Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor
Abstract
1. Introduction
2. Results
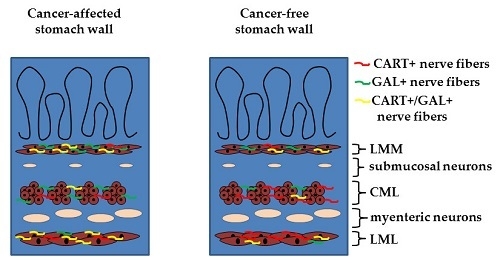
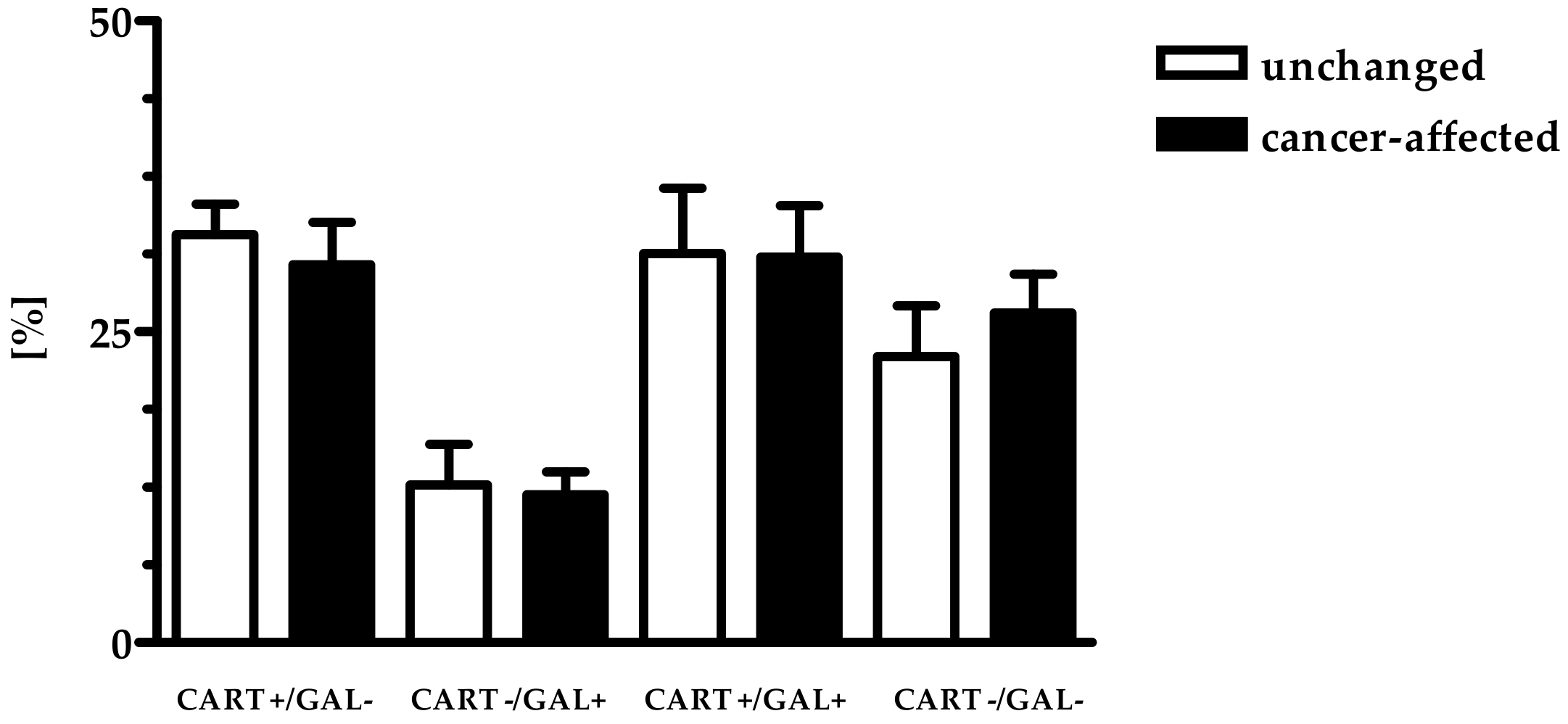
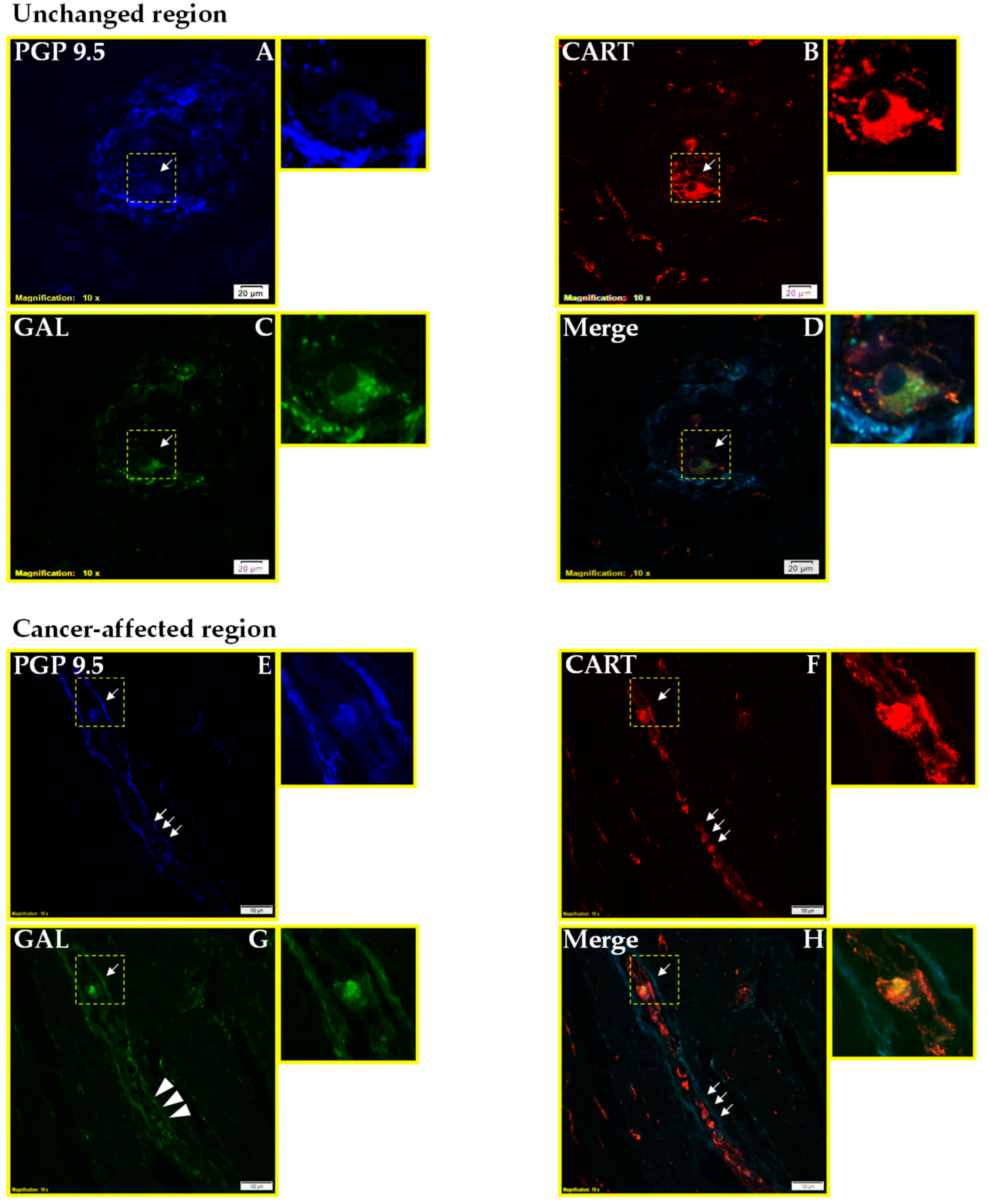
2.1. Co-Localization of CART and GAL in Neurons of Myenteric Plexi (MP) in the Control and Cancer-Affected Areas of the Human Stomach Wall
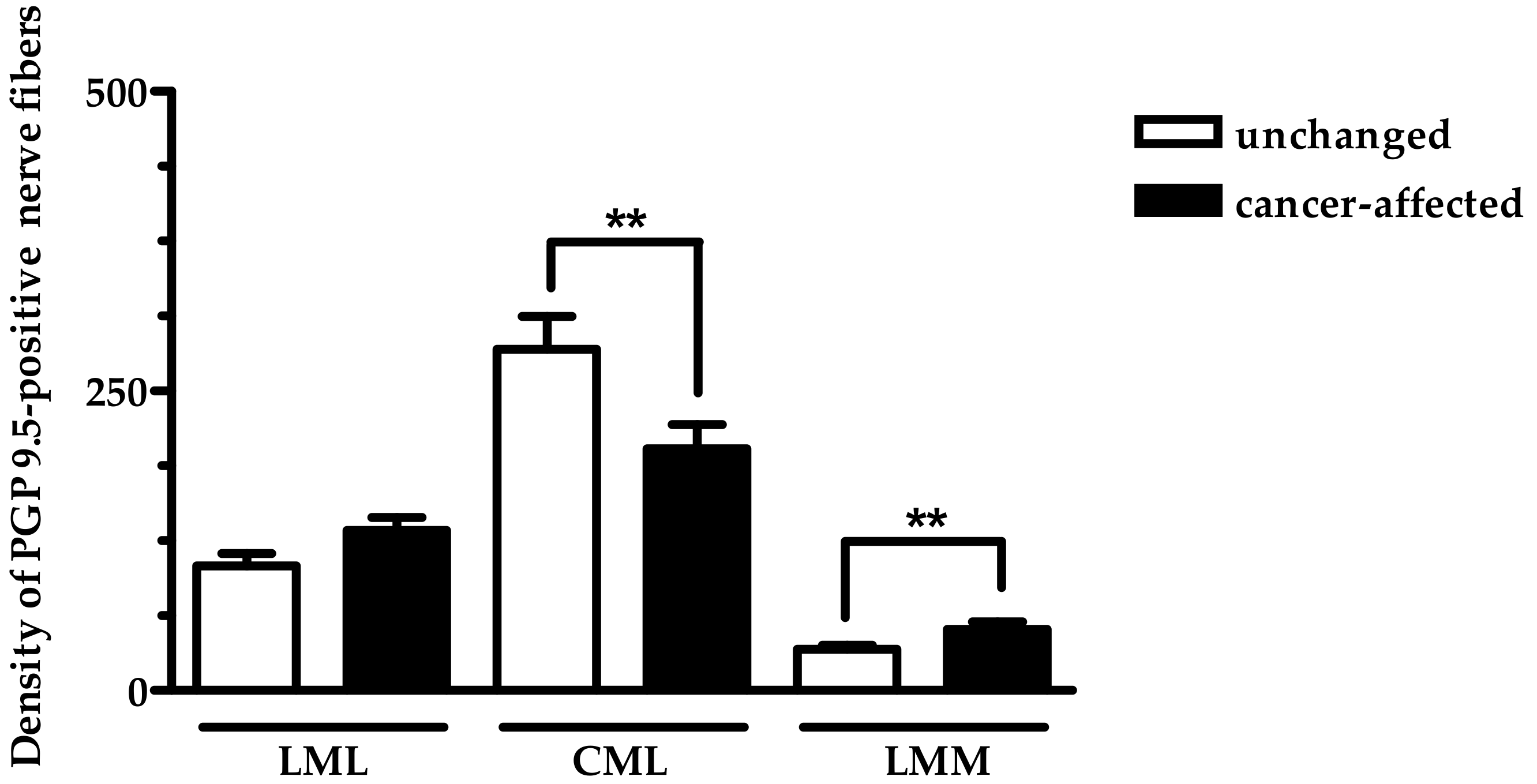
2.2. Density of CART- and GAL-Expressing Fibers in the Muscular Layers in the Control and Cancer-Affected Human Stomach Wall
3. Discussion
3.1. Distribution Pattern of CART with GAL
3.2. Functional Considerations
4. Materials and Methods
4.1. Ethical Statement
4.2. Patient Recruitment and Specimen Collection
4.3. Experimental Procedures
4.3.1. Immunofluorescence Procedures
4.3.2. Counting Neurons and Nerve Fibers
4.3.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CART | cocaine- and amphetamine-regulated transcript |
| GAL | galanin |
| PGP 9.5 | protein gene-product 9.5 |
| IR | immunoreactive |
| + | positive |
| - | negative |
| CRC | colorectal cancer |
| ENS | enteric nervous system |
| CNS | central nervous system |
| MP | myenteric plexi |
| LMM | lamina muscularis mucosae |
| CML | circular muscle layer |
| LML | longitudinal muscle layer |
References
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Poole, D.P.; Cho, H.J.; Callaghan, B.P.; Rivera, L.R. The Innervation of the Gastrointestinal Tract. In Yamada’s Textbook of Gastroenterology; Podolsky, D.K., Camilleri, M., Fitz, J.G., Kalloo, A.N., Shanahan, F., Wang, T.C., Eds.; Whiley Blackwell: Hoboken, NJ, USA, 2015; pp. 239–258. [Google Scholar]
- Schemann, M.; Rohn, M.; Michel, K. Motor control of the stomach. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 41–51. [Google Scholar] [PubMed]
- Costa, M.; Glise, H.; Sjodahl, R. The enteric nervous system in health and disease. Gut 2000, 47 (Suppl. 4), iv1. [Google Scholar] [CrossRef]
- Ekblad, E. CART in the enteric nervous system. Peptides 2006, 27, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Clerc, N.; Kunze, W.A. Memory in the enteric nervous system. Gut 2000, 47 (Suppl. 4), iv60–iv62. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Hirano, I. The enteric nervous system. N. Engl. J. Med. 1996, 334, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Grundy, D.; Schemann, M. Enteric nervous system. Curr. Opin. Gastroenterol. 