Actions of Inonotus obliquus against Hyperuricemia through XOD and Bioactives Screened by Molecular Modeling
Abstract
1. Introduction
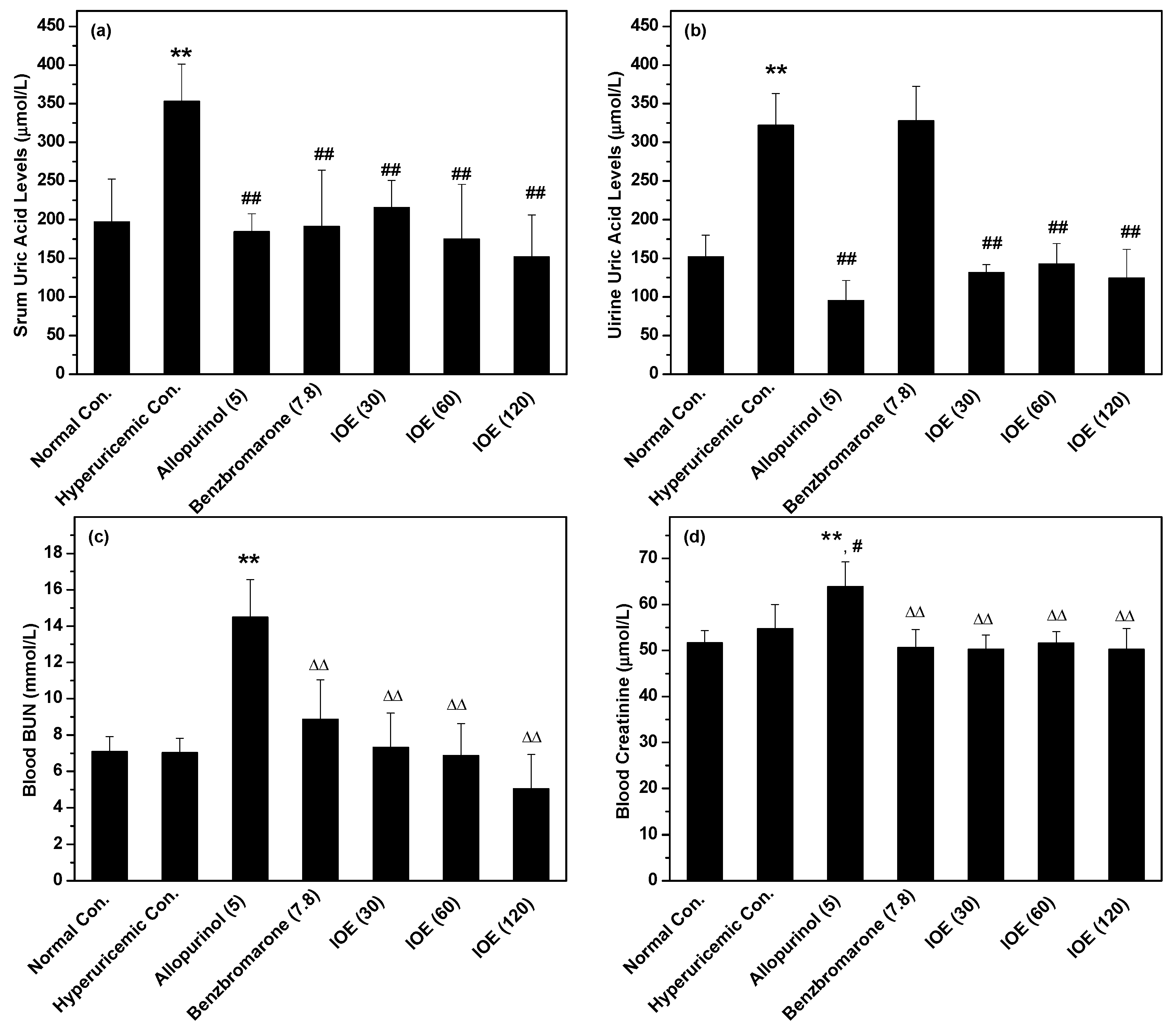
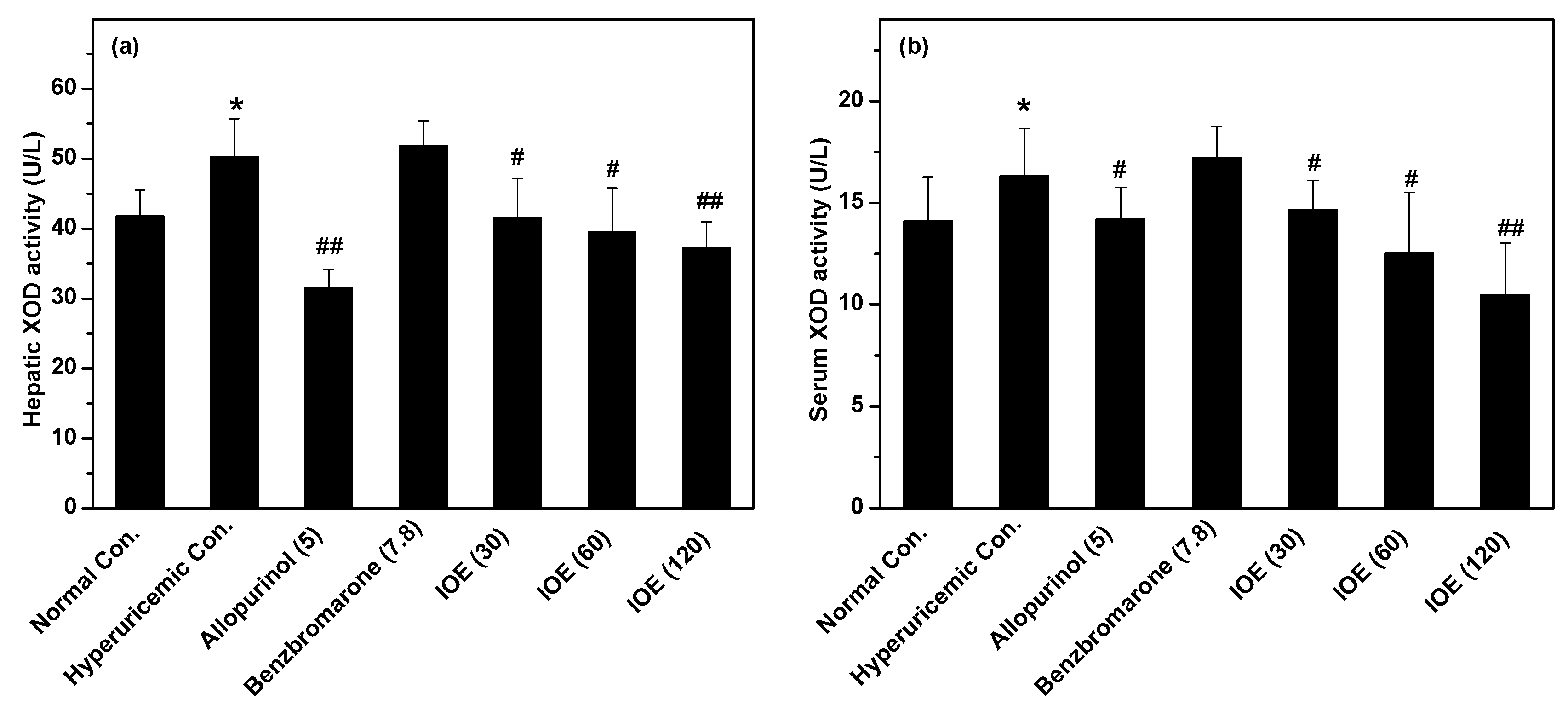
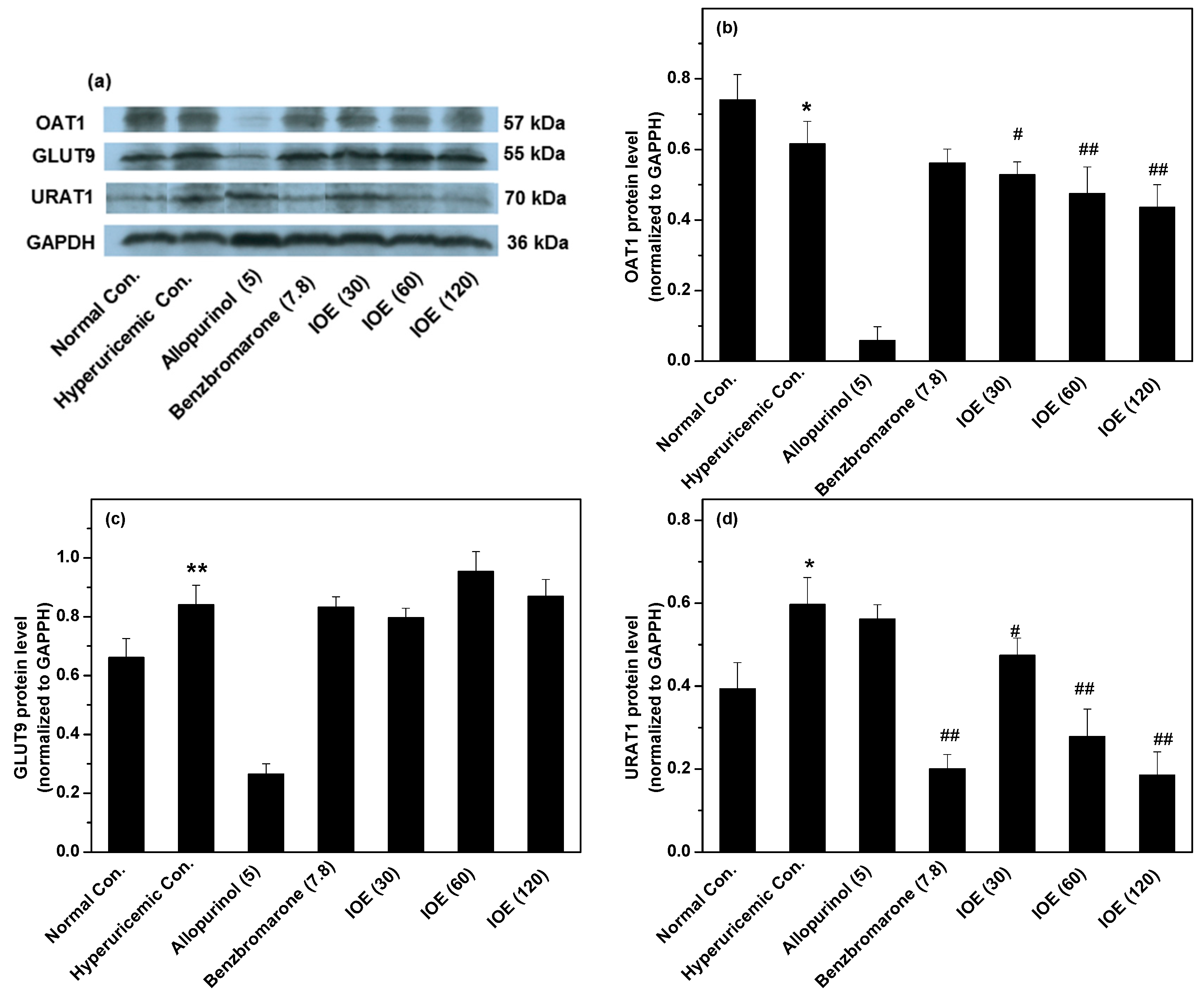
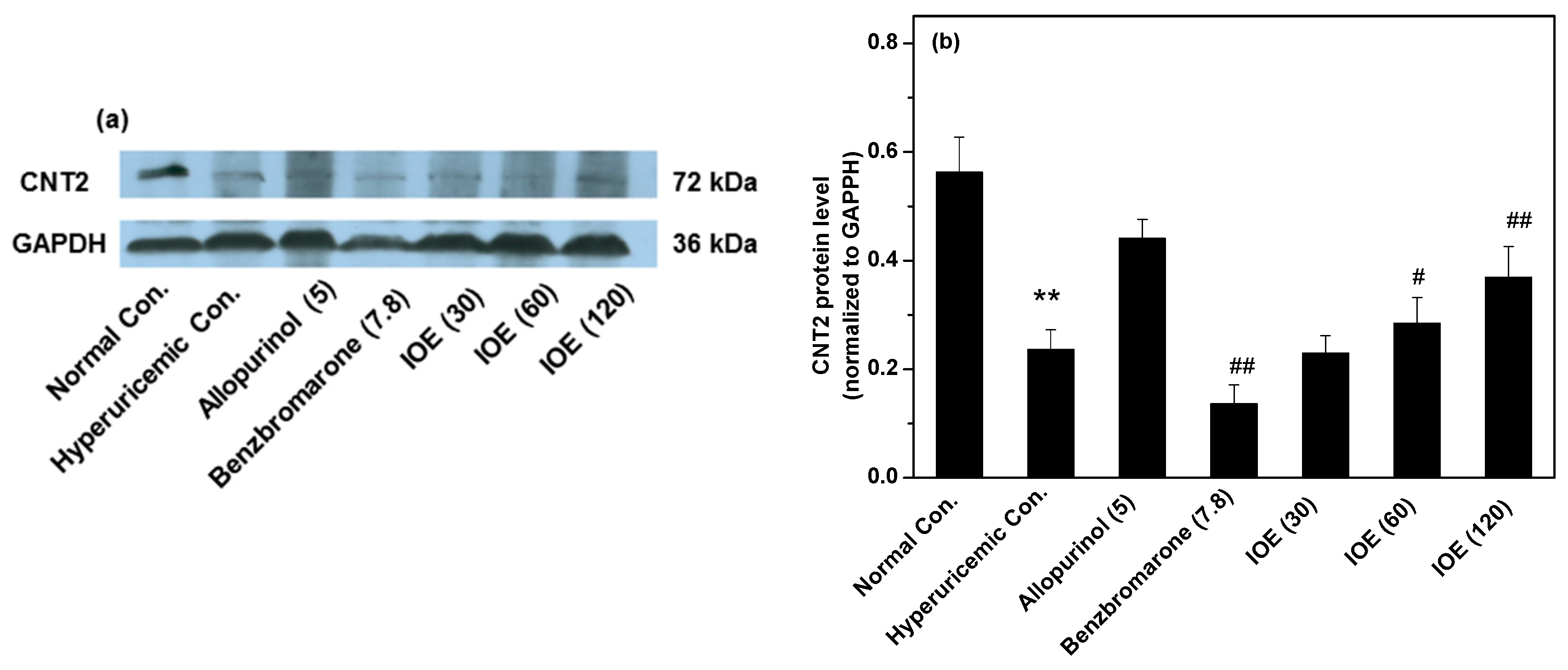
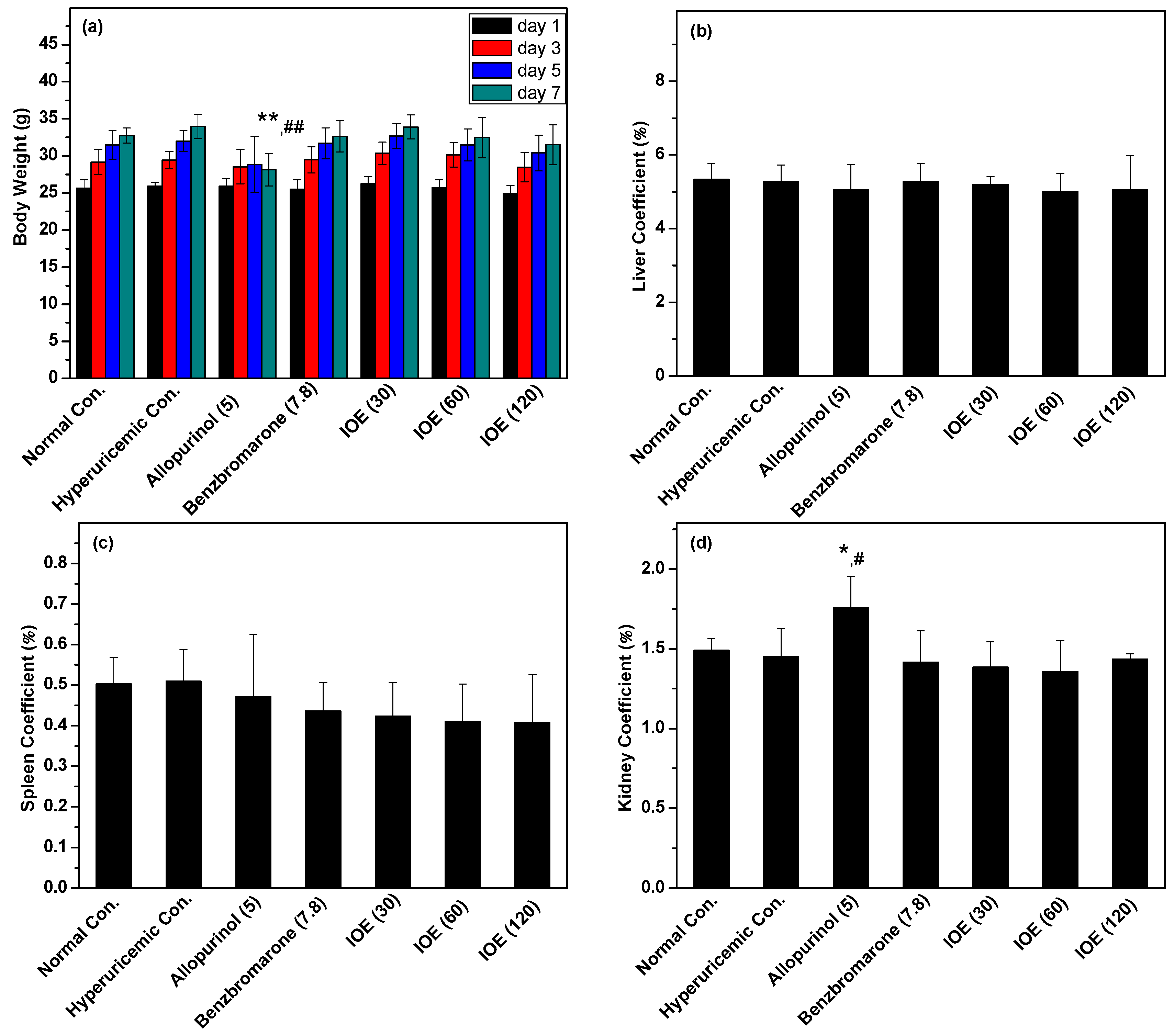
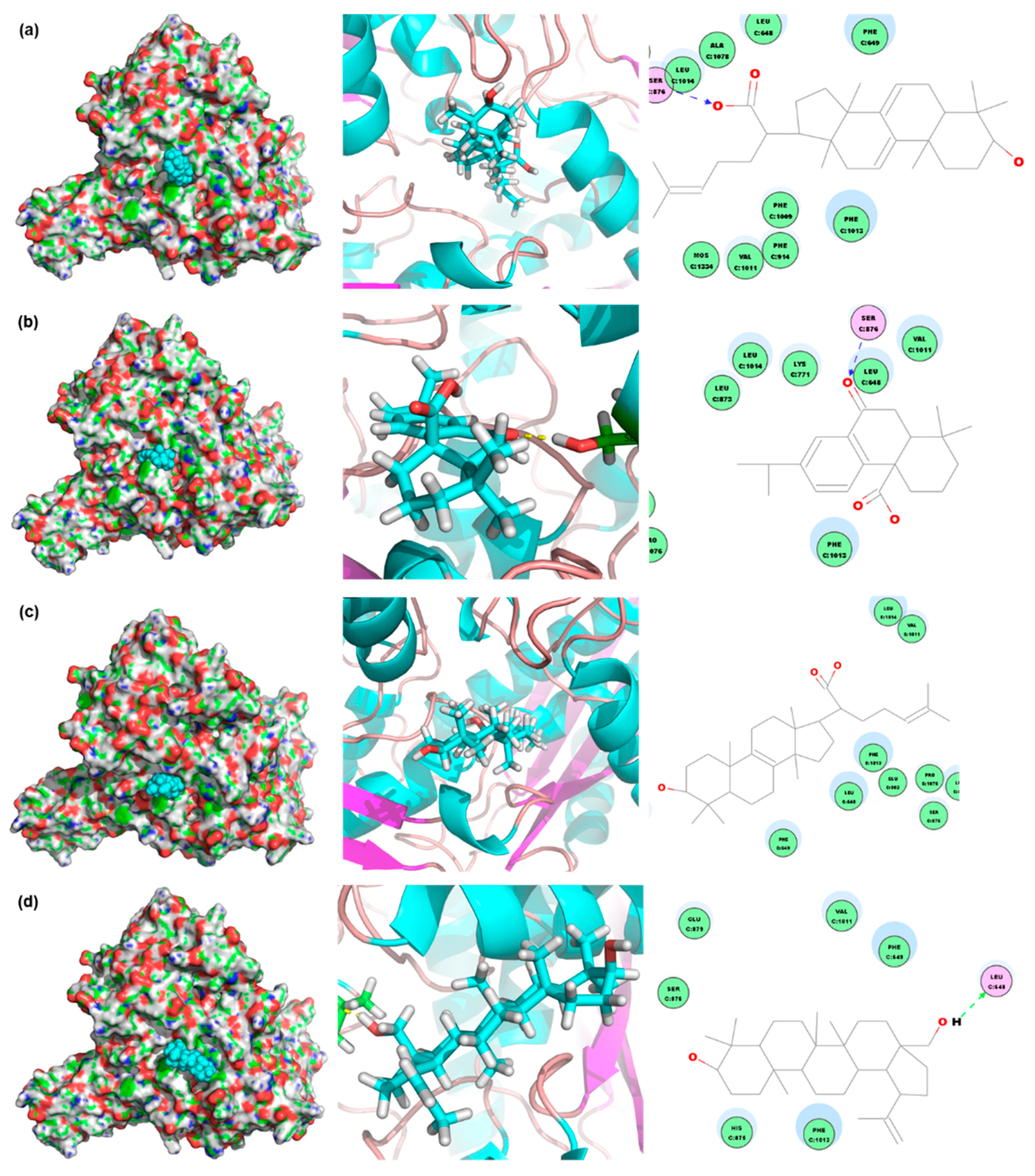
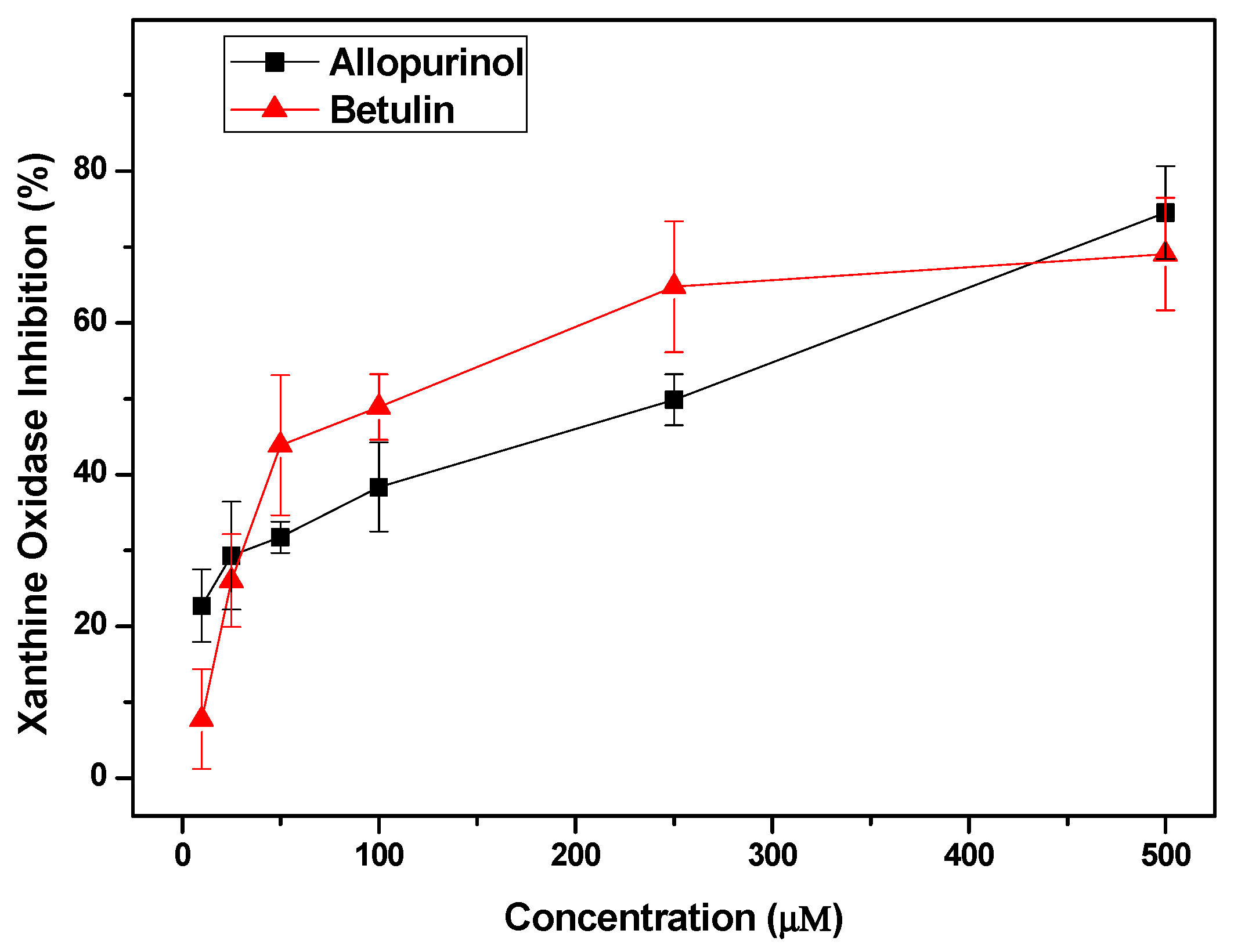
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals, Models, and Drug Administration
4.3. Physiological Parameters
4.4. In vivo XOD Activity, and Renal and Gastrointestinal Tract Transporters
4.5. Compound Database Establishment and Virtual Screening
4.6. Confirming the Anti-Hyperuricemic Effects of Screened Compounds by In Vitro Inhibitory Experiments
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SUA | serum uric acid |
| XOD | xanthine oxidase |
| PBS | phosphate buffer saline |
| BUN | blood urine nitrogen |
| OAT1 | organic anion transporter 1 |
| UART1 | uric acid transporter 1 |
| GLUT9 | glucose transporter 9 |
