Acid Sphingomyelinase Inhibition Stabilizes Hepatic Ceramide Content and Improves Hepatic Biotransformation Capacity in a Murine Model of Polymicrobial Sepsis
Abstract
1. Introduction
2. Results
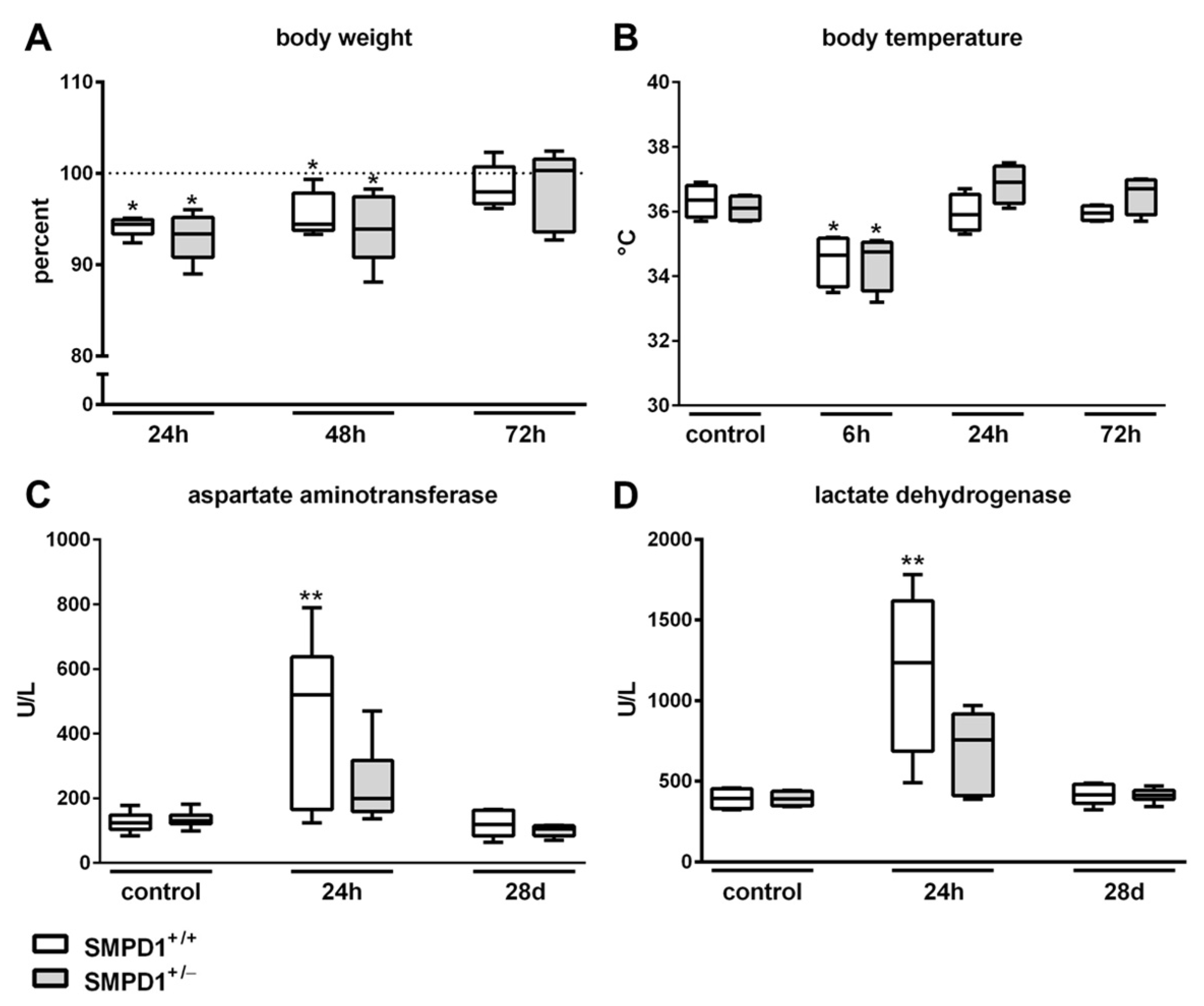
2.1. Parameters of Systemic Inflammation
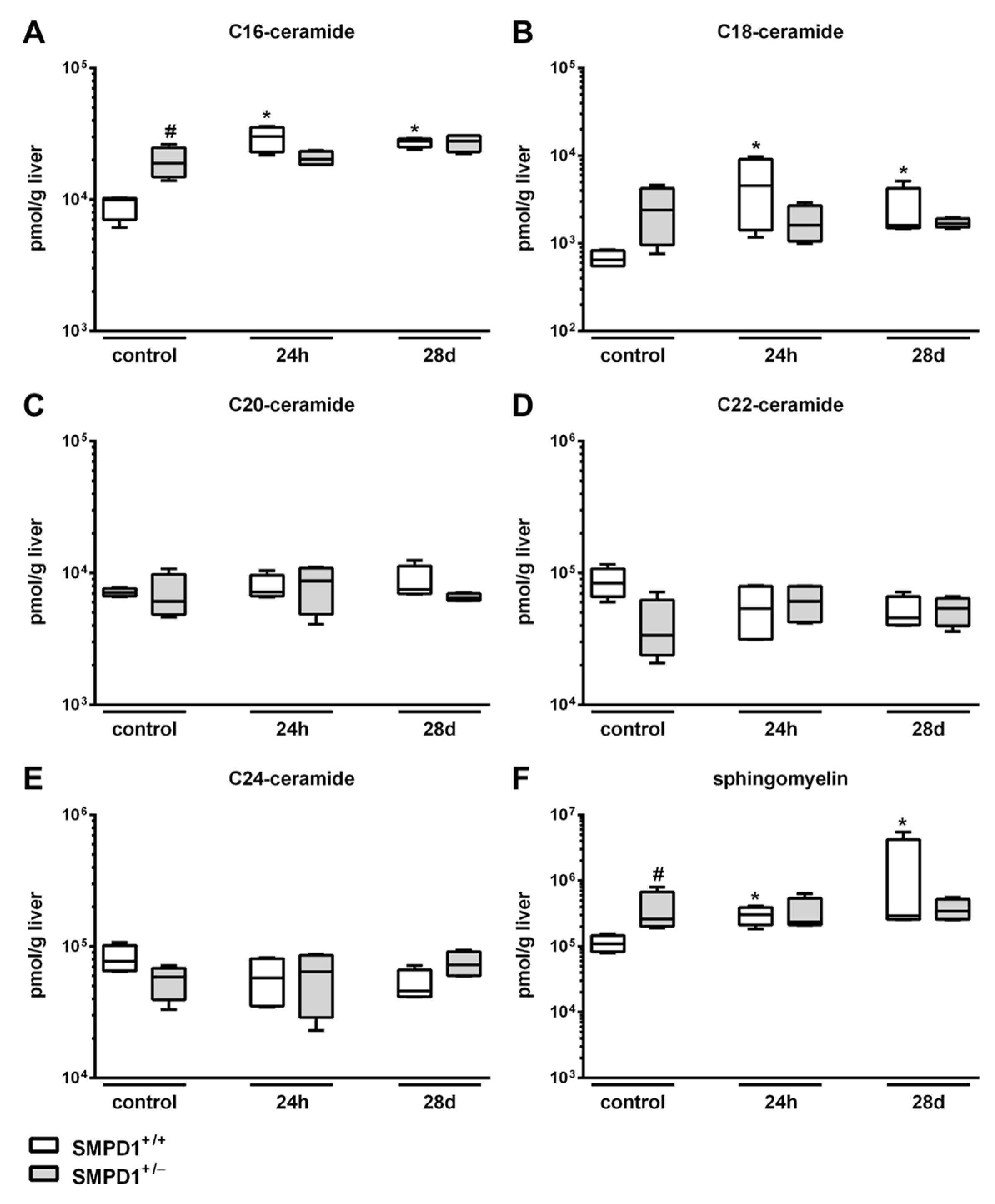
2.2. Sphingomyelin Phosphodiesterase 1 (SMPD1) Triggered Changes in the Composition of Hepatic Ceramides Following Polymicrobial Sepsis Induction
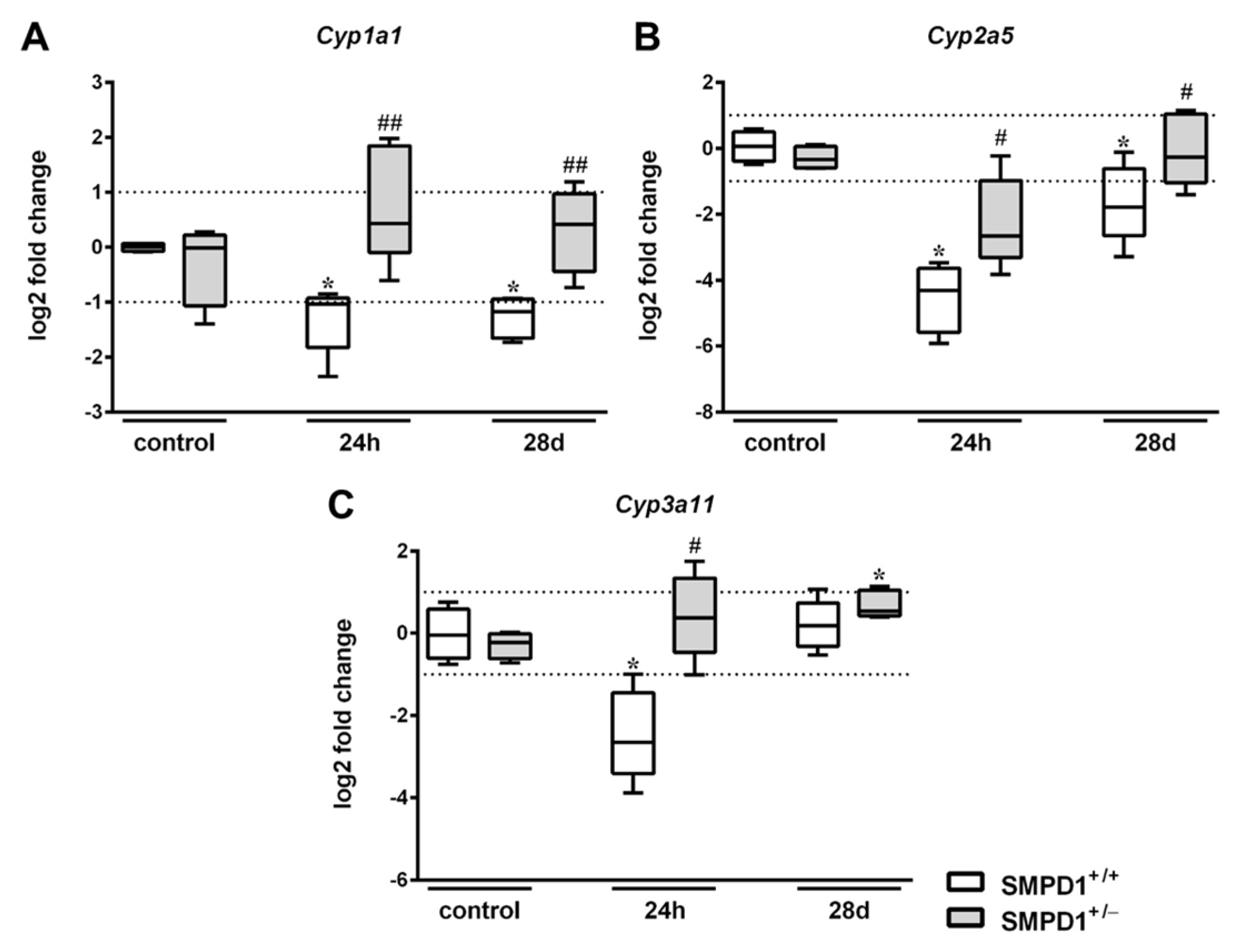
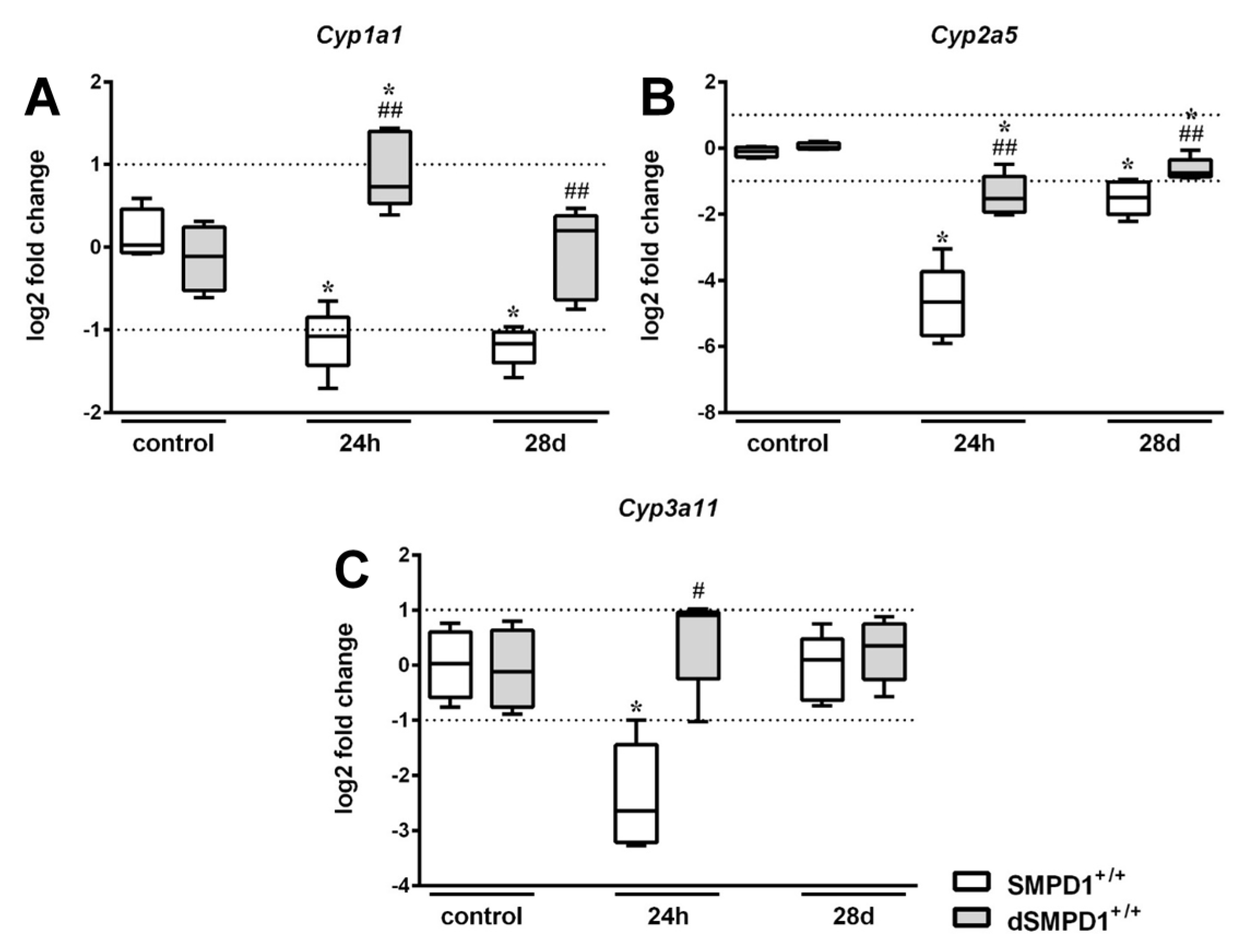
2.3. Hepatic Cytochrome P450 mRNA Expression Following Polymicrobial Sepsis Induction
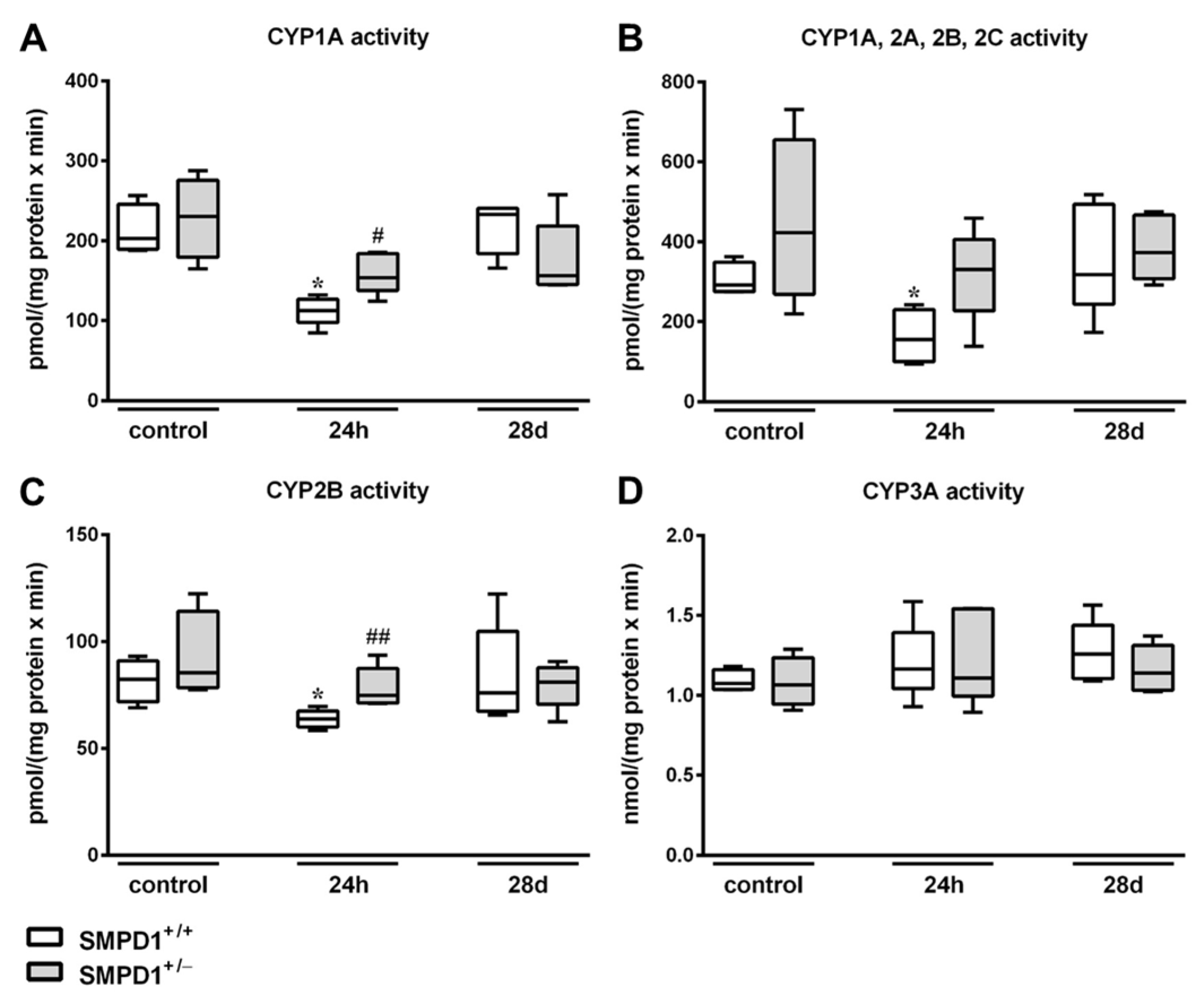
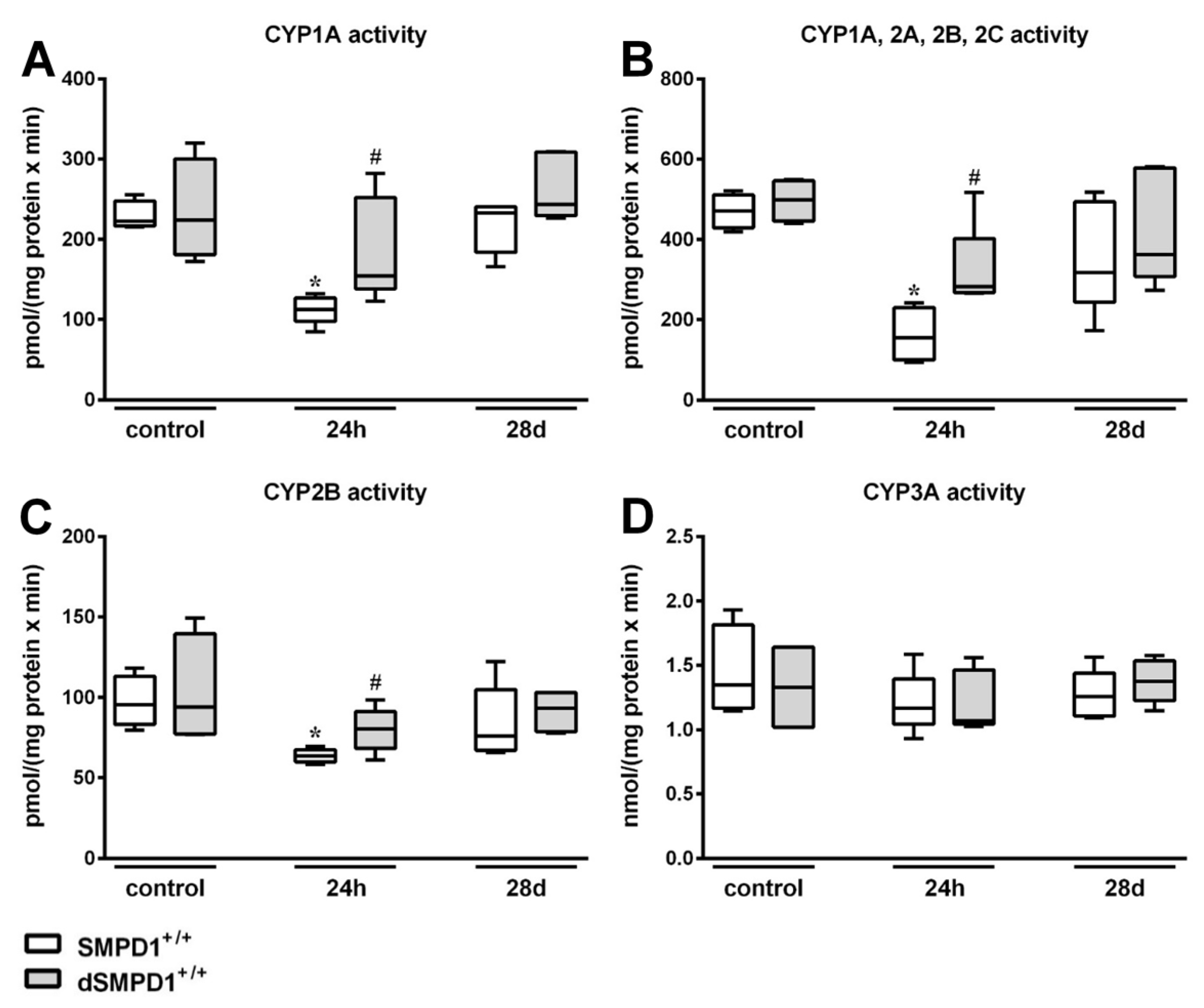
2.4. Hepatic Cytochrome P450 Activity Following Polymicrobial Sepsis Induction
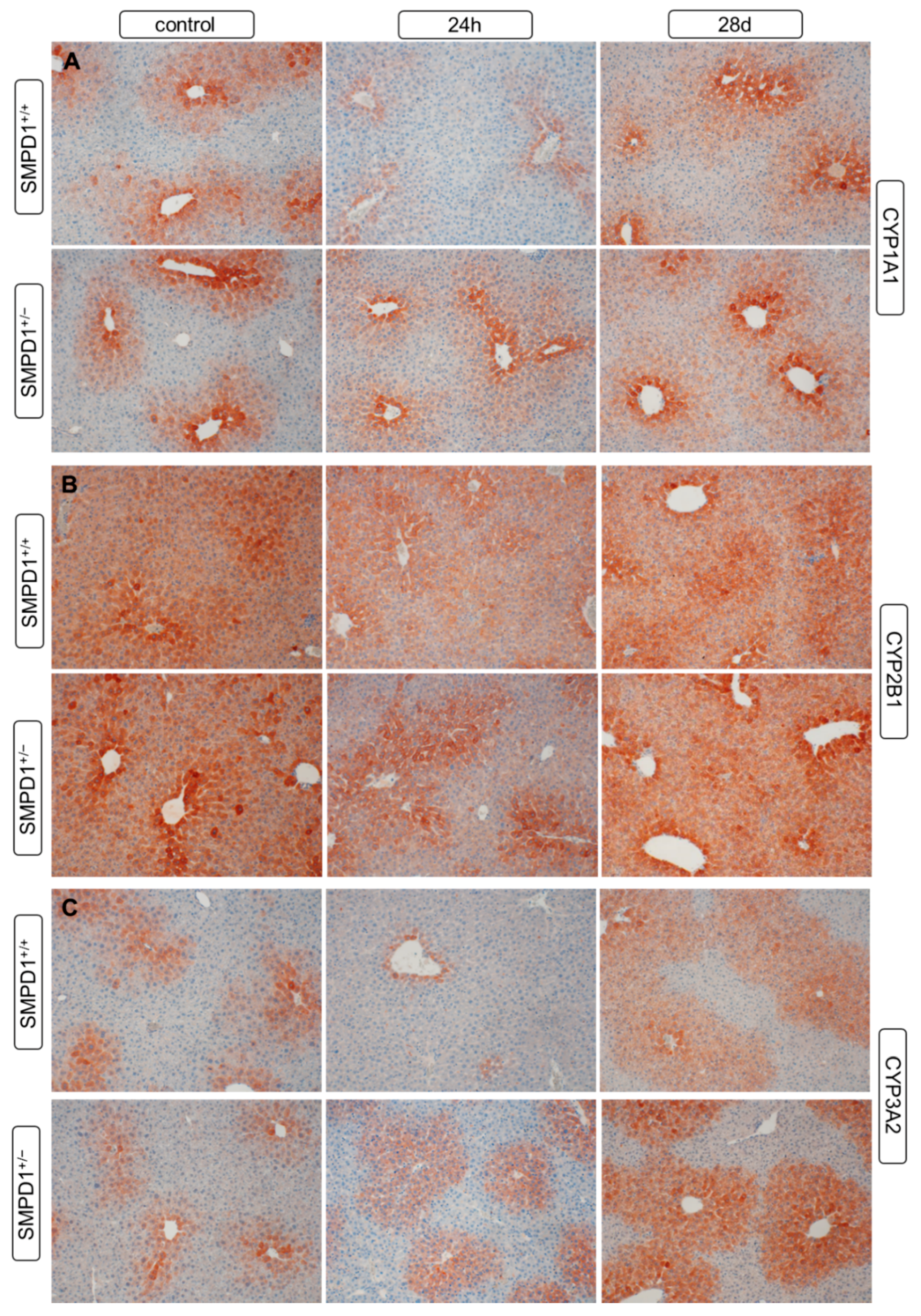
2.5. Hepatic Cytochrome P450 Isoforms Expression after Polymicrobial Sepsis Induction
2.6. Functional Inhibition of SMPD1 with Desipramine Improves Transcriptional Expression of Hepatic Monooxygenases during Host Response
2.7. Functional Inhibition of SMPD1 with Desipramine Improves Hepatic Monooxygenase Activities during Host Response
3. Discussion
4. Material and Methods
4.1. Animals and Sepsis Model
4.2. Laboratory Markers
4.3. Mass Spectrometry
4.4. Real-Time PCR
| Cyp1a1 | forward: | TGAGAAGGGCCACATCCGG |
| reverse: | CCAAAGAGGTCCAAAACAATCG | |
| Cyp2a5 | forward: | ATCGGGTGATTGGCAGGAAC |
| reverse: | TGGGGATCATGTCTGCAAATCT | |
| Cyp3a11 | forward: | AGCAGGGATGGACCTGG |
| reverse: | CGGTAGAGGAGCACCAA | |
| Actb | forward: | GCTCTTTTCCAGCCTTCCTT |
| reverse: | CGGATGTCAACGTCACACTT |
4.5. Immunohistochemistry
4.6. Cytochrome P450 (CYP) Enzyme Activities
4.7. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.K.; Rubenstein, A.R.; Radin, G.T.; Wiener, R.S.; Walkey, A.J. Two decades of mortality trends among patients with severe sepsis: A comparative meta-analysis. Crit. Care Med. 2014, 42, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, Z. Cooperation of liver cells in health and disease. Adv. Anat. Embryol. Cell Biol. 2001, 161, 1–151. [Google Scholar]
