Epigallocatechin Gallate-Modified Gelatins with Different Compositions Alter the Quality of Regenerated Bones
Abstract
1. Introduction
2. Results
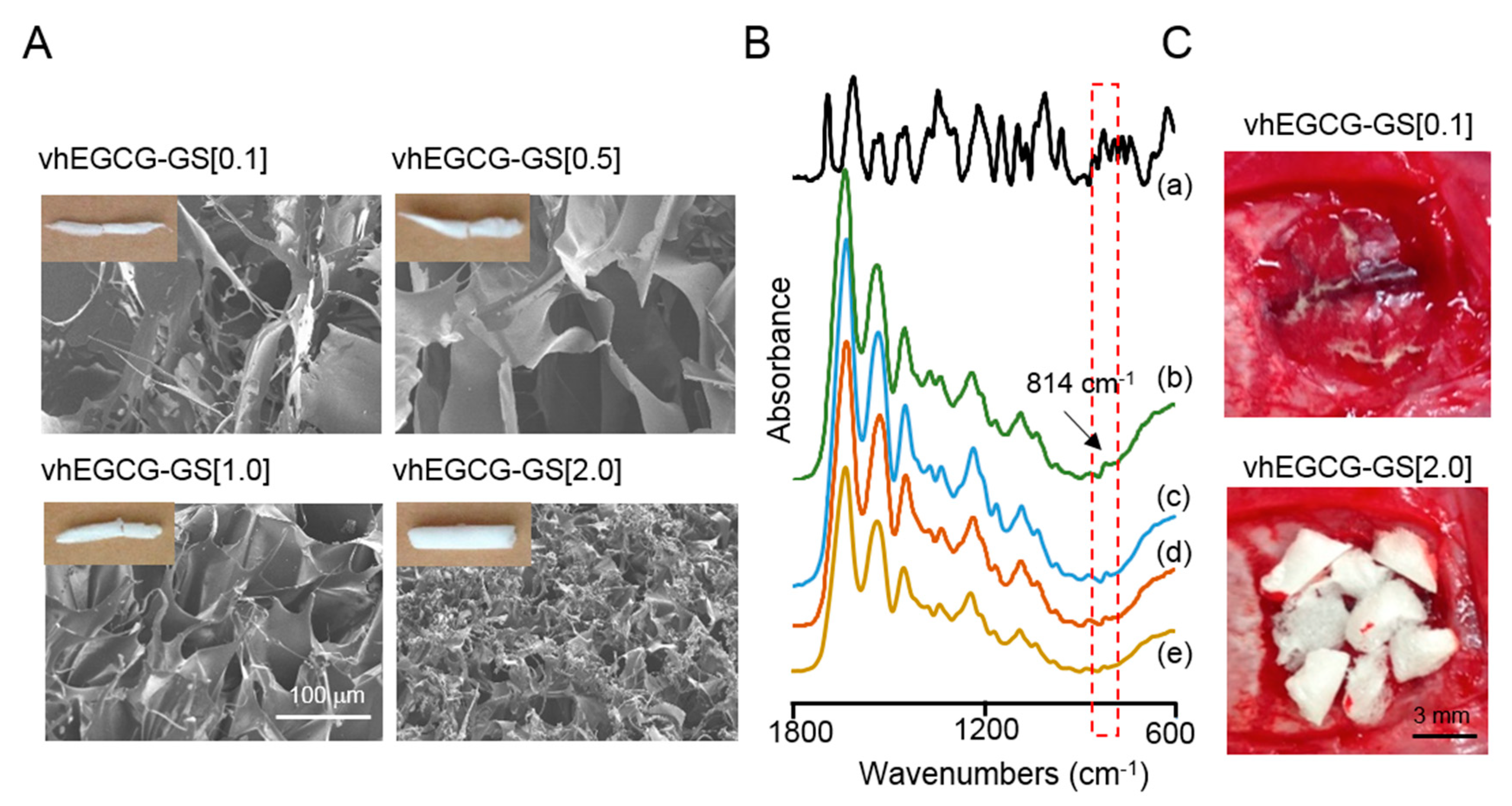
2.1. Characterization of Prepared Sponges
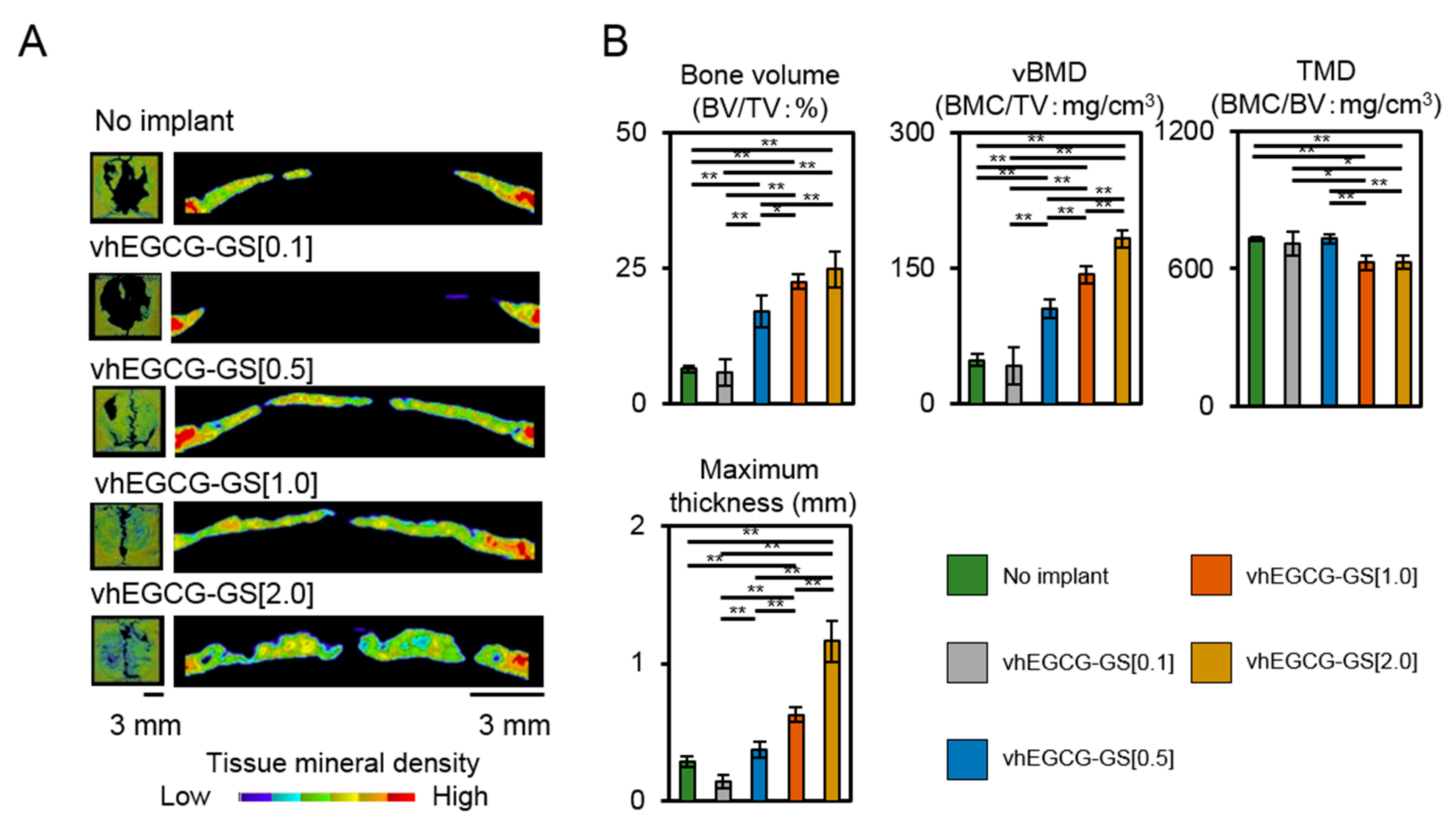
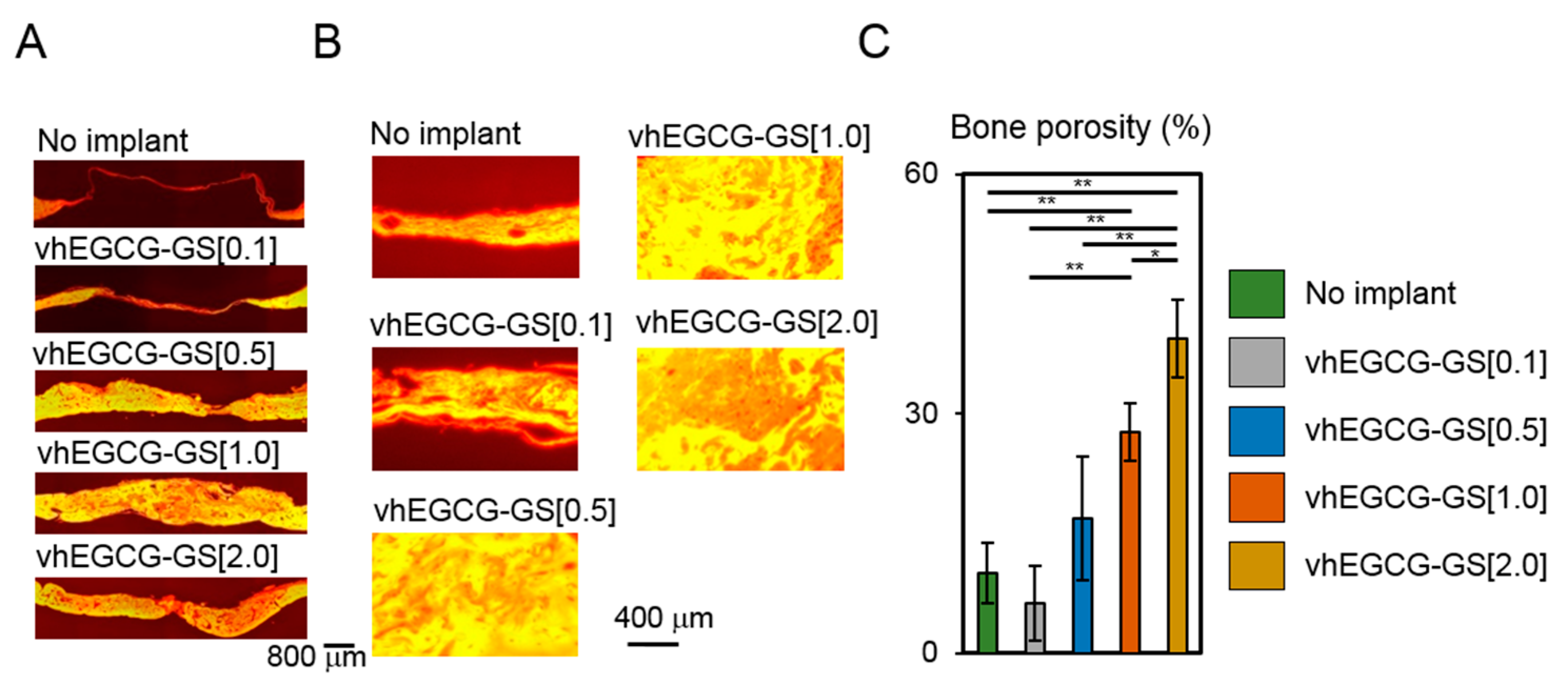
2.2. Bone Formation in Critical Sized Defects of Rat Calvaria
2.3. Porosity of Regenerated Bone
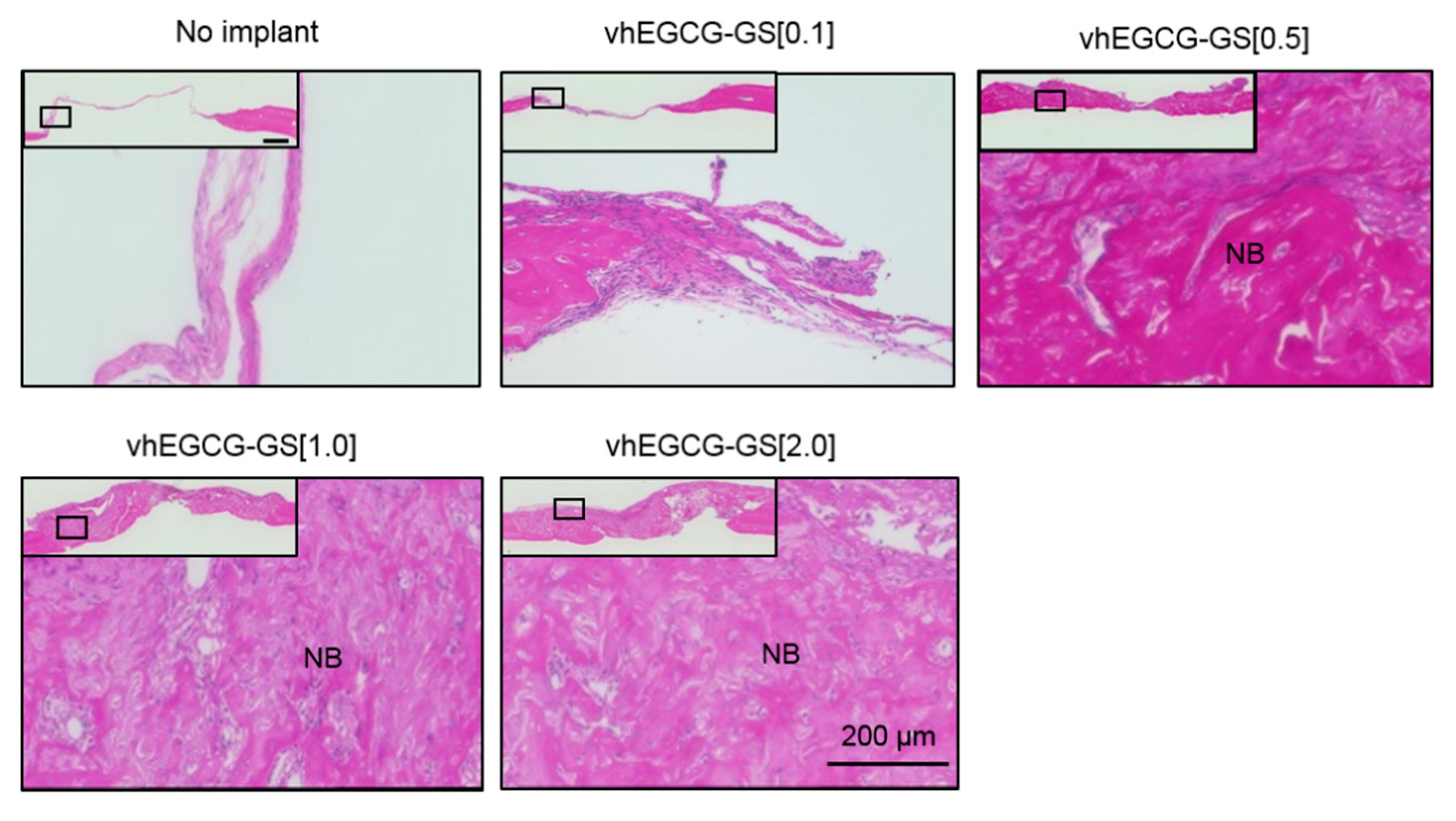
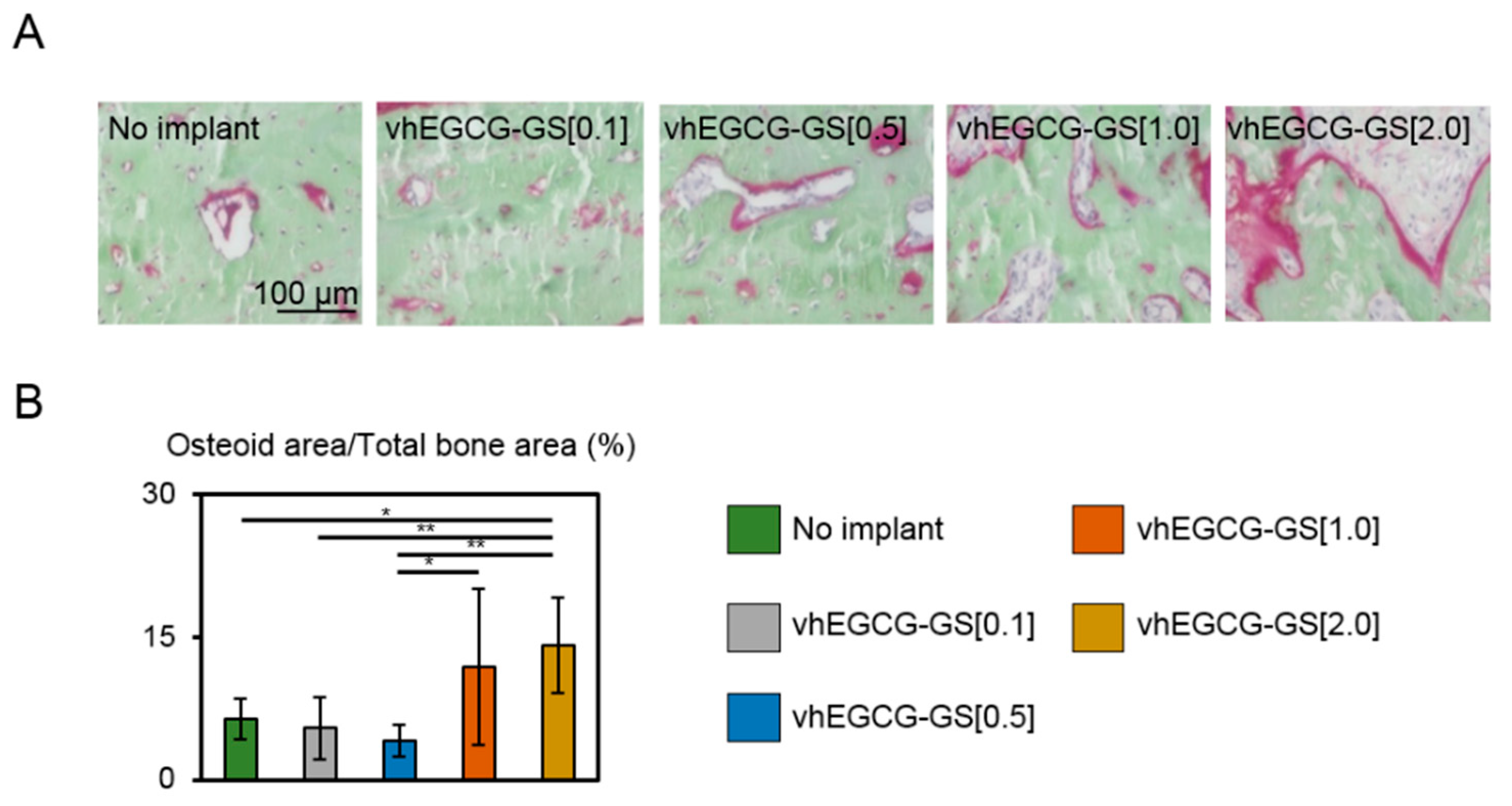
2.4. Osteoid Formation
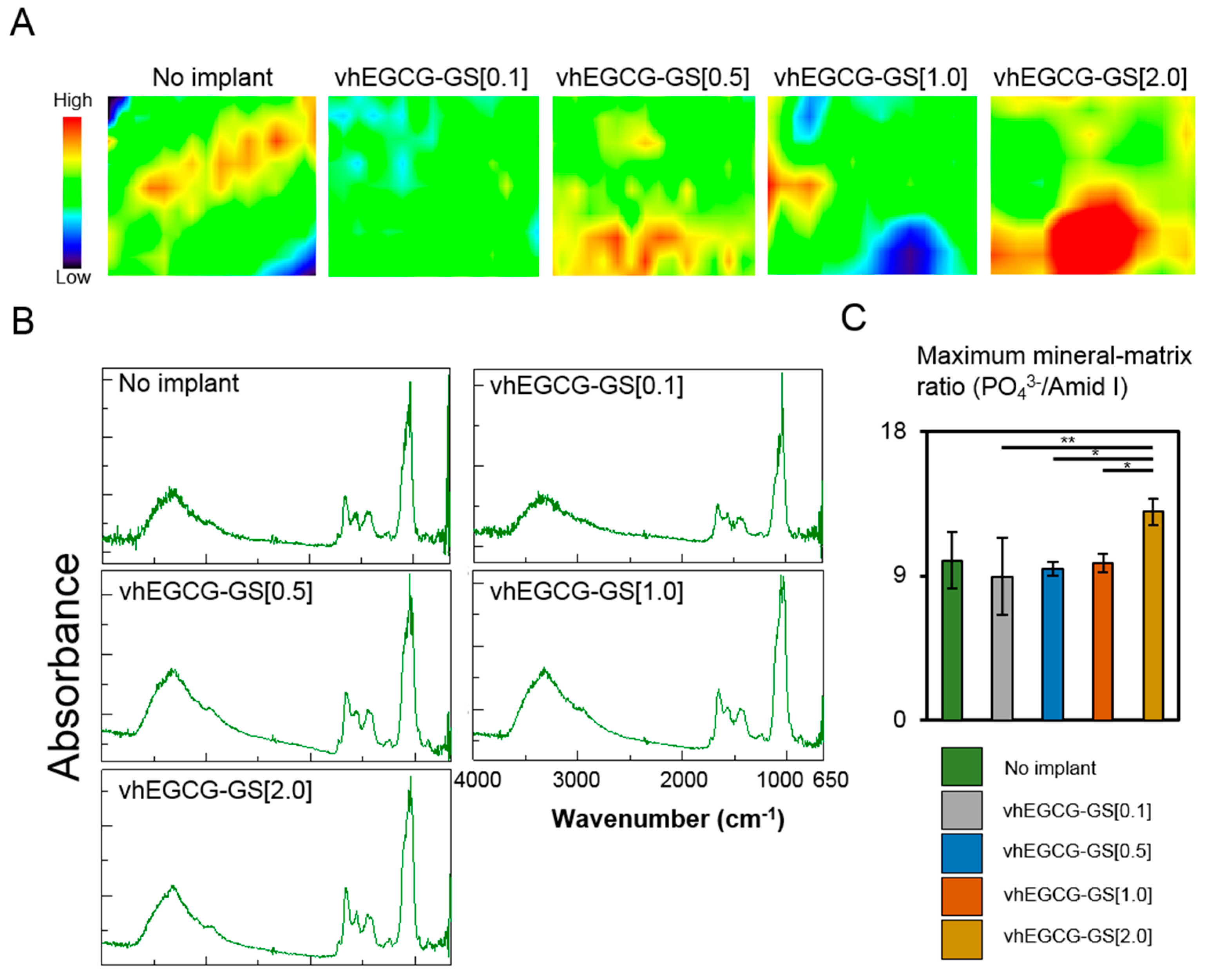
2.5. FTIR Microscopic and Imaging Analysis
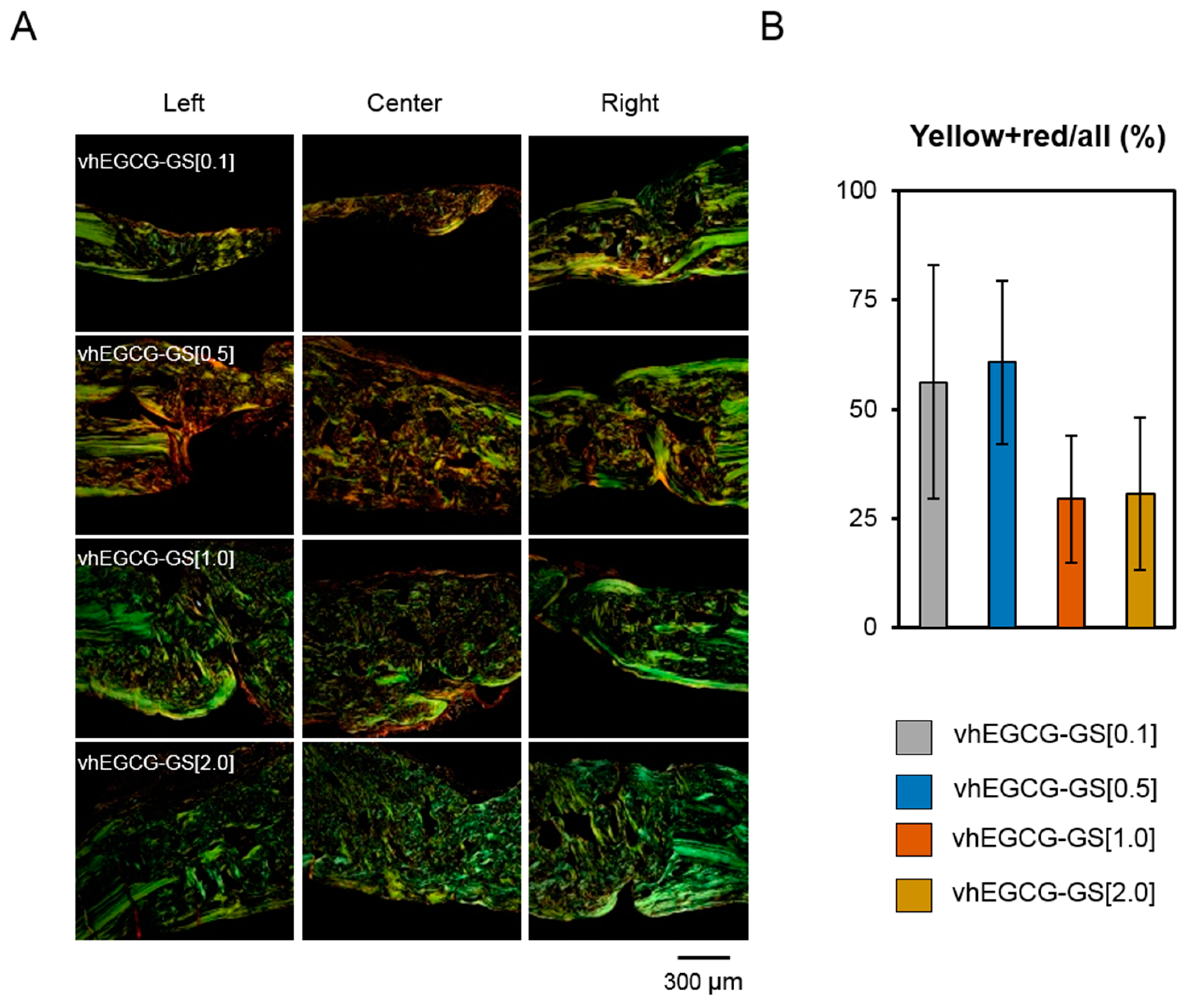
2.6. Picrosirius Red Staining and Polarized Microscopic Observation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of vhEGCG-GSs
4.3. Characterization of Sponges
4.4. Implantation of Sponges
4.5. Bone Histomorphometric Analysis using Microcomputed Tomography
4.6. Hematoxylin–Eosin and Picrosirius Red Staining
4.7. V.Goldner Staining and FTIR Imaging
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| GS | Gelatin sponge |
| vhGS | Vacuum heated gelatin sponge |
| EGCG | Epigallocatechin gallate |
| EGCG-GS | Epigallocatechin gallate-modified gelatin sponge |
| vhEGCG-GS | Vacuum heated EGCG-GS |
References
- Landis, W.J. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 1995, 16, 533–544. [Google Scholar] [CrossRef]
- Szpalski, C.; Barr, J.; Wetterau, M.; Saadeh, P.B.; Warren, S.M. Cranial bone defects: Current and future strategies. Neurosurg. Focus 2010, 29, E8. [Google Scholar] [CrossRef] [PubMed]