2007, 23, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A. Peptidyl hormones of endocrine cells origin in the gut—Their discovery and physiological relevance. J. Physiol. Pharmacol. 2015, 66, 11–27. [Google Scholar] [PubMed]
- Kozlowska, A.; Kwiatkowski, P.; Oponowicz, A.; Majewski, M.; Kmiec, Z.; Godlewski, J. Myenteric plexuses atrophy in the vicinity of colorectal cancer tissue is not caused by apoptosis or necrosis. Folia Histochem. Cytobiol. 2016, 54, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Kozera, P.; Majewski, M.; Godlewski, J. Co-expression of caspase-3 or caspase-8 with galanin in the human stomach section affected by carcinoma. Apoptosis 2018, 23, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Godlewski, J.; Kieżun, J.; Kraziński, B.E.; Kmieć, Z. Colorectal cancer patients exhibit increased levels of galanin in serum and colon tissues. Oncol. Lett. 2016, 12, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Kaleczyc, J. Somatostatin, substance P and calcitonin gene-related peptide-positive intramural nerve structures of the human large intestine affected by carcinoma. Folia Histochem. Cytobiol. 2010, 48, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Łakomy, I.M. Changes in vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide and neuropeptide Y-ergic structures of the enteric nervous system in the carcinoma of the human large intestine. Folia Histochem. Cytobiol. 2010, 48, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Pidsudko, Z. Characteristic of galaninergic components of the enteric nervous system in the cancer invasion of human large intestine. Ann. Anat. 2012, 194, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Oponowicz, A.; Kozłowska, A.; Gonkowski, S.; Godlewski, J.; Majewski, M. Changes in the distribution of cocaine- and amphetamine-regulated transcript-containing neural structures in the human colon affected by the neoplastic process. Int. J. Mol. Sci. 2018, 19, 414. [Google Scholar] [CrossRef] [PubMed]
- Couceyro, P.; Paquet, M.; Koylu, E.; Kuhar, M.J.; Smith, Y. Cocaine- and amphetamine-regulated transcript (CART) peptide immunoreactivity in myenteric plexus neurons of the rat ileum and co-localization with choline acetyltransferase. Synapse 1998, 30, 1–8. [Google Scholar] [CrossRef]
