Hormone Replacement Therapy: Would it be Possible to Replicate a Functional Ovary?
Abstract
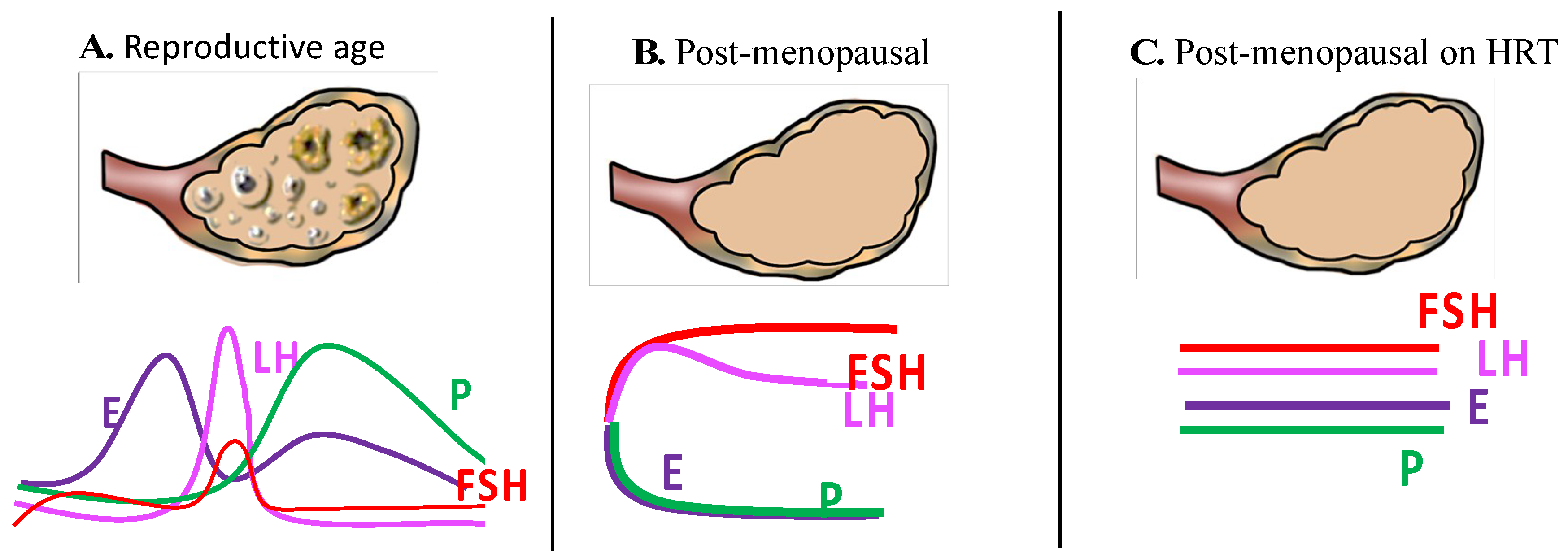
1. Background
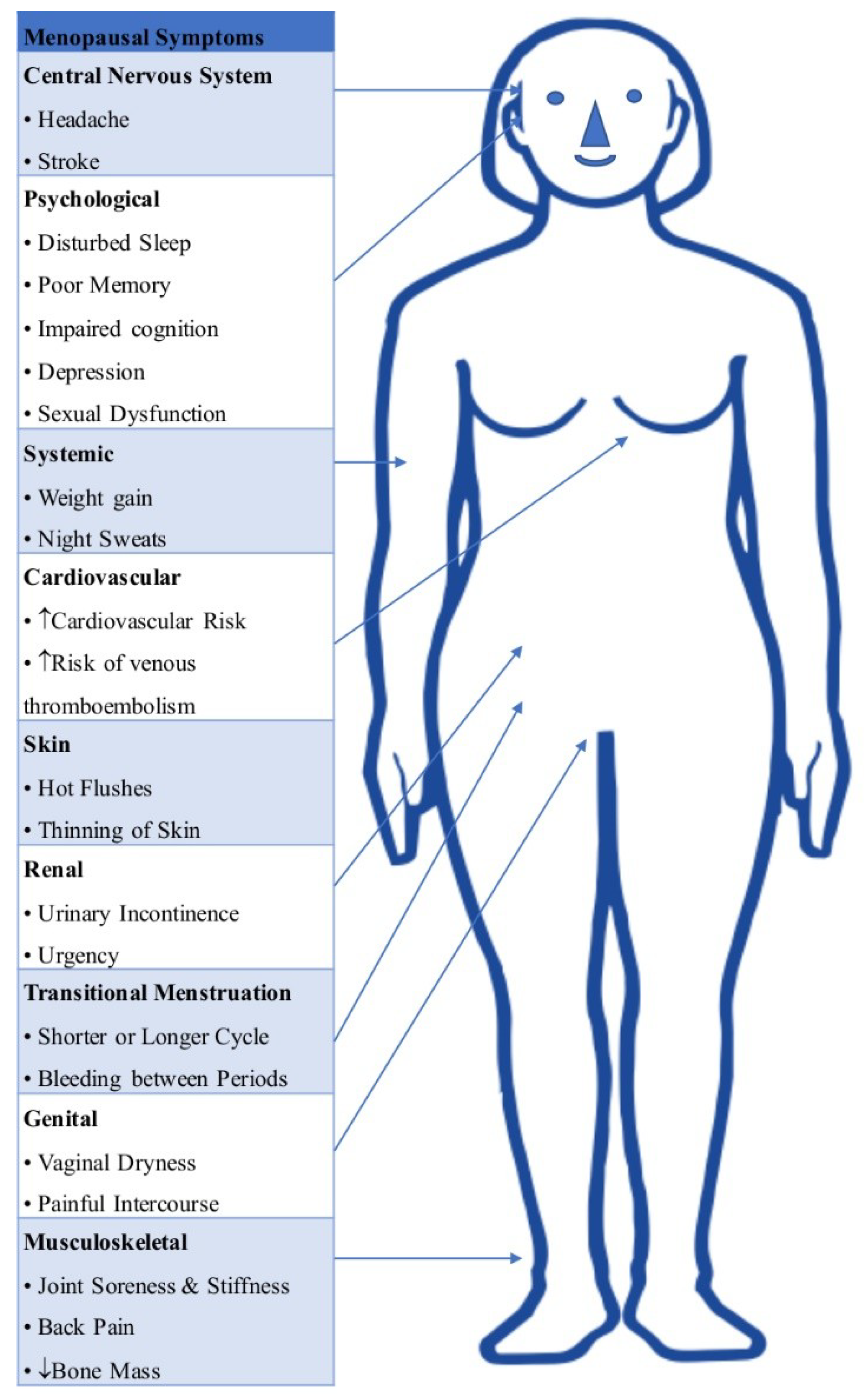
2. Health Concerns with Menopause/Need for HRT
2.1. Coronary Artery Disease
2.2. Metabolic Effects
2.3. Osteoporosis
2.4. Vasomotor Symptoms (VMS)
2.5. Vulvovaginal Atrophy (VVA)
2.6. Cognition and Dementia
2.7. Sleep Disturbances
2.8. Overall Effects of HRT
3. Current Preparations of HRT in Clinical Practice
Bio-Identical HRT (BHRT)
4. Adverse Effects of HRT
4.1. Venous Thromboembolism (VTE) and Stroke
4.2. Gynaecological Cancers
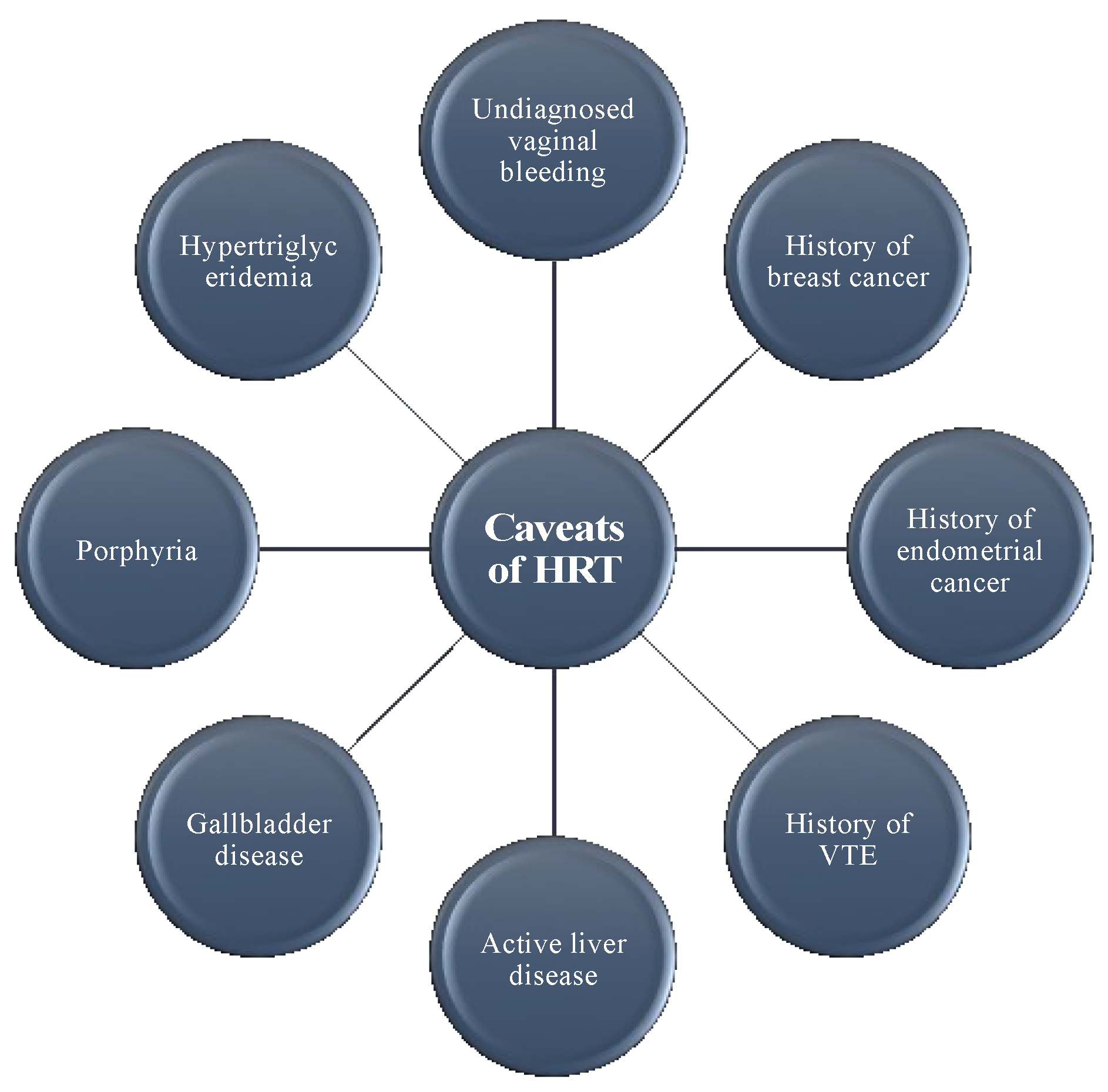
5. Caveats with HRT
6. The Role of Other Hormones Secreted by the Ovary
6.1. Androgens
6.2. Anti-Müllerian Hormone
6.3. Inhibin
6.4. Insulin-Like Growth Factor
6.5. Relaxin
7. Limitations of Current HRT
8. Emerging Opportunities
8.1. Cell-Based Hormone Delivery
8.2. Brain-Selective Oestrogen Therapy
8.3. Ovarian Tissue Transplantation
9. Conclusion
Funding
Conflicts of Interest
References
- Jameson, J.L.; de Kretser, D.M.; Marshall, J.C.; De Groot, L.J. Endocrinology Adult and Pediatric: Reproductive Endocrinology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 978-0-32-322152-8. [Google Scholar]
- Jacobsen, B.K.; Heuch, I.; Kvale, G. Age at natural menopause and all-cause mortality: A 37-year follow-up of 19,731 Norwegian women. Am. J. Epidemiol. 2003, 157, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Ossewaarde, M.E.; Bots, M.L.; Verbeek, A.L.M.; Peeters, P.H.M.; van der Graaf, Y.; Grobbee, D.E.; van der Schouw, Y.T. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 2005, 16, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.B. The Timing of the Age at which Natural Menopause Occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [PubMed]
- De Kleijn, M.J.J.; van der Schouw, Y.T.; Verbeek, A.L.M.; Peeters, P.H.M.; Banga, J.-D.; van der Graaf, Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am. J. Epidemiol. 2002, 155, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Iso, H.; Toyoshima, H.; Date, C.; Yamamoto, A.; Kikuchi, S.; Kondo, T.; Watanabe, Y.; Koizumi, A.; Inaba, Y.; et al. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: The JACC study. J. Epidemiol. 2006, 16, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Atsma, F.; Bartelink, M.-L.E.L.; Grobbee, D.E.; van der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Lisabeth, L.D.; Beiser, A.S.; Brown, D.L.; Murabito, J.M.; Kelly-Hayes, M.; Wolf, P.A. Age at natural menopause and risk of ischemic stroke: The Framingham heart study. Stroke 2009, 40, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Parashar, S.; Reid, K.J.; Spertus, J.A.; Shaw, L.J.; Vaccarino, V. Early menopause predicts angina after myocardial infarction. Menopause 2010, 17, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Bidoli, E.; Franceschi, S.; Schinella, D.; Tesio, F.; La Vecchia, C.; Zecchin, R. Menopause, menstrual and reproductive history, and bone density in northern Italy. J. Epidemiol. Community Health 1996, 50, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Van Der Voort, D.J.M.; van Der Weijer, P.H.M.; Barentsen, R. Early menopause: Increased fracture risk at older age. Osteoporos. Int. 2003, 14, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Monninkhof, E.M.; van der Schouw, Y.T.; Peeters, P.H. Early age at menopause and breast cancer: Are leaner women more protected? A prospective analysis of the Dutch DOM cohort. Breast Cancer Res. Treat. 1999, 55, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; La Vecchia, C.; Booth, M.; Tzonou, A.; Negri, E.; Parazzini, F.; Trichopoulos, D.; Beral, V. Pooled analysis of 3 European case-control studies of ovarian cancer: II. Age at menarche and at menopause. Int. J. Cancer 1991, 49, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-H.; Xiang, Y.-B.; Ruan, Z.-X.; Zheng, W.; Cheng, J.-R.; Dai, Q.; Gao, Y.-T.; Shu, X.-O. Menstrual and reproductive factors and endometrial cancer risk: Results from a population-based case-control study in urban Shanghai. Int. J. Cancer 2004, 108, 613–619. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Statistics 2009; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Felty, Q. Estrogen-induced DNA synthesis in vascular endothelial cells is mediated by ROS signaling. BMC Cardiovasc. Disord. 2006, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Li, H.; Durand, J.; Oparil, S.; Chen, Y.F. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation 1996, 93, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Hsia, J.; Johnson, K.C.; Rossouw, J.E.; Assaf, A.R.; Lasser, N.L.; Trevisan, M.; Black, H.R.; Heckbert, S.R.; Detrano, R.; et al. Estrogen plus progestin and the risk of coronary heart disease. N. Engl. J. Med. 2003, 349, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.; Furberg, C.; Barrett-Connor, E.; Cauley, J.; Grady, D.; Haskell, W.; Knopp, R.; Lowery, M.; Satterfield, S.; Schrott, H.; et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. RAndomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Allison, M.A.; Rossouw, J.E.; Carr, J.J.; Langer, R.D.; Hsia, J.; Kuller, L.H.; Cochrane, B.B.; Hunt, J.R.; Ludlam, S.E.; et al. Estrogen therapy and coronary-artery calcification. N. Engl. J. Med. 2007, 356, 2591–2602. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Ceda, G.P.; Lauretani, F.; Bandinelli, S.; Ruggiero, C.; Guralnik, J.M.; Metter, E.J.; Ling, S.M.; Paolisso, G.; Valenti, G.; et al. Relationship between higher estradiol levels and 9-year mortality in older women: The Invecchiare in Chianti study. J. Am. Geriatr. Soc. 2009, 57, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Schierbeck, L.L.; Rejnmark, L.; Tofteng, C.L.; Stilgren, L.; Eiken, P.; Mosekilde, L.; Køber, L.; Jensen, J.-E.B. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: Randomised trial. BMJ 2012, 345, e6409. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.C.; Kelleher, J.; Lloyd-Jones, H.; Slack, M.; Schofiel, P.M. A study of hormone replacement therapy in postmenopausal women with ischaemic heart disease: The Papworth HRT atherosclerosis study. BJOG 2002, 109, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.F.; Sheppard, B.L.; Norris, L.A. Haemostatic activation in post-menopausal women taking low-dose hormone therapy: Less effect with transdermal administration? Thromb. Haemostasis 2007, 97, 558–565. [Google Scholar] [CrossRef]
- Hsia, J.; Criqui, M.H.; Rodabough, R.J.; Langer, R.D.; Resnick, H.E.; Phillips, L.S.; Allison, M.; Bonds, D.E.; Masaki, K.; Caralis, P.; et al. Estrogen plus progestin and the risk of peripheral arterial disease: The Women’s Health Initiative. Circulation 2004, 109, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Chedraui, P.; Hidalgo, L.; Chavez, D.; Morocho, N.; Alvarado, M.; Huc, A. Quality of life among postmenopausal Ecuadorian women participating in a metabolic syndrome screening program. Maturitas 2007, 56, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Gurka, M.J.; Vishnu, A.; Santen, R.J.; DeBoer, M.D. Progression of Metabolic Syndrome Severity during the Menopausal Transition. J. Am. Heart Assoc. 2016, 5, e003609. [Google Scholar] [CrossRef] [PubMed]
- Ben Ali, S.; Belfki-Benali, H.; Aounallah-Skhiri, H.; Traissac, P.; Maire, B.; Delpeuch, F.; Achour, N.; Ben Romdhane, H. Menopause and metabolic syndrome in tunisian women. BioMed Res. Int. 2014, 2014, 457131. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Kannel, W.B.; Silbershatz, H.; D’Agostino, R.B. Clustering of metabolic factors and coronary heart disease. Arch. Intern. Med. 1999, 159, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.T.; LaRosa, J.; Barnabei, V.; Kessler, C.; Levin, G.; Smith-Roth, A.; Griffin, M.; Stoy, D.B.; Bush, T.; Zacur, H.; et al. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA 1995, 273, 199. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Lin, F.; Grady, D.; Bittner, V.; Cauley, J.A.; Barrett-Connor, E. Glycemic effects of postmenopausal hormone therapy: The Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2003, 138, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.L.; Bonds, D.E.; Rodabough, R.J.; Tinker, L.; Phillips, L.S.; Allen, C.; Bassford, T.; Burke, G.; Torrens, J.; Howard, B. V Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004, 47, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.; Toraldo, G.; Weitzmann, M.N.; Roggia, C.; Gao, Y.; Qian, W.P.; Sierra, O.; Pacifici, R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc. Natl. Acad. Sci. USA 2003, 100, 10405–10410. [Google Scholar] [CrossRef] [PubMed]