- Peres, J.A.; Lamano, T. Strategies for stimulation of new bone formation: A critical review. Braz. Dent. J. 2011, 22, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K. Growth factors in oral and maxillofacial surgery: Potentials and challenges. J. Korean Assoc. Oral. Maxillofac. Surg. 2013, 39, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Kawai, T.; Anada, T.; Kamakura, S.; Suzuki, O. Quality of regenerated bone enhanced by implantation of octacalcium phosphate-collagen composite. Tissue Eng. Part C Methods 2010, 16, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filova, E.; Novak, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnova, V.; Lukasova, V.; Bartos, M.; Necas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Honda, Y.; Kakinoki, S.; Yamaoka, T.; Baba, S. Surface modification of porous alpha-tricalcium phosphate granules with heparin enhanced their early osteogenic capability in a rat calvarial defect model. Dent. Mater. J. 2018, 37, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.P.; Mendelsohn, R.; Boskey, A.L. Infrared assessment of bone quality: A review. Clin. Orthop. Relat. Res. 2011, 469, 2170–2178. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E. Methods for assessing bone quality: A review. Clin. Orthop. Relat. Res. 2011, 469, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Nakano, T.; Ishimoto, T.; Sasaki, M.; Inoue, M.; Yasutake, M.; Sawase, T. Optimally oriented grooves on dental implants improve bone quality around implants under repetitive mechanical loading. Acta Biomater. 