- Rękawek, W.; Sobiech, P.; Gonkowski, S.; Żarczyńska, K.; Snarska, A.; Waśniewski, T.; Wojtkiewicz, J. Distribution and chemical coding patterns of cocaine- and amphetamine-regulated transcript-immunoreactive (CART-IR) neurons in the enteric nervous system of the porcine stomach cardia. Pol. J. Vet. Sci. 2015, 18, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Wierup, N.; Gunnarsdóttir, A.; Ekblad, E.; Sundler, F. Characterisation of CART-containing neurons and cells in the porcine pancreas, gastro-intestinal tract, adrenal and thyroid glands. BMC Neurosci. 2007, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Zacharko-Siembida, A.; Arciszewski, M.B. Immunoreactivity to cocaine- and amphetamine-regulated transcript in the enteric nervous system of the pig and wild boar stomach. Anat. Histol. Embryol. 2014, 43, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Landerholm, K.; Shcherbina, L.; Falkmer, S.E.; Järhult, J.; Wierup, N. Expression of cocaine- and amphetamine-regulated transcript is associated with worse survival in small bowel carcinoid tumors. Clin. Cancer Res. 2012, 18, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Nagayoshi, K.; Ueki, T.; Tashiro, K.; Mizuuchi, Y.; Manabe, T.; Araki, H.; Oda, Y.; Kuhara, S.; Tanaka, M. Galanin plays an important role in cancer invasiveness and is associated with poor prognosis in stage II colorectal cancer. Oncol. Rep. 2015, 33, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Mancino, M.; Ametller, E.; Gascon, P.; Almendro, V. The neuronal influence on tumor progression. Biochim. Biophys. Acta 2011, 1816, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Gonkowski, S.; Landowski, P.; Kamińska, B.; Całka, J. Immunohistochemical distribution of cocaine and amphetamine regulatory peptide-like immunoreactive (CART-LI) nerve fibers in the circular muscle layer and their relationship to other peptides in the human caecum. Acta Histochem. 