| CNT2 | gastrointestinal concentrative nucleoside transporter 2 |
| PO | potassium oxonate |
| HX | hypoxanthine |
| IOE | ethanol extract of Inonotus obliquus |
References
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal plants of the russian pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Hur, S.J.; An, C.S.; Jeon, Y.H.; Jeoung, Y.J.; Bak, J.P.; Lim, B.O. Anti-inflammatory effects of Inonotus obliquus in colitis induced by dextran sodium sulfate. J. Biomed. Biotechnol. 2010, 2010, 943516. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.M.; Zhang, L.Y.; Zhang, X.; Bai, H.B.; Liang, D.E.; Ma, L.F.; Shan, W.G.; Zhan, Z.J. Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus. Phytochemistry 2014, 108, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, C.; Pan, H.H.; Kang, J.; Yu, X.T.; Wang, H.Q.; Li, B.M.; Xie, Y.Z.; Chen, R.Y. Chemical constituents from Inonotus obliquus and their biological activities. J. Nat. Prod. 2014, 77, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, H.; Dong, P.; Lu, X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013, 139, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Mai, Q.; Ma, J.; Xu, M.; Wang, X.; Cui, T.; Qiu, F.; Han, G. Triterpenoids from Inonotus obliquus and their antitumor activities. Fitoterapia 2015, 101, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Yamada, T.; Tanaka, R. An unusual lanostane-type triterpenoid, spiroinonotsuoxodiol, and other triterpenoids from Inonotus obliquus. Phytochemistry 2010, 71, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Takahashi, T.; Uesugi, A.; Tanaka, R.; Kobayashi, S. Inotodiol, a lanostane triterpenoid, from Inonotus obliquus inhibits cell proliferation through caspase-3-dependent apoptosis. Anticancer Res. 2008, 28, 2691–2696. [Google Scholar] [PubMed]
- Glamoclija, J.; Ciric, A.; Nikolic, M.; Fernandes, A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.; Sokovic, M.; van Griensven, L.J. Chemical characterization and biological activity of chaga (Inonotus Obliquus), a medicinal “mushroom”. J. Ethnopharmacol. 