- Ho-Pham, L.T.; Nguyen, N.D.; Vu, B.Q.; Pham, H.N.; Nguyen, T.V. Prevalence and risk factors of radiographic vertebral fracture in postmenopausal Vietnamese women. Bone 2009, 45, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.; Robbins, J.; Chen, Z.; Al, E. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The women’s health initiative randomized trial. JAMA 2003, 290, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Pina, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: A guideline from the american heart association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef] [PubMed]
- ACOG. ACOG Practice Bulletin No. 141: Management of menopausal symptoms. Obstet. Gynecol. 2014, 123, 202–216. [Google Scholar] [CrossRef] [PubMed]
- NAMS. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017, 24, 728–753. [Google Scholar] [CrossRef] [PubMed]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.J.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Hormone Therapy for the Primary Prevention of Chronic Conditions in Postmenopausal Women: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 318, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Hot Flashes: Epidemiology and Physiologya. Ann. N. Y. Acad. Sci. 1990, 592, 52–86. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Sanders, R.J. Risk of long-term hot flashes after natural menopause: Evidence from the Penn Ovarian Aging Study cohort. Menopause 2014, 21, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Avis, N.E.; Crawford, S.L.; Greendale, G.; Bromberger, J.T.; Everson-Rose, S.A.; Gold, E.B.; Hess, R.; Joffe, H.; Kravitz, H.M.; Tepper, P.G.; et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern. Med. 2015, 175, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.C.; Matthews, K.A.; Chang, Y.; Santoro, N.; Barinas-Mitchell, E.; von Känel, R.; Landsittel, D.P.; Jennings, J.R. Changes in heart rate variability during vasomotor symptoms among midlife women. Menopause 2016, 23, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.C.; Johnson, B.D.; Shufelt, C.L.; Braunstein, G.D.; Berga, S.L.; Stanczyk, F.Z.; Pepine, C.J.; Bittner, V.; Reis, S.E.; Thompson, D.V.; et al. Menopausal symptoms and cardiovascular disease mortality in the Women’s Ischemia Syndrome Evaluation (WISE). Menopause 2017, 24, 126–132. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, A.; Lester, S.; Moore, V. Oral oestrogen replacement therapy versus placebo for hot flushes. Cochrane Database Syst. Rev. 2001, CD002978. [Google Scholar] [CrossRef]
- Abdi, F.; Mobedi, H.; Mosaffa, N.; Dolatian, M.; Ramezani Tehrani, F. Hormone Therapy for Relieving Postmenopausal Vasomotor Symptoms: A Systematic Review. Arch. Iran. Med. 2016, 19, 141–146. [Google Scholar] [PubMed]
- Dennerstein, L.; Dudley, E.C.; Hopper, J.L.; Guthrie, J.R.; Burger, H.G. A prospective population-based study of menopausal symptoms. Obstet. Gynecol. 2000, 96, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Suckling, J.; Lethaby, A.; Kennedy, R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database of Syst. Rev. 2006, CD001500. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists Women’s Health Care Physicians Sexual dysfunction. Obstet. Gynecol. 2004, 104, 85S–91S. [CrossRef]
- The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013, 20, 884–888. [Google Scholar] [CrossRef]