2014, 116, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S.; Zielonka, L.; Dabrowski, M.; Calka, J. T2 Toxin-induced changes in cocaine- and amphetamine-regulated transcript (cart)-like immunoreactivity in the enteric nervous system within selected fragments of the porcine digestive tract. Neurotox. Res. 2017, 31, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Wattchow, D.A.; Furness, J.B.; Costa, M. Distribution and coexistence of peptides in nerve fibers of the external muscle of the human gastrointestinal tract. Gastroenterology 1988, 95, 32–41. [Google Scholar] [CrossRef]
- Ekblad, E.; Bauer, A.J. Role of vasoactive intestinal peptide and inflammatory mediators in enteric neuronal plasticity. Neurogastroenterol. Motil. 2004, 16, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Kuol, N.; Stojanovska, L.; Apostolopoulos, V.; Nurgali, K. Role of the nervous system in cancer metastasis. J. Exp. Clin. Cancer Res. 2018, 37, 5. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Nat. Rev. Neurosci. 2008, 9, 747–758. [Google Scholar] [CrossRef]
- Rogge, G.; Jones, D.; Hubert, G.W.; Lin, Y.; Kuhar, M.J. CART peptides: Regulators of body weight, reward and other functions. Neurogastroenterol. Motil. 2003, 15, 545–557. [Google Scholar]
- Zhang, M.; Han, L.; Xu, Y. Roles of cocaine- and amphetamine-regulated transcript in the central nervous system. Clin. Exp. Pharmacol. Physiol. 2012, 39, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, E.; Kuhar, M.; Wierup, N.; Sundler, F. Cocaine- and amphetamine-regulated transcript: Distribution and function in rat gastrointestinal tract. Neurogastroenterol. Motil. 2003, 15, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, A.; Inui, A.; Yuzuriha, H.; Nagata, T.; Kaga, T.; Ueno, N.; Fujino, M.A.; Kasuga, M. Cocaine-amphetamine-regulated transcript influences energy metabolism, anxiety and gastric emptying in mice. Horm. Metab. Res. 2001, 33, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Yamada, H.; Motomura, W.; Kohgo, Y. Cocaine-amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotropin-releasing factor system. Endocrinology 2000, 141, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Smedh, U.; Moran, T.H. Peptides that regulate food intake: Separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1418–R1426. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Wang, L.; Zhang, J.; Qian, L.; Li, Q.; Li, J.; Qian, J.; Gu, S.; Han, L.; Xu, P.; et al. Cocaine- and amphetamine-regulated transcript peptide increases mitochondrial respiratory chain complex II activity and protects against oxygen-glucose deprivation in neurons. Brain Res. 2014, 1582, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Ardeshiri, A.; Jacks, R.; Yang, S.; Hurn, P.D.; Alkayed, N.J. Mitochondrial mechanism of neuroprotection by CART. Eur. J. Neurosci. 2007, 26, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Hu, S.; Liu, L.; Chen, M.; Wang, L.; Zeng, X.; Zhu, S. CART attenuates endoplasmic reticulum stress response induced by cerebral ischemia and reperfusion through upregulating BDNF synthesis and secretion. Biochem. Biophys. Res. Commun. 2013, 436, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Schiffman, S.S.; Pappas, T.N. Role of neuropeptides in the regulation of feeding behavior: A review of cholecystokinin, bombesin, neuropeptide Y, and galanin. Neurosci. Biobehav. Rev. 1994, 18, 313–323. [Google Scholar] [CrossRef]
- Ekblad, E.; Arnbjornsson, E.; Ekman, R.; Hakanson, R.; Sundler, F. Neuropeptides in the human appendix: Distribution and motor effects. Dig. Dis. Sci. 1989, 34, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Melander, T.; Hokfelt, T.; Rokaeus, A.; Fahrenkrug, J.; Tatemoto, K.; Mutt, V. Distribution of galanin-like immunoreactivity in the gastrointestinal tract of several mammalian species. Cell Tissue Res. 1985, 239, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.T.; Kalra, P.S.; Kalra, S.P. Neuropeptide Y stimulates feeding but inhibits sexual behaviour in rats. Endocrinology 1989, 117, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, S.F. Possible contribution of brain peptides to the development of eating disorders and obesity. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 85. [Google Scholar]
- Yuan, C.S.; Dey, L.; Xie, J.T.; Aung, H.H. Gastric effects of galanin and its interaction with leptin on brain stem neuronal activity. J. Pharmacol. Exp. Ther. 2002, 301, 488–493. [Google Scholar] [CrossRef] [PubMed]
- O’Donohue, T.L.; Chronwall, B.M.; Pruss, M.; Mezey, E.; Kiss, J.Z.; Eiden, L.E.; Massari, V.J.; Tessel, R.E.; Pickel, V.M.; DiMaggio, D. Neuropeptide Y and peptide YY neuronal and endocrine systems. Peptides 1995, 6, 755–768. [Google Scholar] [CrossRef]
