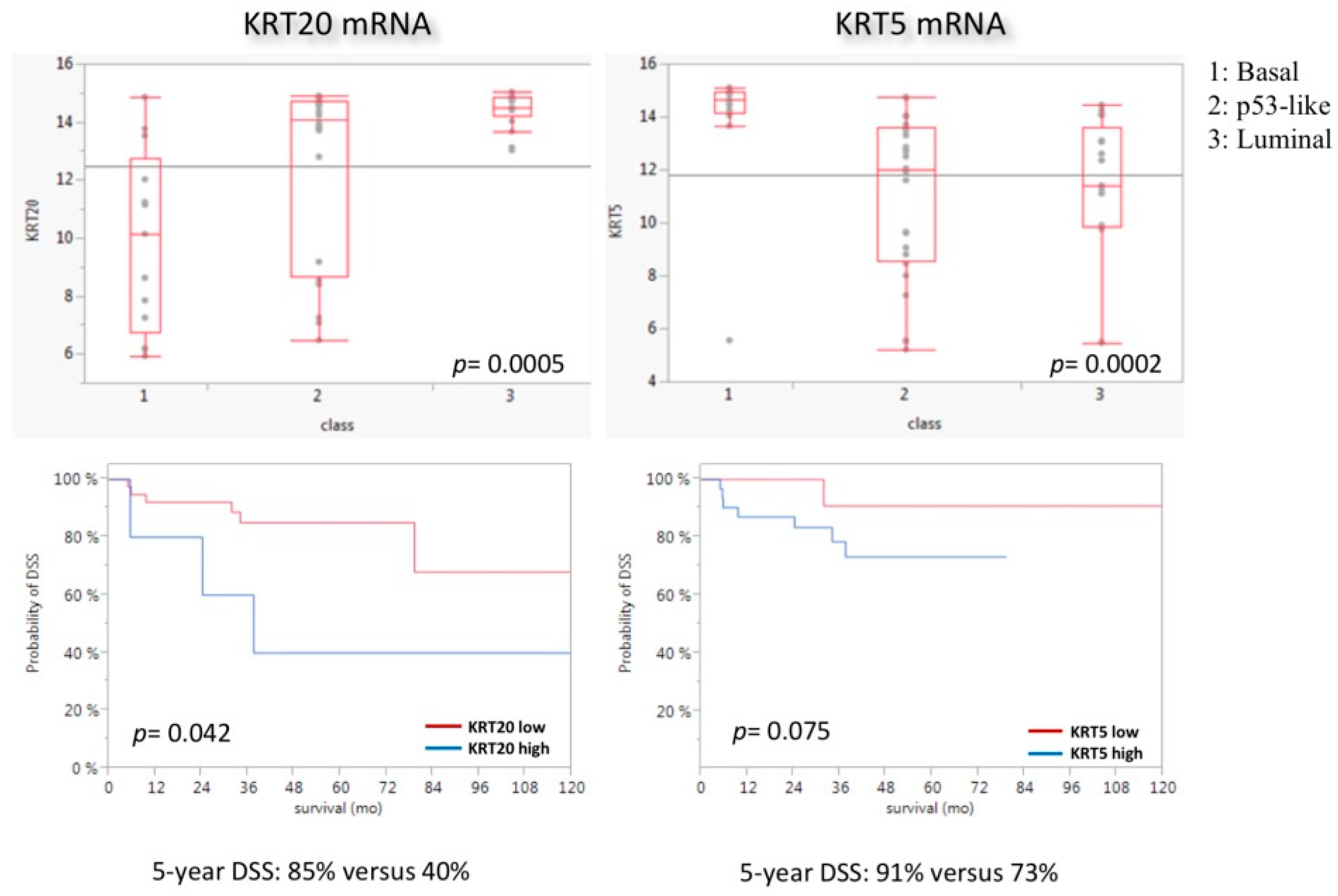
3.4. KRT5 and KRT20 mRNA Expression in the MDACC Cohort
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| UBC | urothelial bladder cancer |
| MIBC | muscle-invasive bladder cancer |
| CK | cytokeratin |
| KRT | keratin |
| MKI67 | marker of proliferation Ki67 |
| CALM2 | calmodulin 2 |
| FFPE | formalin-fixed paraffin-embedded |
| MDACC | MD Anderson Cancer Center |
| PFS | progression-free survival |
| RFS | recurrence-free survival |
| DSS | disease-specific survival |
| NMIBC | non-muscle-invasive bladder cancer |
| RT-qPCR | reverse-transcription quantitative polymerase chain reaction |
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.B.; McConkey, D.J.; Dinney, C.P. New strategies in muscle-invasive bladder cancer: On the road to personalized medicine. Clin. Cancer Res. 2011, 17, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Hollenbeck, B.K.; Holmang, S.; Lee, R.; Kim, S.P.; Stenzl, A.; Lotan, Y. The economics of bladder cancer: Costs and considerations of caring for this disease. Eur. Urol. 2014, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Shariat, S.F.; Novara, G.; Skinner, E.C.; Fradet, Y.; Bastian, P.J.; Kamat, A.M.; Kassouf, W.; Karakiewicz, P.I.; Fritsche, H.M.; et al. Discrepancy between clinical and pathological stage: External validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011, 107, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Seiler, R.; Ashab, H.A.; Erho, N.; van Rhijn, B.W.; Winters, B.; Douglas, J.; Van Kessel, K.E.; Fransen van de Putte, E.E.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Blaveri, E.; Simko, J.P.; Korkola, J.E.; Brewer, J.L.; Baehner, F.; Mehta, K.; Devries, S.; Koppie, T.; Pejavar, S.; Carroll, P.; et al. Bladder cancer outcome and subtype classification by gene expression. Clin. Cancer Res. 2005, 11, 4044–4055. [Google Scholar] [CrossRef] [PubMed]
- Dyrskjot, L.; Thykjaer, T.; Kruhoffer, M.; Jensen, J.L.; Marcussen, N.; Hamilton-Dutoit, S.; Wolf, H.; Orntoft, T.F. Identifying distinct classes of bladder carcinoma using microarrays. Nat. Genet. 2003, 33, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, E.J.; Kim, S.K.; Kim, Y.J.; Ha, Y.S.; Jeong, P.; Kim, M.J.; Yun, S.J.; Lee, K.M.; Moon, S.K.; et al. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol. Cancer 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Leem, S.H.; Lee, S.Y.; Kim, S.C.; Park, E.S.; Kim, S.B.; Kim, S.K.; Kim, Y.J.; Kim, W.J.; Chu, I.S. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J. Clin. Oncol. 2010, 28, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carbayo, M.; Socci, N.D.; Lozano, J.; Saint, F.; Cordon-Cardo, C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J. Clin. Oncol. 2006, 24, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sjodahl, G.; Lauss, M.; Lovgren, K.; Chebil, G.; Gudjonsson, S.; Veerla, S.; Patschan, O.; Aine, M.; Ferno, M.; Ringner, M.; et al. A molecular taxonomy for urothelial carcinoma. Clin. Cancer Res. 2012, 18, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 2014, 507, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Breyer, J.; Otto, W.; Wirtz, R.M.; Wullich, B.; Keck, B.; Erben, P.; Kriegmair, M.C.; Stoehr, R.; Eckstein, M.; Laible, M.; et al. ERBB2 Expression as Potential Risk-Stratification for Early Cystectomy in Patients with pT1 Bladder Cancer and Concomitant Carcinoma in situ. Urol. Int. 2016, 98, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Hoyer, S.; Ulhoi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Rinaldetti, S.; Rempel, E.; Worst, T.S.; Eckstein, M.; Steidler, A.; Weiss, C.A.; Bolenz, C.; Hartmann, A.; Erben, P. Subclassification, survival prediction and drug target analyses of chemotherapy-naive muscle-invasive bladder cancer with a molecular screening. Oncotarget 2018, 9, 25935–25945. [Google Scholar] [CrossRef] [PubMed]
- Dadhania, V.; Zhang, M.; Zhang, L.; Bondaruk, J.; Majewski, T.; Siefker-Radtke, A.; Guo, C.C.; Dinney, C.; Cogdell, D.E.; Zhang, S.; et al. Meta-Analysis of the Luminal and Basal Subtypes of Bladder Cancer and the Identification of Signature Immunohistochemical Markers for Clinical Use. EBioMedicine 2016, 12, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Breyer, J.; Wirtz, R.M.; Otto, W.; Erben, P.; Kriegmair, M.C.; Stoehr, R.; Eckstein, M.; Eidt, S.; Denzinger, S.; Burger, M.; et al. In stage pT1 non-muscle-invasive bladder cancer (NMIBC), high KRT20 and low KRT5 mRNA expression identify the luminal subtype and predict recurrence and survival. Virchows Arch. 2017, 470, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Humphrey, P.A.; Ulbright, T.M.; Reuter, V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 2016. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26935559 (accessed on 28 February 2018).