- Guengerich, F.P. Cytochrome p450 and chemical toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Harder, D.R.; Campbell, W.B.; Roman, R.J. Role of cytochrome p-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J. Vasc. Res. 1995, 32, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Cytochrome p450 and the individuality of species. Arch. Biochem. Biophys. 1999, 369, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.; Zhou, M.; Wu, R.; Wang, P. The role of hepatic cytochrome p-450 in sepsis. Int. J. Clin. Exp. Med. 2009, 2, 203–211. [Google Scholar] [PubMed]
- Abdel-Razzak, Z.; Loyer, P.; Fautrel, A.; Gautier, J.C.; Corcos, L.; Turlin, B.; Beaune, P.; Guillouzo, A. Cytokines downregulate expression of major cytochrome p-450 enzymes in adult human hepatocytes in primary culture. Mol. Pharmacol. 1993, 44, 707–715. [Google Scholar] [PubMed]
- Muntane-Relat, J.; Ourlin, J.C.; Domergue, J.; Maurel, P. Differential effects of cytokines on the inducible expression of cyp1a1, cyp1a2, and cyp3a4 in human hepatocytes in primary culture. Hepatology 1995, 22, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Groger, M.; Rennert, K.; Giszas, B.; Weiss, E.; Dinger, J.; Funke, H.; Kiehntopf, M.; Peters, F.T.; Lupp, A.; Bauer, M.; et al. Monocyte-induced recovery of inflammation-associated hepatocellular dysfunction in a biochip-based human liver model. Sci. Rep. 2016, 6, 21868. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.T. Regulation of cytochrome p450 by inflammatory mediators: Why and how? Drug Metab. Dispos. 2001, 29, 207–212. [Google Scholar] [PubMed]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef] [PubMed]
- Harbrecht, B.G.; Frye, R.F.; Zenati, M.S.; Branch, R.A.; Peitzman, A.B. Cytochrome p-450 activity is differentially altered in severely injured patients. Crit. Care Med. 2005, 33, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Shedlofsky, S.I.; Israel, B.C.; McClain, C.J.; Hill, D.B.; Blouin, R.A. Endotoxin administration to humans inhibits hepatic cytochrome p450-mediated drug metabolism. J. Clin. Investig. 1994, 94, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.S.; Freir, N.M.; Venkatesh, B.; Robertson, T.A.; Roberts, M.S.; Jones, M. A preliminary study of atorvastatin plasma concentrations in critically ill patients with sepsis. Intensive Care Med. 2009, 35, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.A.; Bunck, A.C.; Bockmeyer, C.L.; Brunkhorst, F.M.; Losche, W.; Kinscherf, R.; Deigner, H.P. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J. 2005, 19, 1719–1721. [Google Scholar] [CrossRef] [PubMed]