- Kato, S.; Korolkiewicz, R.; Rekowski, P.; Szyk, A.; Sugawa, Y.; Takeuchi, K. Inhibition of gastric acid secretion by galanin in rats. Relation to endogenous histamine release. Regul. Pept. 1998, 74, 53–59. [Google Scholar] [CrossRef]
- Kisfalvi, I.Jr.; Burghardt, B.; Bálint, A.; Zelles, T.; Vizi, E.S.; Varga, G. Antisecretory effects of galanin and its putative antagonists M15, M35 and C7 in the rat stomach. J. Physiol. Paris 2000, 94, 37–42. [Google Scholar] [CrossRef]
- Guerrini, S.; Raybould, H.E.; Reeve, J.R.; Morgan-Ross, T.; Scott, M.; Reitz, A.B.; Lee, D.H.S.; Plata-Salaman, C.R.; Wong, H.E.; Walsh, J.H.; et al. Galanin affects gastric motility in rats through different galanin receptors. Reg. Peptides 2000, 1, 22. [Google Scholar] [CrossRef]
- Iishi, H.; Tatsuta, M.; Baba, M.; Uehara, H.; Nakaizumi, A. Protection by galanin against gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Res. 1994, 54, 3167–3170. [Google Scholar] [PubMed]
- Yoon, D.; Bae, K.; Lee, M.K.; Kim, J.H.; Yoon, K.A. Galanin is an epigenetically silenced tumor suppressor gene in gastric cancer cells. PLoS ONE 2018, 13, e0193275. [Google Scholar] [CrossRef] [PubMed]
- Bharne, A.P.; Upadhya, M.A.; Shelkar, G.P.; Singru, P.S.; Subhedar, N.K.; Kokare, D.M. Neuroprotective effect of cocaine- and amphetamine-regulated transcript peptide in spinal cord injury in mice. Neuropharmacology 2013, 67, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Iishi, H.; Tatsuta, M.; Baba, M.; Uehara, H.; Yano, H.; Nakaizumi, A. Experimental cancer chemoprevention by galanin against colon carcinogenesis induced by azoxymethane in wistar rats. Int. J. Cancer 1995, 61, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Konturek, S.J. The role of capsaicin-sensitive sensory neurons and nitric oxide in regulation of gastric mucosal growth. J. Physiol. Pharmacol. 1995, 46, 351–362. [Google Scholar] [PubMed]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Dembiński, M.; Cieszkowski, J.; Konturek, S.J.; Polus, A.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes in young mature rats. J. Physiol. Pharmacol. 2006, 57, 425–437. [Google Scholar] [PubMed]
- Warzecha, Z.; Ceranowicz, D.; Dembiński, A.; Ceranowicz, P.; Cieszkowski, J.; Kuwahara, A.; Kato, I.; Dembiński, M.; Konturek, P.C. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med. Sci. Monit. 2012, 18, BR181–BR187. [Google Scholar] [CrossRef] [PubMed]
- Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Sendur, R.; Cieszkowski, J.; Ceranowicz, D.; Pawlik, W.W.; Kuwahara, A.; Kato, I.; Konturek, P.C. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J. Physiol. Pharmacol. 2009, 60, 87–98. [Google Scholar] [PubMed]
- Zhu, C.Z.; Liu, D.; Kang, W.M.; Yu, J.C.; Ma, Z.Q.; Ye, X.; Li, K. Ghrelin and gastrointestinal stromal tumors. World J. Gastroenterol. 2017, 23, 1758–1763. [Google Scholar] [CrossRef] [PubMed]
- Matuszyk, A.; Ceranowicz, P.; Warzecha, Z.; Cieszkowski, J.; Bonior, J.; Jaworek, J.; Kuśnierz-Cabala, B.; Konturek, P.; Ambroży, T.; Dembiński, A. Obestatin accelerates the healing of acetic acid-induced colitis in rats. Oxid. Med. Cell. Longev. 2016, 2016, 2834386. [Google Scholar] [CrossRef] [PubMed]
- Konarska, K.; Cieszkowski, J.; Warzecha, Z.; Ceranowicz, P.; Chmura, A.; Kuśnierz-Cabala, B.; Gałązka, K.; Kowalczyk, P.; Miskiewicz, A.; Konturek, T.J.; et al. Treatment with obestatin-A ghrelin gene-encoded peptide-reduces the severity of experimental colitis evoked by trinitrobenzene sulfonic acid. Int. J. Mol. Sci. 2018, 19, 1643. [Google Scholar] [CrossRef] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Cieszkowski, J.; Dembiński, M.; Ptak-Belowska, A.; Kuwahara, A.; Kato, I. Administration of obestatin accelerates the healing of chronic gastric ulcers in rats. Med. Sci. Monit. 2011, 17, BR196–BR200. [Google Scholar] [CrossRef] [PubMed]
- Alén, B.O.; Leal-López, S.; Alén, M.O.; Viaño, P.; García-Castro, V.; Mosteiro, C.S.; Beiras, A.; Casanueva, F.F.; Gallego, R.; García-Caballero, T.; et al. The role of the obestatin/GPR39 system in human gastric adenocarcinomas. Oncotarget 2016, 7, 5957–5971. [Google Scholar] [CrossRef] [PubMed]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Warzecha, A.M.; Pawlik, W.W.; Dembiński, M.; Rembiasz, K.; Sendur, P.; Kuśnierz-Cabala, B.; Tomaszewska, R.; et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur. J. Pharmacol. 2008, 591, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kownacki, P.; Konturek, P.C. Role of sensory nerves in gastroprotective effect of anandamide in rats. J. Physiol. Pharmacol. 2011, 62, 207–217. [Google Scholar] [PubMed]