- Crandall, C.J.; Hovey, K.M.; Andrews, C.A.; Chlebowski, R.T.; Stefanick, M.L.; Lane, D.S.; Shifren, J.; Chen, C.; Kaunitz, A.M.; Cauley, J.A.; et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause 2018, 25, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Allred, D.C.; Ardoin, S.P.; Archer, D.F.; Boyd, N.; Braunstein, G.D.; Burger, H.G.; Colditz, G.A.; Davis, S.R.; Gambacciani, M.; et al. Postmenopausal hormone therapy: An Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2010, 95, s1–s66. [Google Scholar] [CrossRef] [PubMed]
- Hannestad, Y.S.; Rortveit, G.; Sandvik, H.; Hunskaar, S. A community-based epidemiological survey of female urinary incontinence:: The Norwegian EPINCONT Study. J. Clin. Epidemiol. 2000, 53, 1150–1157. [Google Scholar] [CrossRef]
- Ouslander, J.G.; Greendale, G.A.; Uman, G.; Lee, C.; Paul, W.; Schnelle, J. Effects of oral estrogen and progestin on the lower urinary tract among female nursing home residents. J. Am. Geriatr. Soc. 2001, 49, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Shepherd, A.; Brookes, S.; Abrams, P. The effect of oestrogen supplementation on post-menopausal urinary stress incontinence: A double-blind placebo-controlled trial. Br. J. Obstet. Gynaecol. 1999, 106, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.; Rekers, H.; Tapp, A.; Barnick, C.; Shepherd, A.; Schussler, B.; Kerr-Wilson, R.; van Geelan, J.; Barlebo, H.; Walter, S. Oestriol in the treatment of postmenopausal urgency: A multicentre study. Maturitas 1993, 18, 47–53. [Google Scholar] [CrossRef]
- Grady, D.; Applegate, W.; Bush, T.; Furberg, C.; Riggs, B.; Hulley, S.B. Heart and Estrogen/progestin Replacement Study (HERS): Design, methods, and baseline characteristics. Control. Clin. Trials 1998, 19, 314–335. [Google Scholar] [CrossRef]
- Paganini-Hill, A.; Henderson, V.W. Estrogen deficiency and risk of Alzheimer’s disease in women. Am. J. Epidemiol. 1994, 140, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Jacobs, D.; Stern, Y.; Marder, K.; Schofield, P.; Gurland, B.; Andrews, H.; Mayeux, R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet 1996, 348, 429–432. [Google Scholar] [CrossRef]
- Manly, J.J.; Merchant, C.A.; Jacobs, D.M.; Small, S.A.; Bell, K.; Ferin, M.; Mayeux, R. Endogenous estrogen levels and Alzheimer’s disease among postmenopausal women. Neurology 2000, 54, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Luine, V.N. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp. Neurol. 1985, 89, 484–490. [Google Scholar] [CrossRef]
- Shao, H.; Breitner, J.C.S.; Whitmer, R.A.; Wang, J.; Hayden, K.; Wengreen, H.; Corcoran, C.; Tschanz, J.; Norton, M.; Munger, R.; et al. Hormone therapy and Alzheimer disease dementia: New findings from the Cache County Study. Neurology 2012, 79, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Norbury, R.; Travis, M.J.; Erlandsson, K.; Waddington, W.; Ell, P.J.; Murphy, D.G.M. Estrogen therapy and brain muscarinic receptor density in healthy females: A SPET study. Horm. Behav. 2007, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M. Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause 2013, 20, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, S.A.; Legault, C.; Rapp, S.R.; Thal, L.; Wallace, R.B.; Ockene, J.K.; Hendrix, S.L.; Jones, B.N., 3rd; Assaf, A.R.; Jackson, R.D.; et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA 2003, 289, 2651–2662. [Google Scholar] [CrossRef] [PubMed]
- Rapp, S.R.; Espeland, M.A.; Shumaker, S.A.; Henderson, V.W.; Brunner, R.L.; Manson, J.E.; Gass, M.L.S.; Stefanick, M.L.; Lane, D.S.; Hays, J.; et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA 2003, 289, 2663–2672. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.H.S.; Geist, C.L.; Kenna, H.A.; Williams, K.; Wroolie, T.; Powers, B.; Brooks, J.; Rasgon, N.L. Differences in regional brain metabolism associated with specific formulations of hormone therapy in postmenopausal women at risk for AD. Psychoneuroendocrinology 2011, 36, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Hogervorst, E.; Richards, M.; Yesufu, A.; Yaffe, K. Hormone replacement therapy for cognitive function in postmenopausal women. Cochrane Database Syst. Rev. 2008, CD003122. [Google Scholar] [CrossRef] [PubMed]