- Fucho, R.; Martinez, L.; Baulies, A.; Torres, S.; Tarrats, N.; Fernandez, A.; Ribas, V.; Astudillo, A.M.; Balsinde, J.; Garcia-Roves, P.; et al. Asmase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage nonalcoholic steatohepatitis. J. Hepatol. 2014, 61, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.A.; Schenck, M.; Nicolay, J.P.; Becker, J.U.; Kempe, D.S.; Lupescu, A.; Koka, S.; Eisele, K.; Klarl, B.A.; Rubben, H.; et al. Liver cell death and anemia in wilson disease involve acid sphingomyelinase and ceramide. Nat. Med. 2007, 13, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.; Tarrats, N.; Morales, A.; Dominguez, M.; Bataller, R.; Caballeria, J.; Garcia-Ruiz, C.; Fernandez-Checa, J.C.; Mari, M. Acidic sphingomyelinase controls hepatic stellate cell activation and in vivo liver fibrogenesis. Am. J. Pathol. 2010, 177, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Claus, R.A.; Dorer, M.J.; Bunck, A.C.; Deigner, H.P. Inhibition of sphingomyelin hydrolysis: Targeting the lipid mediator ceramide as a key regulator of cellular fate. Curr. Med. Chem. 2009, 16, 1978–2000. [Google Scholar] [CrossRef] [PubMed]
- Goggel, R.; Winoto-Morbach, S.; Vielhaber, G.; Imai, Y.; Lindner, K.; Brade, L.; Brade, H.; Ehlers, S.; Slutsky, A.S.; Schutze, S.; et al. Paf-mediated pulmonary edema: A new role for acid sphingomyelinase and ceramide. Nat. Med. 2004, 10, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kott, M.; Elke, G.; Reinicke, M.; Winoto-Morbach, S.; Schadler, D.; Zick, G.; Frerichs, I.; Weiler, N.; Schutze, S. Acid sphingomyelinase serum activity predicts mortality in intensive care unit patients after systemic inflammation: A prospective cohort study. PLoS ONE 2014, 9, e112323. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Hupe, D.C.; Otto, G.P.; Sprenger, M.; Bunck, A.C.; Dorer, M.J.; Bockmeyer, C.L.; Deigner, H.P.; Graler, M.H.; Claus, R.A. Acid sphingomyelinase promotes endothelial stress response in systemic inflammation and sepsis. Mol. Med. 2016, 22, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, C.; Kadow, S.; Henry, B.D.; Steinmann, J.; Becker, K.A.; Riehle, A.; Beckmann, N.; Wilker, B.; Li, P.L.; et al. Acid sphingomyelinase inhibition protects mice from lung edema and lethal staphylococcus aureus sepsis. J. Mol. Med. 2015, 93, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Witt, C.J.; Jbeily, N.; Hurtado-Oliveros, J.; Giszas, B.; Lupp, A.; Graler, M.H.; Bruns, T.; Stallmach, A.; Gonnert, F.A.; et al. Acid sphingomyelinase inhibition prevents development of sepsis sequelae in the murine liver. Sci. Rep. 2017, 7, 12348. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Kollmey, A.S.; Schrepper, A.; Kohl, M.; Blass, M.F.; Stehr, S.N.; Lupp, A.; Graler, M.H.; Claus, R.A. Adjustment of dysregulated ceramide metabolism in a murine model of sepsis-induced cardiac dysfunction. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef] [PubMed]
- Goni, F.M.; Alonso, A. Sphingomyelinases: Enzymology and membrane activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Canals, D.; Idkowiak-Baldys, J.; Simbari, F.; Roddy, P.; Perry, D.M.; Kitatani, K.; Luberto, C.; Hannun, Y.A. Regulated secretion of acid sphingomyelinase: Implications for selectivity of ceramide formation. J. Biol. Chem. 2010, 285, 35706–35718. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, H. Sepsis and septic shock: Pathogenesis and treatment perspectives. J. Crit. Care 2017, 40, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Hoffmann, J.N.; Juers, M.; Ostermann, H.; Kienast, J.; Briegel, J.; Strauss, R.; Keinecke, H.O.; Warren, B.L.; Opal, S.M.; et al. High-dose antithrombin iii in the treatment of severe sepsis in patients with a high risk of death: Efficacy and safety. Crit. Care Med. 2006, 34, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Marti-Carvajal, A.J.; Sola, I.; Gluud, C.; Lathyris, D.; Cardona, A.F. Human recombinant protein c for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst. Rev. 2012, 12, CD004388. [Google Scholar] [CrossRef] [PubMed]