- Eckstein, M.; Wirtz, R.M.; Pfannstil, C.; Wach, S.; Stoehr, R.; Breyer, J.; Erlmeier, F.; Gunes, C.; Nitschke, K.; Weichert, W.; et al. A multicenter round robin test of PD-L1 expression assessment in urothelial bladder cancer by immunohistochemistry and RT-qPCR with emphasis on prognosis prediction after radical cystectomy. Oncotarget 2018, 9, 15001–15014. [Google Scholar] [CrossRef] [PubMed]
- Tramm, T.; Sorensen, B.S.; Overgaard, J.; Alsner, J. Optimal reference genes for normalization of qRT-PCR data from archival formalin-fixed, paraffin-embedded breast tumors controlling for tumor cell content and decay of mRNA. Diagn. Mol. Pathol. 2013, 22, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kriegmair, M.C.; Balk, M.; Wirtz, R.; Steidler, A.; Weis, C.A.; Breyer, J.; Hartmann, A.; Bolenz, C.; Erben, P. Expression of the p53 Inhibitors MDM2 and MDM4 as Outcome Predictor in Muscle-invasive Bladder Cancer. Anticancer Res. 2016, 36, 5205–5213. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J., Jr.; Chang, H.Y.; van de Rijn, M.; et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14016–14021. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.L.; Kurtova, A.; Chan, K.S. Normal and neoplastic urothelial stem cells: Getting to the root of the problem. Nat. Rev. Urol. 2012, 9, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Reis-Filho, J.S.; Simpson, P.T.; Martins, A.; Preto, A.; Gartner, F.; Schmitt, F.C. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch. 2003, 443, 122–132. [Google Scholar] [PubMed]
- Guo, C.C.; Dadhania, V.; Zhang, L.; Majewski, T.; Bondaruk, J.; Sykulski, M.; Wronowska, W.; Gambin, A.; Wang, Y.; Zhang, S.; et al. Gene Expression Profile of the Clinically Aggressive Micropapillary Variant of Bladder Cancer. Eur. Urol. 2016, 70, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Comperat, E.; Roupret, M.; Yaxley, J.; Reynolds, J.; Varinot, J.; Ouzaid, I.; Cussenot, O.; Samaratunga, H. Micropapillary urothelial carcinoma of the urinary bladder: A clinicopathological analysis of 72 cases. Pathology 2010, 42, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, I.A.; Miocinovic, R.; Stephenson, A.J.; Garcia, J.A.; Gong, M.C.; Campbell, S.C.; Hansel, D.E.; Fergany, A.F. Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology 2011, 77, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Kamat, A.M.; Gee, J.R.; Dinney, C.P.; Grossman, H.B.; Swanson, D.A.; Millikan, R.E.; Detry, M.A.; Robinson, T.L.; Pisters, L.L. The case for early cystectomy in the treatment of nonmuscle invasive micropapillary bladder carcinoma. J. Urol. 2006, 175, 881–885. [Google Scholar] [CrossRef]
- Bertz, S.; Wach, S.; Taubert, H.; Merten, R.; Krause, F.S.; Schick, S.; Ott, O.J.; Weigert, E.; Dworak, O.; Rodel, C.; et al. Micropapillary morphology is an indicator of poor prognosis in patients with urothelial carcinoma treated with transurethral resection and radiochemotherapy. Virchows Arch. 2016, 469, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Christoph, F.; Muller, M.; Schostak, M.; Soong, R.; Tabiti, K.; Miller, K. Quantitative detection of cytokeratin 20 mRNA expression in bladder carcinoma by real-time reverse transcriptase-polymerase chain reaction. Urology 2004, 64, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Gazquez, C.; Ribal, M.J.; Marin-Aguilera, M.; Kayed, H.; Fernandez, P.L.; Mengual, L.; Alcaraz, A. Biomarkers