- Resnick, S.M.; Maki, P.M.; Rapp, S.R.; Espeland, M.A.; Brunner, R.; Coker, L.H.; Granek, I.A.; Hogan, P.; Ockene, J.K.; Shumaker, S.A. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J. Clin. Endocrinol. Metab. 2006, 91, 1802–1810. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.F.; Sun, D.M.; Shao, H.F.; Li, C.B.; Teng, Y.C. Poor sleep in middle-aged women is not associated with menopause per se. Braz. J. Med. Biol. Res. 2016, 49, e4718. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, D.; Goldin, J.; Hickey, M. Sleep disturbance in menopause. Intern. Med. J. 2012, 42, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Prairie, B.A.; Wisniewski, S.R.; Luther, J.; Hess, R.; Thurston, R.C.; Wisner, K.L.; Bromberger, J.T. Symptoms of depressed mood, disturbed sleep, and sexual problems in midlife women: Cross-sectional data from the Study of Women’s Health Across the Nation. J. Women’s Health 2015, 24, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Reed-Kane, D. Natural hormone replacement therapy: What it is and what consumers really want. Int. J. Pharm. Compd. 2001, 5, 332–335. [Google Scholar] [PubMed]
- Hankinson, S.E.; Willett, W.C.; Manson, J.E.; Colditz, G.A.; Hunter, D.J.; Spiegelman, D.; Barbieri, R.L.; Speizer, F.E. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 1998, 90, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Key, T.; Appleby, P.; Barnes, I.; Reeves, G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002, 94, 606–616. [Google Scholar] [PubMed]
- Lippert, C.; Seeger, H.; Mueck, A.O. The effect of endogenous estradiol metabolites on the proliferation of human breast cancer cells. Life Sci. 2003, 72, 877–883. [Google Scholar] [CrossRef]
- Gleason, C.E.; Dowling, N.M.; Wharton, W.; Manson, J.A.E.; Miller, V.M.; Atwood, C.S.; Brinton, E.A.; Cedars, M.I.; Lobo, R.A.; Merriam, G.R.; et al. Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS–Cognitive and Affective Study. PLoS Med. 2015, 12, e1001833. [Google Scholar] [CrossRef] [PubMed]
- Cintron, D.; Lipford, M.; Larrea-Mantilla, L.; Spencer-Bonilla, G.; Lloyd, R.; Gionfriddo, M.R.; Gunjal, S.; Farrell, A.M.; Miller, V.M.; Murad, M.H. Efficacy of menopausal hormone therapy on sleep quality: Systematic review and meta-analysis. Endocrine 2017, 55, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Al-Safi, Z.A.; Santoro, N. Menopausal hormone therapy and menopausal symptoms. Fertil. Steril. 2018, 101, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Høibraaten, E.; Abdelnoor, M.; Sandset, P.M. Hormone Replacement Therapy with Estradiol and Risk of Venous Thromboembolism A Population-based Case-control Study. Thromb. Haemost. 1999, 82, 1218–1221. [Google Scholar] [PubMed]
- Canonico, M.; Oger, E.; Plu-Bureau, G.; Conard, J.; Meyer, G.; Levesque, H.; Trillot, N.; Barrellier, M.-T.; Wahl, D.; Emmerich, J.; et al. Hormone therapy and venous thromboembolism among postmenopausal women: Impact of the route of estrogen administration and progestogens: The ESTHER study. Circulation 2007, 115, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Canonico, M.; Fournier, A.; Carcaillon, L.; Olie, V.; Plu-Bureau, G.; Oger, E.; Mesrine, S.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Scarabin, P.-Y. Postmenopausal hormone therapy and risk of idiopathic venous thromboembolism: Results from the E3N cohort study. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Hsia, J.; Cauley, J.A.; Richards, C.; Harris, F.; Fong, J.; Barrett-Connor, E.; Hulley, S.B. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen-progestin Replacement Study (HERS). Circulation 2001, 103, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.W.; Gray, L.J. Association between hormone replacement therapy and subsequent stroke: A meta-analysis. BMJ 2005, 330, 342. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Manson, J.E.; Stampfer, M.J.; Rexrode, K. Postmenopausal hormone therapy and stroke: Role of time since menopause and age at initiation of hormone therapy. Arch. Intern. Med. 2008, 168, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Renoux, C.; Dell’aniello, S.; Garbe, E.; Suissa, S. Transdermal and oral hormone replacement therapy and the risk of stroke: A nested case-control study. BMJ 2010, 340, c2519. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Chlebowski, R.T.; Rohan, T.E.; Manson, J.E.; Aragaki, A.K.; Kaunitz, A.; Stefanick, M.L.; Simon, M.S.; Johnson, K.C.; Wactawski-Wende, J.; O’sullivan, M.J.; et al. Breast cancer after use of estrogen plus progestin and estrogen alone: Analyses of data from 2 women’s health initiative randomized clinical trials. JAMA Oncol. 2015, 1, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Schoemaker, M.J.; Wright, L.; McFadden, E.; Griffin, J.; Thomas, D.; Hemming, J.; Wright, K.; Ashworth, A.; Swerdlow, A.J. Menopausal hormone therapy and breast cancer: What is the true size of the increased risk? Br. J. Cancer 2016, 115, 607. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.A.; Chia, V.M.; Taylor, A.; O’Malley, C.; Kelsh, M.; Mohamed, M.; Mowat, F.S.; Goff, B. An international assessment of ovarian cancer incidence and mortality. Gynecol. Oncol. 2013, 130, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [CrossRef]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.L.; Judd, H.L.; Kaunitz, A.M.; Barad, D.H.; Beresford, S.A.A.; Pettinger, M.; Liu, J.; McNeeley, S.G.; Lopez, A.M. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: The Women’s Health Initiative randomized trial. JAMA 2003, 290, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.; Gebretsadik, T.; Kerlikowske, K.; Ernster, V.; Petitti, D. Hormone replacement therapy and endometrial cancer risk: A meta-analysis. Obstet. Gynecol. 1995, 85, 304–313. [Google Scholar] [CrossRef]
- Sjögren, L.L.; Mørch, L.S.; Løkkegaard, E. Hormone replacement therapy and the risk of endometrial cancer: A systematic review. Maturitas 2016, 91, 25–35. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, A.H. HRT in difficult circumstances: Are there any absolute contraindications? Climacteric 2011, 14, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Loria, P.; Petraglia, F.; Concari, M.; Bertolotti, M.; Martella, P.; Luisi, S.; Grisolia, C.; Foresta, C.; Volpe, A.; Genazzani, A.R.; et al. Influence of age and sex on serum concentrations of total dimeric activin A. Eur. J. Endocrinol. 1998, 139, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Reame, N.E.; Lukacs, J.L.; Olton, P.; Ansbacher, R.; Padmanabhan, V. Differential Effects of Aging on Activin A and its Binding Protein, Follistatin, across the Menopause Transition. Fertil. Steril. 2007, 88, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Sherwin, B.B.; Alexander, G.M.; Davidson, D.W.; Walker, A. Oral contraceptives, androgens, and the sexuality of young women: II. The role of androgens. Arch. Sex. Behav. 1991, 20, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Appelt, H.; Strauss, B. Effects of antiandrogen treatment on the sexuality of women with hyperandrogenism. Psychother. Psychosom. 1984, 42, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Buster, J.E.; Kingsberg, S.A.; Aguirre, O.; Brown, C.; Breaux, J.G.; Buch, A.; Rodenberg, C.A.; Wekselman, K.; Casson, P. Testosterone patch for low sexual desire in surgically menopausal women: A randomized trial. Obstet. Gynecol. 2005, 105, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Panay, N.; Al-Azzawi, F.; Bouchard, C.; Davis, S.R.; Eden, J.; Lodhi, I.; Rees, M.; Rodenberg, C.A.; Rymer, J.; Schwenkhagen, A.; et al. Testosterone treatment of HSDD in naturally menopausal women: The ADORE study. Climacteric 2010, 13, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Maclaran, K.; Panay, N. Managing low sexual desire in women. Women’s Health 2011, 7, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.A.; Speroff, L. Clinical Gynecologic Endocrinology and Infertility, 8th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; ISBN 978-1-45-114847-3. [Google Scholar]