2017, 48, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Compston, J. Bone quality: What is it and how is it measured? Arq. Bras. Endocrinol. Metabol. 2006, 50, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Kan, T.; Ishimoto, T.; Ohashi, Y.; Fujitani, W.; Umakoshi, Y.; Hattori, T.; Higuchi, Y.; Tane, M.; Nakajima, H. Evaluation of bone quality near metallic implants with and without lotus-type pores for optimal biomaterial design. Mater. Trans. 2006, 47, 2233–2239. [Google Scholar] [CrossRef]
- Nakada, H.; Numata, Y.; Sakae, T.; Kimura-Suda, H.; Tanimoto, Y.; Saeki, H.; Teranishi, M.; Kato, T.; LeGeros, R. Changes in bone quality associated with the mineralization of new bone formed around implants—Using xps, polarized microscopy, and ftir imaging. J. Hard Tissue Biol. 2010, 19, 101–110. [Google Scholar] [CrossRef]
- La, W.G.; Kwon, S.H.; Lee, T.J.; Yang, H.S.; Park, J.; Kim, B.S. The effect of the delivery carrier on the quality of bone formed via bone morphogenetic protein-2. Artif. Organs 2012, 36, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Sun, J.; Chen, Y.; Zong, M.; Li, S.; Wang, Y. Egcg attenuates uric acid-induced inflammatory and oxidative stress responses by medicating the notch pathway. Oxid. Med. Cell. Longev. 2015, 2015, 214836. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Ochiai, K. Exploring the possible applications of catechin (gel) for oral care of the elderly and disabled individuals. Jpn. Dent. Sci. Rev. 2012, 48, 126–134. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (egcg), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jie, G.; Zhang, J.; Zhao, B. Significant longevity-extending effects of EGCG on Caenorhabditis elegans under stress. Free Radic. Biol. Med. 2009, 46, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Peairs, A.; Dai, R.; Gan, L.; Shimp, S.; Rylander, M.N.; Li, L.; Reilly, C.M. Epigallocatechin-3-gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cell. Mol. Immunol. 2010, 7, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wu, H.; Xu, G.; Zheng, L.; Zhao, J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: An in vitro study. Cell Tissue Res. 2014, 356, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kaida, K.; Honda, Y.; Hashimoto, Y.; Tanaka, M.; Baba, S. Application of green tea catechin for inducing the osteogenic differentiation of human dedifferentiated fat cells in vitro. Int. J. Mol. Sci. 2015, 16, 27988–28000. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yu, B.; Liu, F. Epigallocatechin-3-gallate promotes osteoblastic activity in human osteoblast-like cells. Trop. J. Pharm. Res. 2016, 15, 313. [Google Scholar] [CrossRef]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local controlled release of polyphenol conjugated with gelatin facilitates bone formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Wang, X.; Liang, C.; Yuan, F.; Gao, Y. Preparation and functional evaluation of chitosan-egcg conjugates. J. Appl. Polym. Sci. 2014, 39732, 1–8. [Google Scholar] [CrossRef]