vs conventional histological analysis to detect lymph node micrometastases in bladder cancer: A real improvement? BJU Int. 2012, 110, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Retz, M.; Rotering, J.; Nawroth, R.; Buchner, A.; Stockle, M.; Gschwend, J.E.; Lehmann, J. Long-term follow-up of bladder cancer patients with disseminated tumour cells in bone marrow. Eur. Urol. 2011, 60, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, R.M.; Sihto, H.; Isola, J.; Heikkila, P.; Kellokumpu-Lehtinen, P.L.; Auvinen, P.; Turpeenniemi-Hujanen, T.; Jyrkkio, S.; Lakis, S.; Schlombs, K.; et al. Biological subtyping of early breast cancer: A study comparing RT-qPCR with immunohistochemistry. Breast Cancer Res. Treat. 2016, 157, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, A.; Al-Batran, S.E.; Wirtz, R.M.; Werner, D.; Zirlik, S.; Wiest, G.; Eschbach, C.; Claas, S.; Hartmann, A.; Ficker, J.H.; et al. The validation of estrogen receptor 1 mRNA expression as a predictor of outcome in patients with metastatic non-small cell lung cancer. Int. J. Cancer 2014, 134, 2314–2321. [Google Scholar] [CrossRef] [PubMed]
- Sikic, D.; Breyer, J.; Hartmann, A.; Burger, M.; Erben, P.; Denzinger, S.; Eckstein, M.; Stöhr, R.; Wach, S.; Wullich, B.; et al. High androgen receptor mRNA expression is independently associated with prolonged cancer-specific and recurrence-free survival in stage T1 bladder cancer. Transl. Oncol. 2017, 10, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.R.; Xiao, Y.; Spoerke, J.M.; Fridlyand, J.; Koeppen, H.; Fuentes, E.; Huw, L.Y.; Abbas, I.; Gower, A.; Schleifman, E.B.; et al. Development of a robust RNA-based classifier to accurately determine ER, PR, and HER2 status in breast cancer clinical samples. Breast Cancer Res. Treat. 2014, 148, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Balar, A.V.; Milowsky, M.I.; Bochner, B.H.; Dalbagni, G.; Donat, S.M.; Herr, H.W.; Huang, W.C.; Taneja, S.S.; Woods, M.; et al. Multicenter Prospective Phase II Trial of Neoadjuvant Dose-Dense Gemcitabine Plus Cisplatin in Patients With Muscle-Invasive Bladder Cancer. J. Clin. Oncol. 2018, 36, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total | KRT5 High | KRT5 Low | KRT20 High | KRT20 Low | p-Value |
|---|---|---|---|---|---|---|
| Cohort size (n) | 122 | 89 | 33 | 48 | 74 | |
| Mean age (years) | 67.9 | 67.9 | 68.4 | 67.5 | 67.9 | a: n.s. b: n.s. |
| Gender (n) | ||||||
| Male | 89 (73%) | 66 (74%) | 23 (70%) | 34 (71%) | 55 (74%) | a: n.s. |
| Female | 33 (27%) | 23 (26%) | 10 (30%) | 14 (29%) | 19 (26%) | b: n.s. |
| Adjuvant chemotherapy | 22 (18%) | 14 (16%) | 8 (24%) | 9 (19%) | 13 (17%) | n.s. |
| Pathological characteristics | ||||||
| pTis (concomitant) | 43 (35%) | 34 (38%) | 9 (27%) | 19 (40%) | 24 (32%) | a: n.s. b: n.s. |
| pT2 | 33 (27%) | 28 (31%) | 5 (15%) | 14 (29%) | 19 (26%) | a: 0.027 |
| pT3 | 62 (51%) | 44 (50%) | 18 (55%) | 25 (52%) | 37 (50%) | b: n.s. |
| pT4 | 27 (22%) | 17 (19%) | 10 (30%) | 9 (19%) | 18 (24%) | |
| pN0 | 74 (60%) | 60 (67%) | 14 (42%) | 20 (42%) | 54 (73%) | a: 0.0005 |
| pN1-2 | 48 (37%) | 29 (33%) | 19 (57%) | 28 (58%) | 20 (27%) | b: 0.002 |
| L0 | 62 (51%) | 52 (58%) | 10 (30%) | 17 (35%) | 45 (61%) | a: 0.005 |
| L1 | 60 (49%) | 37 (52%) | 23 (70%) | 31 (65%) | 29 (39%) | b: 0.006 |
| V0 | 104 (85%) | 81 (91%) | 23 (70%) | 42 (87%) | 61 (84%) | a: 0.005 |