2015, 162, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Ding, S.; Ai, L.; Deng, K. Antitumor and immunomodulatory activity of water-soluble polysaccharide from Inonotus obliquus. Carbohydr. Polym. 2012, 90, 870–874. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.S.; Kim, S.H.; Moon, S.Y.; Chung, M.J.; Cui, C.B.; Han, E.K.; Chung, C.K.; Choe, M. Antimutagenic effects of subfractions of chaga mushroom (Inonotus Obliquus) extract. Mutat. Res. 2009, 672, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, W.; Li, W.; Wang, Y.; Wang, C. Study on effect of the extract of Inonotus obliquus on rats hyperuricemia. Food Sci. Technol. 2010, 06, 218–221. [Google Scholar]
- Yong, T.; Zhang, M.; Chen, D.; Shuai, O.; Chen, S.; Su, J.; Jiao, C.; Feng, D.; Xie, Y. Actions of water extract from cordyceps militaris in hyperuricemic mice induced by potassium oxonate combined with hypoxanthine. J. Ethnopharmacol. 2016, 194, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Su, J.; Shuai, O.; Jiao, C.; Zuo, D. Hypouricemic effects of ganoderma applanatum in hyperuricemia mice through oat1 and glut9. Front. Pharmacol. 2017, 8, 996. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hall, J.; Hille, R. X-ray crystal structure of arsenite-inhibited xanthine oxidase: Μ-sulfido,μ-oxo double bridge between molybdenum and arsenic in the active site. J. Am. Chem. Soc. 2011, 133, 12414–12417. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using gold. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.H.; Chen, C.J.; Wu, S.H.; Hsieh, J.F. Inhibition of xanthine oxidase by rhodiola crenulata extracts and their phytochemicals. J. Agric. Food Chem. 2014, 62, 3742–3749. [Google Scholar] [CrossRef] [PubMed]
- Prusis, P.; Dambrova, M.; Andrianov, V.; Rozhkov, E.; Semenikhina, V.; Piskunova, I.; Ongwae, E.; Lundstedt, T.; Kalvinsh, I.; Wikberg, J.E.S. Synthesis and quantitative structure−activity relationship of hydrazones of n-amino-n′-hydroxyguanidine as electron acceptors for xanthine oxidase. J. Med. Chem. 2004, 47, 3105–3110. [Google Scholar] [CrossRef] [PubMed]