- MacLaughlin, D.T.; Donahoe, P.K. Müllerian Inhibiting Substance/anti-Müllerian hormone: A potential therapeutic agent for human ovarian and other cancers. Futur. Oncol. 2010, 6, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; MacLaughlin, D.T.; Donahoe, P.K. Mullerian inhibiting substance/anti-Mullerian hormone: A novel treatment for gynecologic tumors. Obstet. Gynecol. Sci. 2014, 57, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Suh, M.J.; Yoon, J.H.; Kim, M.R.; Ryu, K.S.; Nam, S.W.; Donahoe, P.K.; Maclaughlin, D.T.; Kim, J.H. Identification of characteristic molecular signature of Mullerian inhibiting substance in human HPV-related cervical cancer cells. Int. J. Oncol. 2011, 39, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, M.; Farkkila, A.; Tauriala, H.; Kauppinen, M.; MacLaughlin, D.T.; Unkila-Kallio, L.; Butzow, R.; Heikinheimo, M. Anti-Mullerian hormone inhibits growth of AMH type II receptor-positive human ovarian granulosa cell tumor cells by activating apoptosis. Lab. Investig. 2011, 91, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Pepin, D.; Sosulski, A.; Zhang, L.; Wang, D.; Vathipadiekal, V.; Hendren, K.; Coletti, C.M.; Yu, A.; Castro, C.M.; Birrer, M.J.; et al. AAV9 delivering a modified human Mullerian inhibiting substance as a gene therapy in patient-derived xenografts of ovarian cancer. Proc. Nal. Acad. Sci. USA 2015, 112, E4418–E4427. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, T.D.; Pieretti-Vanmarcke, R.; Nicolaou, F.; Thanos, A.; Trichonas, G.; Koufomichali, X.; Anago, K.; Donahoe, P.K.; Teixeira, J.; MacLaughlin, D.T.; et al. Development of an efficiently cleaved, bioactive, highly pure FLAG-tagged recombinant human Mullerian Inhibiting Substance. Protein Expr. Purif. 2010, 70, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Finegold, M.J.; Su, J.G.; Hsueh, A.J.; Bradley, A. Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 1992, 360, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Finegold, M.J.; Mather, J.P.; Krummen, L.; Lu, H.; Bradley, A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc. Nal. Acad. Sci. USA 1994, 91, 8817–8821. [Google Scholar] [CrossRef]
- Pangas, S.A.; Woodruff, T.K. Production and purification of recombinant human inhibin and activin. J. Endocrinol. 2002, 172, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Conti, E.; Musumeci, M.B.; Assenza, G.E.; Quarta, G.; Autore, C.; Volpe, M. Recombinant human insulin-like growth factor-1: A new cardiovascular disease treatment option? Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Saukkonen, T.; Amin, R.; Williams, R.M.; Fox, C.; Yuen, K.C.; White, M.A.; Umpleby, A.M.; Acerini, C.L.; Dunger, D.B. Dose-dependent effects of recombinant human insulin-like growth factor (IGF)-I/IGF binding protein-3 complex on overnight growth hormone secretion and insulin sensitivity in type 1 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Milne, P.; Saleh, T.M. Relaxin pretreatment decreases infarct size in male rats after middle cerebral artery occlusion. Ann. N. Y. Acad. Sci. 2005, 1041, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Connell, B.; Saleh, T.M. Relaxin-induced reduction of infarct size in male rats receiving MCAO is dependent on nitric oxide synthesis and not estrogenic mechanisms. Neurosci. Lett. 2006, 393, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Danielson, L.A.; Conrad, K.P. Time course and dose response of relaxin-mediated renal vasodilation, hyperfiltration, and changes in plasma osmolality in conscious rats. J. Appl. Physiol. 2003, 95, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.P.; Debrah, D.O.; Novak, J.; Danielson, L.A.; Shroff, S.G. Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 2004, 145, 3289–3296. [Google Scholar] [CrossRef] [PubMed]