- Matsui, H.; Shimokawa, O.; Kaneko, T.; Nagano, Y.; Rai, K.; Hyodo, I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 2011, 48, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 28, 676–682. [Google Scholar] [CrossRef] [PubMed]
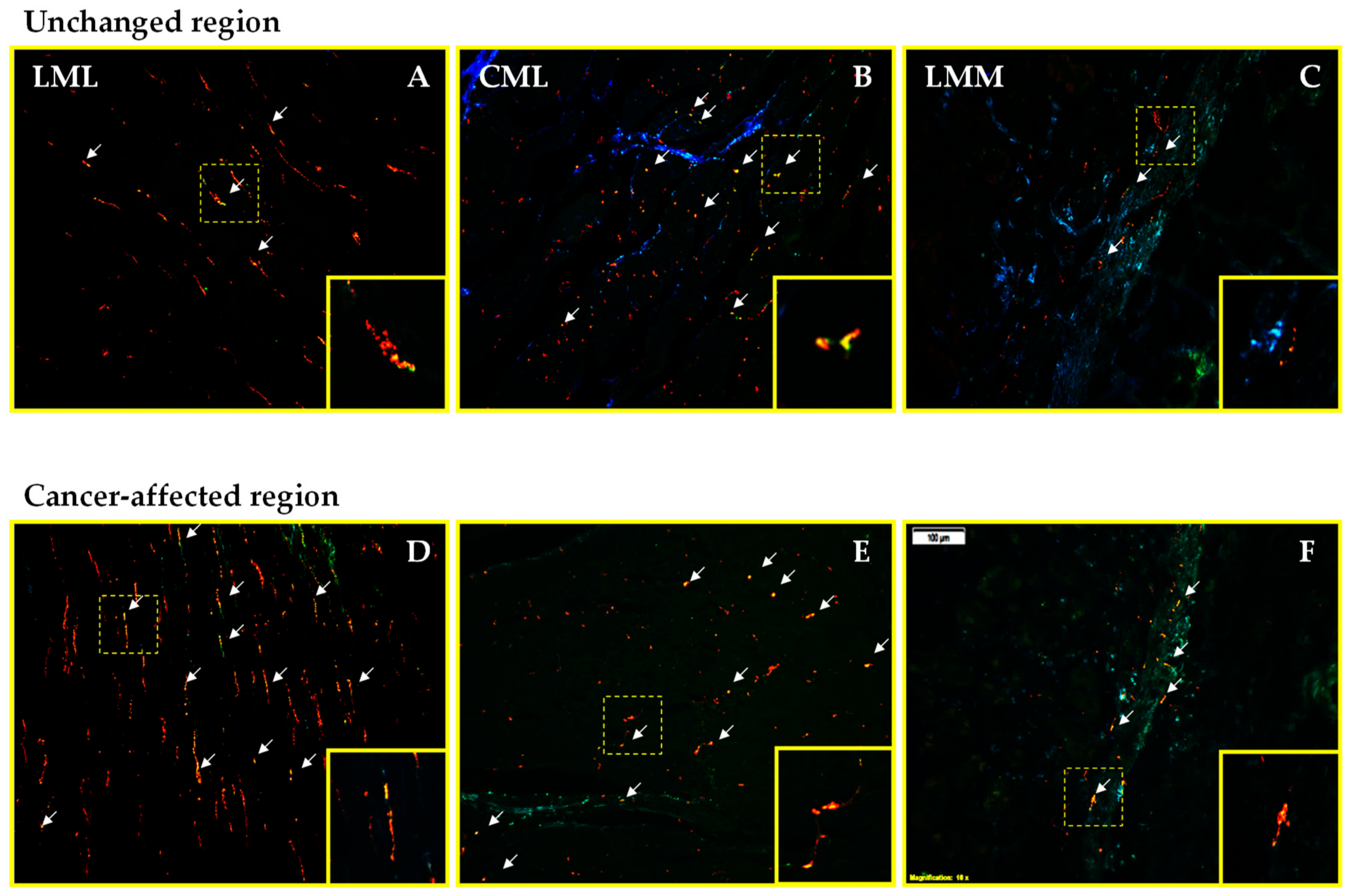
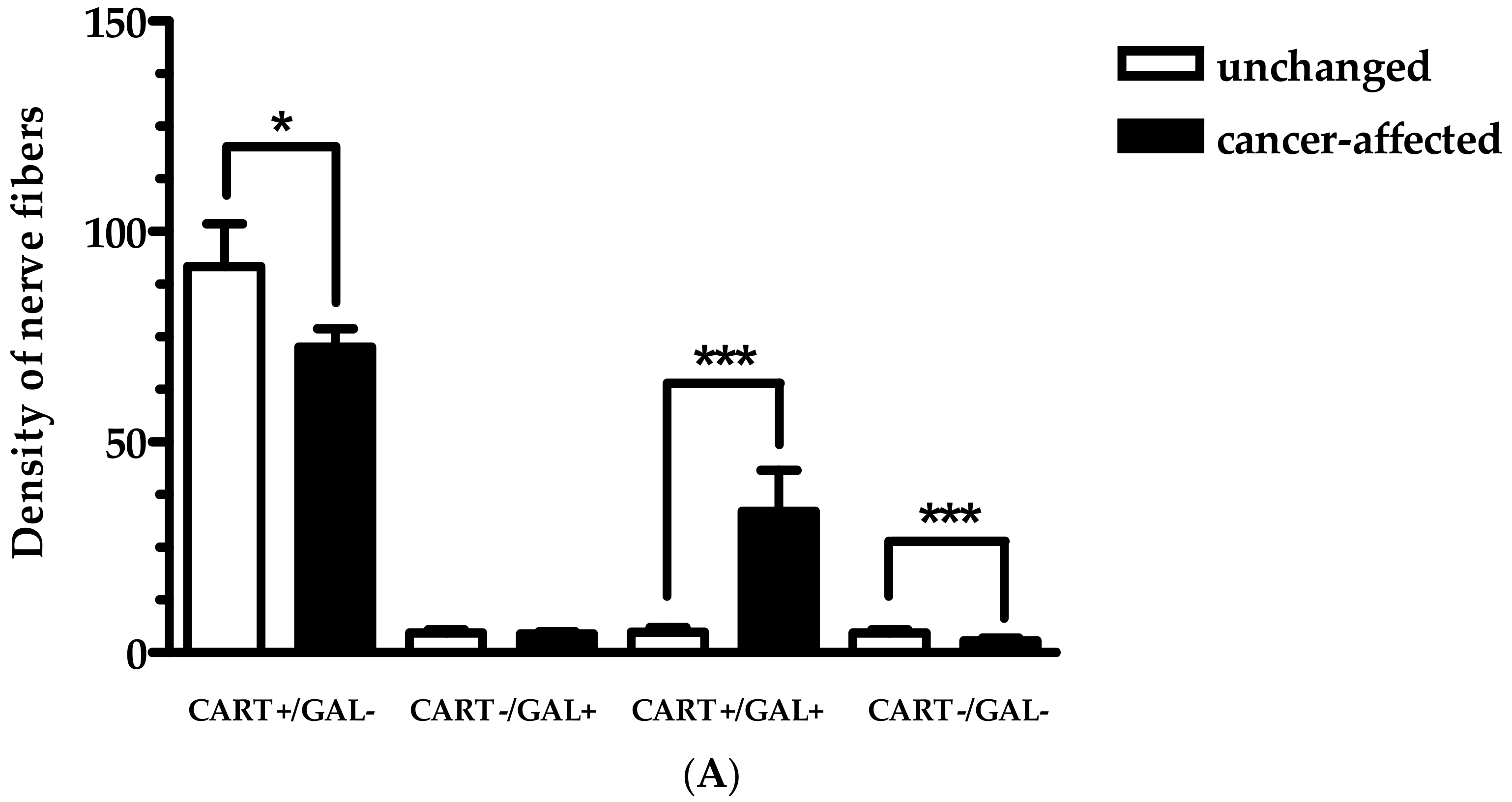
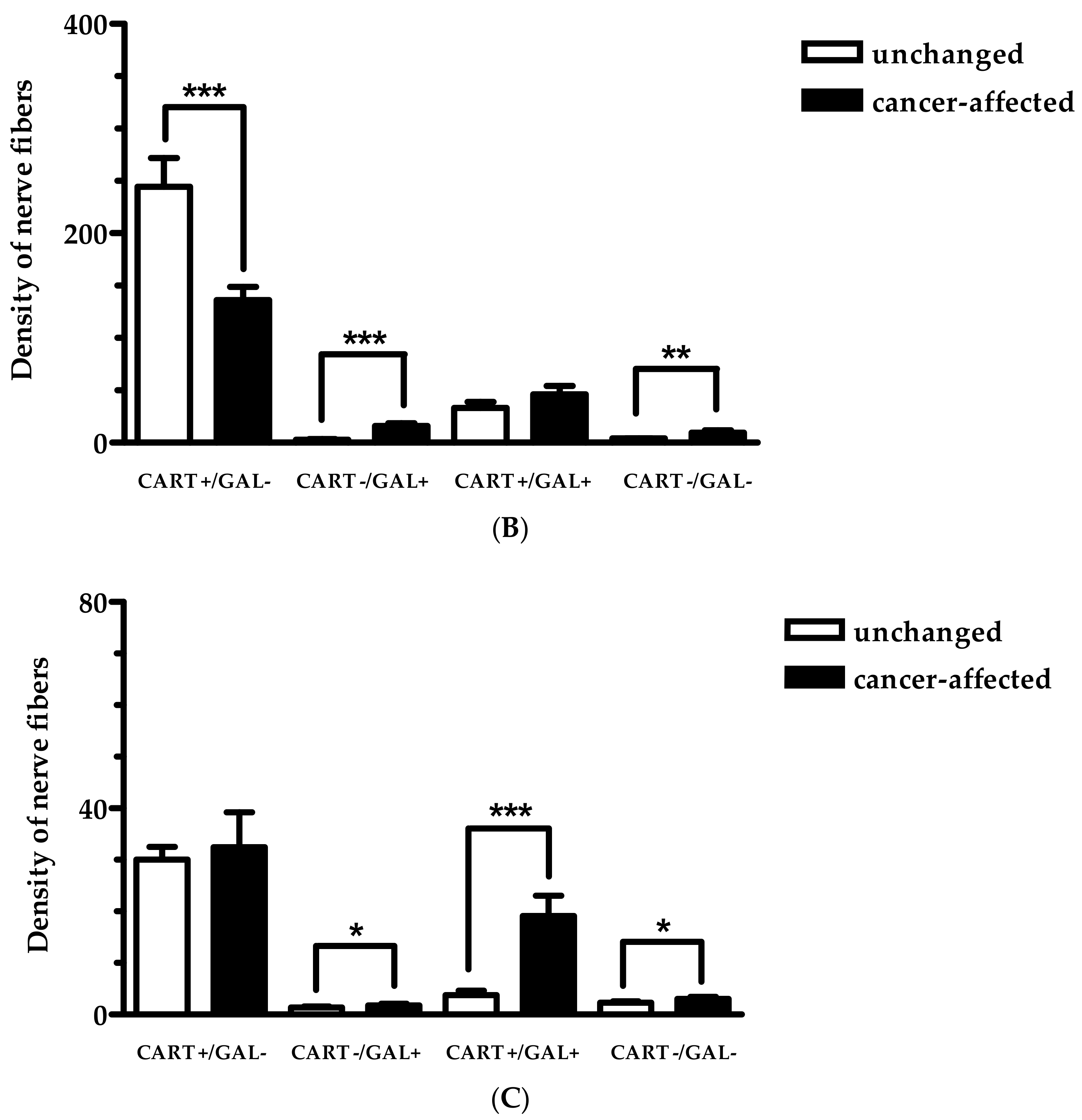
| The Number of PGP 9.5-IR Neurons Counted Inside Studied MP | |
|---|---|
| Total number of PGP 9.5-IR neurons | 1265 [100%] |
| Cancer-affected tissue | 601 [47.5%] |
| Surgical margin | 664 [52.5%] |
| Antisera | Code | Host Species | Dilution | Supplier |
|---|---|---|---|---|
| Primary Antibody | ||||
| CART (61–102) | H-003-61 | Rabbit | 1:6000 | Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA |
| Galanin | T-5034 | Guinea pig | 1:1200 | Bachem AG, Bubendorf, CH |
| PGP 9.5 | 7863-2004 | Mouse | 1:950 | Biogenesis, Kingstone, NH, USA |
| Secondary Antibody | ||||
| Biotinylated polyclonal anti-rabbit | E0432 | Goat | 1:1000 | Dako, Glostrup, DK, |
| Fluorescein-conjugated AffiniPure anti-guinea pig | 706-096-148 | Donkey | 1:450 | Jackson Immunoresearch, West Grove, PA, USA |
| AMCA-AffiniPure anti-mouse | 715-156-151 | Donkey | 1:75 | Jackson Immunoresearch, West Grove, PA, USA |
| CyTM3-conjugated streptavidin | 016-160-084 | - | 1:4500 | Jackson Immunoresearch, West Grove, PA, USA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, A.; Godlewski, J.; Majewski, M. Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor. Int. J. Mol. Sci. 2018, 19, 3357. https://doi.org/10.3390/ijms19113357
Kozłowska A, Godlewski J, Majewski M. Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor. International Journal of Molecular Sciences. 2018; 19(11):3357. https://doi.org/10.3390/ijms19113357
Chicago/Turabian StyleKozłowska, Anna, Janusz Godlewski, and Mariusz Majewski. 2018. "Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor" International Journal of Molecular Sciences 19, no. 11: 3357. https://doi.org/10.3390/ijms19113357
APA StyleKozłowska, A., Godlewski, J., & Majewski, M. (2018). Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor. International Journal of Molecular Sciences, 19(11), 3357. https://doi.org/10.3390/ijms19113357