| V1 | 18 (15%) | 8 (9%) | 10 (30%) | 6 (13%) | 12 (16%) | b: n.s. |
| R0 | 105 (86%) | 77 (86%) | 28 (85%) | 42 (88%) | 63 (85%) | a: n.s. |
| R1 | 17 (14%) | 12 (14%) | 5 (15%) | 6 (12%) | 11 (15%) | b: n.s. |
| Grading | ||||||
| WHO 1973 | ||||||
| G1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| G2 | 27 (22%) | 21 (24%) | 6 (18%) | 11 (23%) | 16 (22%) | a: n.s. |
| G3 | 95 (78%) | 68 (76%) | 27 (82%) | 37 (77%) | 58 (78%) | b: n.s. |
| WHO 2004 | ||||||
| Low grade | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | a: n.s. |
| High grade | 122 (100%) | 89 (100%) | 33(100%) | 48 (100%) | 74 (100%) | b: n.s. |
| Characteristic | Epi-Typer Class 1 | Epi-Typer Class 2 | p-Value | |||
| Cohort size (n) | 103 | 19 | ||||
| Mean age (years) | 67.9 | 71 | n.s. | |||
| Gender (n) | ||||||
| Male | 75 (73%) | 14 (74%) | n.s. | |||
| Female | 28 (27%) | 5 (26%) | ||||
| Adjuvant chemotherapy | 17 (17%) | 5 (26%) | n.s. | |||
| Pathological T stage | ||||||
| pTis (concomitant) | 37 (36%) | 6 (31%) | n.s. | |||
| pT2 | ||||||
| pT3 | ||||||
| pT4 | ||||||
| Pathological characteristics | ||||||
| pN0 | 69 (67%) | 5 (26%) | 0.0009 | |||
| pN1-2 | 34 (33%) | 14 (74%) | ||||
| L0 | 58 (56%) | 4 (21%) | 0.004 | |||
| L1 | 45 (44%) | 15 (79%) | ||||
| V0 | 90 (87%) | 14 (74%) | n.s. | |||
| V1 | 13 (13%) | 5 (26%) | ||||
| R0 | 89 (86%) | 16 (84%) | n.s. | |||
| R1 | 14 (14%) | 3 (16%) | ||||
| Grading WHO 1973 | ||||||
| G1 | 0 (0%) | 0 (0%) | n.s. | |||
| G2 | 22 (21%) | 5 (26%) | n.s. | |||
| G3 | 81 (79%) | 14 (74%) | n.s. | |||
| Grading WHO 2004 | ||||||
| Low grade | 0 (0%) | 0 (0%) | n.s. | |||
| High grade | 103 (100%) | 19 (100%) | n.s. | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckstein, M.; Wirtz, R.M.; Gross-Weege, M.; Breyer, J.; Otto, W.; Stoehr, R.; Sikic, D.; Keck, B.; Eidt, S.; Burger, M.; et al. mRNA-Expression of KRT5 and KRT20 Defines Distinct Prognostic Subgroups of Muscle-Invasive Urothelial Bladder Cancer Correlating with Histological Variants. Int. J. Mol. Sci. 2018, 19, 3396. https://doi.org/10.3390/ijms19113396
Eckstein M, Wirtz RM, Gross-Weege M, Breyer J, Otto W, Stoehr R, Sikic D, Keck B, Eidt S, Burger M, et al. mRNA-Expression of KRT5 and KRT20 Defines Distinct Prognostic Subgroups of Muscle-Invasive Urothelial Bladder Cancer Correlating with Histological Variants. International Journal of Molecular Sciences. 2018; 19(11):3396. https://doi.org/10.3390/ijms19113396
Chicago/Turabian StyleEckstein, Markus, Ralph Markus Wirtz, Matthias Gross-Weege, Johannes Breyer, Wolfgang Otto, Robert Stoehr, Danijel Sikic, Bastian Keck, Sebastian Eidt, Maximilian Burger, and et al. 2018. "mRNA-Expression of KRT5 and KRT20 Defines Distinct Prognostic Subgroups of Muscle-Invasive Urothelial Bladder Cancer Correlating with Histological Variants" International Journal of Molecular Sciences 19, no. 11: 3396. https://doi.org/10.3390/ijms19113396
APA StyleEckstein, M., Wirtz, R. M., Gross-Weege, M., Breyer, J., Otto, W., Stoehr, R., Sikic, D., Keck, B., Eidt, S., Burger, M., Bolenz, C., Nitschke, K., Porubsky, S., Hartmann, A., & Erben, P. (2018). mRNA-Expression of KRT5 and KRT20 Defines Distinct Prognostic Subgroups of Muscle-Invasive Urothelial Bladder Cancer Correlating with Histological Variants. International Journal of Molecular Sciences, 19(11), 3396. https://doi.org/10.3390/ijms19113396