- Ren, P.-G.; Ma, T.; Huang, Z.; Smith, R.L.; Goodman, S.B. Quantitation of bone area in undecalcified frozen sections with fluorescent microscopy. J. Histotech. 2013, 31, 15–17. [Google Scholar] [CrossRef]
- Ota, T.; Nishida, Y.; Ikuta, K.; Kato, R.; Kozawa, E.; Hamada, S.; Sakai, T.; Ishiguro, N. Heat-stimuli-enhanced osteogenesis using clinically available biomaterials. PLoS ONE 2017, 12, e0181404. [Google Scholar] [CrossRef] [PubMed]
- Dayan, D.; Hiss, Y.; Hirshberg, A.; Bubis, J.J.; Wolman, M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 1989, 93, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, Y.Z.; Iwai, Y.; Tamura, J.; Uehara, M. Diamond squid (thysanoteuthis rhombus)-derived chondroitin sulfate stimulates bone healing within a rat calvarial defect. Mar. Drugs 2013, 11, 5024–5035. [Google Scholar] [CrossRef] [PubMed]
- Oest, M.E.; Dupont, K.M.; Kong, H.J.; Mooney, D.J.; Guldberg, R.E. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J. Orthop. Res. 2007, 25, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Maisani, M.; Sindhu, K.R.; Fenelon, M.; Siadous, R.; Rey, S.; Mantovani, D.; Chassande, O. Prolonged delivery of BMP-2 by a non-polymer hydrogel for bone defect regeneration. Drug Deliv. Transl. Res. 2018, 8, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Galois, L.; Malinard, D. Bone ingrowth into two porous ceramics with different pore sizes: An experimental study. Acta Orthop. Belg. 2004, 70, 598–603. [Google Scholar] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, Q.; Nakamoto, T.; Kawazoe, N.; Chen, G. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue engineering. Tissue Eng. Part C Methods 2016, 22, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Pleshko Camacho, N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 2007, 28, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Cossermelli, W.; Brentani, R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch. Histol. Jpn. 1978, 41, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kaku, M.; Rosales Rocabado, J.M.; Kitami, M.; Ida, T.; Akiba, Y.; Yamauchi, M.; Uoshima, K. Mechanical loading stimulates expression of collagen cross-linking associated enzymes in periodontal ligament. J. Cell. Physiol. 2016, 231, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Uto, Y.; Kuroshima, S.; Nakano, T.; Ishimoto, T.; Inaba, N.; Uchida, Y.; Sawase, T. Effects of mechanical repetitive load on bone quality around implants in rat maxillae. PLoS ONE 2017, 12, e0189893. [Google Scholar] [CrossRef] [PubMed]
- Matsui, A.; Anada, T.; Masuda, T.; Honda, Y.; Miyatake, N.; Kawai, T.; Kamakura, S.; Echigo, S.; Suzuki, O. Mechanical stress-related calvaria bone augmentation by onlayed octacalcium phosphate-collagen implant. Tissue