- Dhainaut, J.F.; Marin, N.; Mignon, A.; Vinsonneau, C. Hepatic response to sepsis: Interaction between coagulation and inflammatory processes. Crit. Care Med. 2001, 29, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, P.; Gonnert, F.A.; Westermann, M.; Lambeck, S.; Lupp, A.; Rudiger, A.; Dyson, A.; Carre, J.E.; Kortgen, A.; Krafft, C.; et al. Liver dysfunction and phosphatidylinositol-3-kinase signalling in early sepsis: Experimental studies in rodent models of peritonitis. PLoS Med. 2012, 9, e1001338. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Tacke, F.; Koch, A.; Trautwein, C. Liver—Guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Carcillo, J.A.; Korzekwa, K.R.; Jones, G.S.; Parise, R.A.; Gillespie, D.G.; Whalen, M.J.; Kochanek, P.M.; Branch, R.A.; Kost, C.K., Jr. The cytochrome p450 suicide inhibitor, 1-aminobenzotriazole, sensitizes rats to zymosan-induced toxicity. Res. Commun. Mol. Pathol. Pharmacol. 1998, 102, 57–68. [Google Scholar] [PubMed]
- Nyholm, T.K.; Grandell, P.M.; Westerlund, B.; Slotte, J.P. Sterol affinity for bilayer membranes is affected by their ceramide content and the ceramide chain length. Biochim. Biophys. Acta 2010, 1798, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Chiantia, S.; Kahya, N.; Schwille, P. Raft domain reorganization driven by short- and long-chain ceramide: A combined AFM and FCS study. Langmuir 2007, 23, 7659–7665. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Grassme, H.; Cremesti, A.; Zager, J.; Fong, Y.; Haimovitz-Friedman, A.; Fuks, Z.; Gulbins, E.; Kolesnick, R. Natural ceramide reverses Fas resistance of acid sphingomyelinase−/− hepatocytes. J. Biol. Chem. 2001, 276, 8297–8305. [Google Scholar] [CrossRef] [PubMed]
- Grassme, H.; Jekle, A.; Riehle, A.; Schwarz, H.; Berger, J.; Sandhoff, K.; Kolesnick, R.; Gulbins, E. Cd95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001, 276, 20589–20596. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, K.; Erlich, S.; Perl, D.P.; Ferlinz, K.; Bisgaier, C.L.; Sandhoff, K.; Desnick, R.J.; Stewart, C.L.; Schuchman, E.H. Acid sphingomyelinase deficient mice: A model of types a and b niemann-pick disease. Nat. Genet. 1995, 10, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nikolova-Karakashian, M.; Merrill, A.H., Jr.; Morgan, E.T. Regulation of cytochrome p450 2c11 (cyp2c11) gene expression by interleukin-1, sphingomyelin hydrolysis, and ceramides in rat hepatocytes. J. Biol. Chem. 1995, 270, 25233–25238. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, C.; Colell, A.; Mari, M.; Morales, A.; Calvo, M.; Enrich, C.; Fernandez-Checa, J.C. Defective TNF-α-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J. Clin. Investig. 2003, 111, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Press, A.T.; Trauner, M. The liver in sepsis: Patterns of response and injury. Curr. Opin. Crit. Care 2013, 19, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Fernandez de Gatta, M.; Martin-Suarez, A.; Lanao, J.M. Approaches for dosage individualisation in critically ill patients. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.M.; Roberts, J.A.; Lipman, J. Pharmacokinetics and pharmacodynamics in critically ill patients. Curr. Opin. Anaesthesiol. 2010, 23, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Muehlbacher, M.; Trapp, S.; Pechmann, S.; Friedl, A.; Reichel, M.; Muhle, C.; Terfloth, L.; Groemer, T.W.; Spitzer, G.M.; et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE 2011, 6, e23852. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.L., 3rd; Rice, T.C.; Xia, B.T.; Boone, K.I.; Green, E.A.; Gulbins, E.; Caldwell, C.C. Amitriptyline usage exacerbates the immune suppression following burn injury. Shock 2016, 46, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Roumestan, C.; Michel, A.; Bichon, F.; Portet, K.; Detoc, M.; Henriquet, C.; Jaffuel, D.; Mathieu, M. Anti-inflammatory properties of desipramine and fluoxetine. Respir. Res. 2007, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Horn, A.; Duckers, H.; Yagmur, E.; Sanson, E.; Bruensing, J.; Buendgens, L.; Voigt, S.; Trautwein, C.; Tacke, F. Increased liver stiffness denotes hepatic dysfunction and mortality risk in critically ill non-cirrhotic patients at a medical ICU. Crit. Care 2011, 15, R266. [Google Scholar] [CrossRef] [PubMed]