- Enroth, C.; Eger, B.T.; Okamoto, K.; Nishino, T.; Nishino, T.; Pai, E.F. Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: Structure-based mechanism of conversion. Proc. Natl. Acad. Sci. USA 2000, 97, 10723–10728. [Google Scholar] [CrossRef] [PubMed]
- Halevy, S.; Ghislain, P.-D.; Mockenhaupt, M.; Fagot, J.-P.; Bouwes Bavinck, J.N.; Sidoroff, A.; Naldi, L.; Dunant, A.; Viboud, C.; Roujeau, J.-C. Allopurinol is the most common cause of stevens-johnson syndrome and toxic epidermal necrolysis in europe and israel. J. Am. Acad. Dermatol. 2008, 58, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Schumacher, H.R.; Espinoza, L.R.; Wells, A.F.; MacDonald, P.; Lloyd, E.; Lademacher, C. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: The confirms trial. Arthritis Res. Ther. 2010, 12, R63. [Google Scholar] [CrossRef] [PubMed]
- Eraly, S.A.; Vallon, V.; Rieg, T.; Gangoiti, J.A.; Wikoff, W.R.; Siuzdak, G.; Barshop, B.A.; Nigam, S.K. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol. Genom. 2008, 33, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yuan, Y.; Zhou, Y.; Zhao, M.; Chen, Y.; Cheng, J.; Lu, Y.; Liu, J. Phloretin attenuates hyperuricemia-induced endothelial dysfunction through co-inhibiting inflammation and glut9-mediated uric acid uptake. J. Cell. Mol. Med. 2017, 21, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S.H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of current practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.A.; Zidell, R.H.; Perry, R.W. Relationships between organ weight and body/brain weight in the rat: What is the best analytical endpoint? Toxicol. Pathol. 2004, 32, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P. IX-Urinary Tract. In Histopathology of Preclinical Toxicity Studies, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2000; pp. 545–626. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yong, T.; Chen, S.; Liang, D.; Zuo, D.; Diao, X.; Deng, C.; Wu, Y.; Hu, H.; Xie, Y.; Chen, D. Actions of Inonotus obliquus against Hyperuricemia through XOD and Bioactives Screened by Molecular Modeling. Int. J. Mol. Sci. 2018, 19, 3222. https://doi.org/10.3390/ijms19103222
Yong T, Chen S, Liang D, Zuo D, Diao X, Deng C, Wu Y, Hu H, Xie Y, Chen D. Actions of Inonotus obliquus against Hyperuricemia through XOD and Bioactives Screened by Molecular Modeling. International Journal of Molecular Sciences. 2018; 19(10):3222. https://doi.org/10.3390/ijms19103222
Chicago/Turabian StyleYong, Tianqiao, Shaodan Chen, Danling Liang, Dan Zuo, Xue Diao, Chenling Deng, Yuning Wu, Huiping Hu, Yizhen Xie, and Diling Chen. 2018. "Actions of Inonotus obliquus against Hyperuricemia through XOD and Bioactives Screened by Molecular Modeling" International Journal of Molecular Sciences 19, no. 10: 3222. https://doi.org/10.3390/ijms19103222
APA StyleYong, T., Chen, S., Liang, D., Zuo, D., Diao, X., Deng, C., Wu, Y., Hu, H., Xie, Y., & Chen, D. (2018). Actions of Inonotus obliquus against Hyperuricemia through XOD and Bioactives Screened by Molecular Modeling. International Journal of Molecular Sciences, 19(10), 3222. https://doi.org/10.3390/ijms19103222