Eng. Part A 2010, 16, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.P.; Shane, E.; Lyritis, G.; Skarantavos, G.; Mendelsohn, R.; Boskey, A.L. Bone fragility and collagen cross-links. J. Bone Miner. Res. 2004, 19, 2000–2004. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Yoon, B.S.; Moon, J.H.; Kim, B.; Jun, E.K.; Oh, S.; Kim, H.; Song, H.J.; Noh, J.Y.; Oh, C.; et al. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J. Investig. Dermatol. 2008, 128, 2429–2441. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Honda, Y.; Arima, Y.; Yasui, K.; Inami, K.; Nishiura, A.; Hashimoto, Y.; Matsumoto, N. Interferon-gamma enhances the efficacy of autogenous bone grafts by inhibiting postoperative bone resorption in rat calvarial defects. J. Prosthodont. Res. 2016, 60, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, W.; Taira, M.; Ikeda, K.; Sato, H.; Kihara, H.; Takemoto, S.; Kondo, H. Bone regeneration of rat critical-size calvarial defects using a collagen/porous-apaitite composite: Micro-CT analysis and histological observations. J. Oral Tissue Eng. 2017, 15, 49–60. [Google Scholar]
- Kamakura, S.; Sasaki, K.; Honda, Y.; Anada, T.; Matsui, K.; Echigo, S.; Suzuki, O. Dehydrothermal treatment of collagen influences on bone regeneration by octacalcium phosphate (OCP) collagen composites. J. Tissue Eng. Regen. Med. 2007, 1, 450–456. [Google Scholar] [CrossRef] [PubMed]
| Designation | EGCG (mg) | Gelatin (mg) | Water (mL) | Percentage of Gelatin (%) | Vacuum Heating | Used for Animal Experimentation |