- Gonnert, F.A.; Kunisch, E.; Gajda, M.; Lambeck, S.; Weber, M.; Claus, R.A.; Bauer, M.; Kinne, R.W. Hepatic fibrosis in a long-term murine model of sepsis. Shock 2012, 37, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Gonnert, F.A.; Recknagel, P.; Seidel, M.; Jbeily, N.; Dahlke, K.; Bockmeyer, C.L.; Winning, J.; Losche, W.; Claus, R.A.; Bauer, M. Characteristics of clinical sepsis reflected in a reliable and reproducible rodent sepsis model. J. Surg. Res. 2011, 170, e123–e134. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Asaka, Y.; Kanamura, S. Postnatal development and sublobular distribution of cytochrome p-450 in rat liver: A microphotometric study. J. Histochem. Cytochem. 1993, 41, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Klinger, W. Developmental pharmacology and toxicology: Biotransformation of drugs and other xenobiotics during postnatal development. Eur. J. Drug. Metab. Pharmacokinet. 2005, 30, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Waxman, D.J.; Dannan, G.A.; Guengerich, F.P. Regulation of rat hepatic cytochrome p-450: Age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry 1985, 24, 4409–4417. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.U.; Oh, S.J.; Oh, J.M.; Kang, K.W.; Myung, C.S.; Song, G.Y.; Kim, B.H.; Kim, S.K. Age-related changes in hepatic expression and activity of cytochrome p450 in male rats. Arch. Toxicol. 2010, 84, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.C.; Kim, H.C.; Oh, S.J.; Kim, S.K. Effects of age increase on hepatic expression and activity of cytochrome p450 in male C57BL/6 mice. Arch. Pharm. Res. 2015, 38, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Graler, M.H. Quantification of sphingosine-1-phosphate and related sphingolipids by liquid chromatography coupled to tandem mass spectrometry. Methods Mol. Biol. 2012, 874, 33–44. [Google Scholar] [PubMed]
- Sieber, M.W.; Recknagel, P.; Glaser, F.; Witte, O.W.; Bauer, M.; Claus, R.A.; Frahm, C. Substantial performance discrepancies among commercially available kits for reverse transcription quantitative polymerase chain reaction: A systematic comparative investigator-driven approach. Anal. Biochem. 2010, 401, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kaemmerer, D.; Sanger, J.; Arsenic, R.; D’Haese, J.G.; Neumann, J.; Schmitt-Graeff, A.; Wirtz, R.M.; Schulz, S.; Lupp, A. Evaluation of somatostatin, CXCR4 chemokine and endothelin a receptor expression in a large set of paragangliomas. Oncotarget 2017, 8, 89958–89969. [Google Scholar] [CrossRef] [PubMed]
- Lubet, R.A.; Mayer, R.T.; Cameron, J.W.; Nims, R.W.; Burke, M.D.; Wolff, T.; Guengerich, F.P. Dealkylation of pentoxyresorufin: A rapid and sensitive assay for measuring induction of cytochrome(s) p-450 by phenobarbital and other xenobiotics in the rat. Arch. Biochem. Biophys. 1985, 238, 43–48. [Google Scholar] [CrossRef]
- Aitio, A. A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal. Biochem. 1978, 85, 488–491. [Google Scholar] [CrossRef]
- Pohl, R.J.; Fouts, J.R. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal. Biochem. 1980, 107, 150–155. [Google Scholar] [CrossRef]
- Kleeberg, U.; Klinger, W. Sensitive formaldehyde determination with Nash’s reagent and a ‘tryptophan reaction’. J. Pharmacol. Methods 1982, 8, 19–31. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-Y.; Witt, C.J.; Hurtado-Oliveros, J.; Wickel, J.; Gräler, M.H.; Lupp, A.; Claus, R.A. Acid Sphingomyelinase Inhibition Stabilizes Hepatic Ceramide Content and Improves Hepatic Biotransformation Capacity in a Murine Model of Polymicrobial Sepsis. Int. J. Mol. Sci. 2018, 19, 3163. https://doi.org/10.3390/ijms19103163
Chung H-Y, Witt CJ, Hurtado-Oliveros J, Wickel J, Gräler MH, Lupp A, Claus RA. Acid Sphingomyelinase Inhibition Stabilizes Hepatic Ceramide Content and Improves Hepatic Biotransformation Capacity in a Murine Model of Polymicrobial Sepsis. International Journal of Molecular Sciences. 2018; 19(10):3163. https://doi.org/10.3390/ijms19103163
Chicago/Turabian StyleChung, Ha-Yeun, C. Julius Witt, Jorge Hurtado-Oliveros, Jonathan Wickel, Markus H. Gräler, Amelie Lupp, and Ralf A. Claus. 2018. "Acid Sphingomyelinase Inhibition Stabilizes Hepatic Ceramide Content and Improves Hepatic Biotransformation Capacity in a Murine Model of Polymicrobial Sepsis" International Journal of Molecular Sciences 19, no. 10: 3163. https://doi.org/10.3390/ijms19103163
APA StyleChung, H.-Y., Witt, C. J., Hurtado-Oliveros, J., Wickel, J., Gräler, M. H., Lupp, A., & Claus, R. A. (2018). Acid Sphingomyelinase Inhibition Stabilizes Hepatic Ceramide Content and Improves Hepatic Biotransformation Capacity in a Murine Model of Polymicrobial Sepsis. International Journal of Molecular Sciences, 19(10), 3163. https://doi.org/10.3390/ijms19103163