|---|---|---|---|---|---|---|
| vhEGCG-GS[0.01] | 0.07 | 1 | 10 | 0.01 | + | No |
| vhEGCG-GS[0.1] | 0.07 | 10 | 10 | 0.1 | + | Yes |
| vhEGCG-GS[0.5] | 0.07 | 50 | 10 | 0.5 | + | Yes |
| vhEGCG-GS[1.0] | 0.07 | 100 | 10 | 1 | + | Yes |
| vhEGCG-GS[2.0] | 0.07 | 200 | 10 | 2 | + | Yes |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, E.; Honda, Y.; Suzuki, O.; Tanaka, T.; Matsumoto, N. Epigallocatechin Gallate-Modified Gelatins with Different Compositions Alter the Quality of Regenerated Bones. Int. J. Mol. Sci. 2018, 19, 3232. https://doi.org/10.3390/ijms19103232
Hara E, Honda Y, Suzuki O, Tanaka T, Matsumoto N. Epigallocatechin Gallate-Modified Gelatins with Different Compositions Alter the Quality of Regenerated Bones. International Journal of Molecular Sciences. 2018; 19(10):3232. https://doi.org/10.3390/ijms19103232
Chicago/Turabian StyleHara, Eiki, Yoshitomo Honda, Osamu Suzuki, Tomonari Tanaka, and Naoyuki Matsumoto. 2018. "Epigallocatechin Gallate-Modified Gelatins with Different Compositions Alter the Quality of Regenerated Bones" International Journal of Molecular Sciences 19, no. 10: 3232. https://doi.org/10.3390/ijms19103232
APA StyleHara, E., Honda, Y., Suzuki, O., Tanaka, T., & Matsumoto, N. (2018). Epigallocatechin Gallate-Modified Gelatins with Different Compositions Alter the Quality of Regenerated Bones. International Journal of Molecular Sciences, 19(10), 3232. https://doi.org/10.3390/ijms19103232