- Debrah, D.O.; Conrad, K.P.; Danielson, L.A.; Shroff, S.G. Effects of relaxin on systemic arterial hemodynamics and mechanical properties in conscious rats: Sex dependency and dose response. J. Appl. Physiol. 2005, 98, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Unemori, E.N.; Lewis, M.; Constant, J.; Arnold, G.; Grove, B.H.; Normand, J.; Deshpande, U.; Salles, A.; Pickford, L.B.; Erikson, M.E.; et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen. 2000, 8, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Salvemini, D.; Mugnai, L.; Bello, M.G.; Bani, D.; Mannaioni, P.F. The effect of relaxin on myocardial ischaemia-reperfusion injury and histamine release in vitro and in vivo. Inflamm. Res. 1996, 45 (Suppl. 1), S27–S28. [Google Scholar] [CrossRef]
- Masini, E.; Bani, D.; Bello, M.G.; Bigazzi, M.; Mannaioni, P.F.; Sacchi, T.B. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: Evidence for an involvement of nitric oxide. Endocrinology 1997, 138, 4713–4720. [Google Scholar] [CrossRef] [PubMed]
- Bani, D.; Masini, E.; Bello, M.G.; Bigazzi, M.; Sacchi, T.B. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am. J. Pathol. 1998, 152, 1367–1376. [Google Scholar] [PubMed]
- Bani, D.; Bigazzi, M.; Masini, E.; Bani, G.; Sacchi, T.B. Relaxin depresses platelet aggregation: In vitro studies on isolated human and rabbit platelets. Lab. Investig. 1995, 73, 709–716. [Google Scholar] [PubMed]
- Nistri, S.; Chiappini, L.; Sassoli, C.; Bani, D. Relaxin inhibits lipopolysaccharide-induced adhesion of neutrophils to coronary endothelial cells by a nitric oxide-mediated mechanism. FASEB J. 2003, 17, 2109–2111. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Nistri, S.; Vannacci, A.; Bani Sacchi, T.; Novelli, A.; Bani, D. Relaxin inhibits the activation of human neutrophils: Involvement of the nitric oxide pathway. Endocrinology 2004, 145, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Bani, D.; Ballati, L.; Masini, E.; Bigazzi, M.; Sacchi, T.B. Relaxin counteracts asthma-like reaction induced by inhaled antigen in sensitized guinea pigs. Endocrinology 1997, 138, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, I.; Tang, M.L.K.; Solly, N.; Tregear, G.W.; Samuel, C.S. Investigating the role of relaxin in the regulation of airway fibrosis in animal models of acute and chronic allergic airway disease. Ann. N. Y. Acad. Sci. 2005, 1041, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.A.; Sisson, J.H.; Forget, M.A.; Bennett, R.G.; Hamel, F.G.; Spurzem, J.R. Relaxin stimulates bronchial epithelial cell PKA activation, migration, and ciliary beating. Exp. Biol. Med. 2002, 227, 1047–1053. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F.; et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 2013, 381, 29–39. [Google Scholar] [CrossRef]
- Dschietzig, T.B. Recombinant human relaxin-2: (How) can a pregnancy hormone save lives in acute heart failure? Am. J. Cardiovasc. Drugs 2014, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Longcope, C.; Gorbach, S.; Goldin, B.; Woods, M.; Dwyer, J.; Warram, J. The metabolism of estradiol; oral compared to intravenous administration. J. Steroid Biochem. 1985, 23, 1065–1070. [Google Scholar] [CrossRef]
- Serin, I.S.; Ozcelik, B.; Basbug, M.; Aygen, E.; Kula, M.; Erez, R. Long-term effects of continuous oral and transdermal estrogen replacement therapy on sex hormone binding globulin and free testosterone levels. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 99, 222–225. [Google Scholar] [CrossRef]
- Stomati, M.; Hartmann, B.; Spinetti, A.; Mailand, D.; Rubino, S.; Albrecht, A.; Huber, J.; Petraglia, F.; Genazzani, A.R. Effects of hormonal replacement therapy on plasma sex hormone-binding globulin, androgen and insulin-like growth factor-1 levels in postmenopausal women. J. Endocrinol. Investig. 1996, 19, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Krotz, S.P.; Robins, J.C.; Ferruccio, T.-M.; Moore, R.; Steinhoff, M.M.; Morgan, J.R.; Carson, S. In vitro maturation of oocytes via the pre-fabricated self-assembled artificial human ovary. J. Assist. Reprod. Genet. 2010, 27, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Sittadjody, S.; Saul, J.M.; Joo, S.; Yoo, J.J.; Atala, A.; Opara, E.C. Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials 2013, 34, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xia, X.; Miao, W.; Luan, X.; Sun, L.; Jin, Y.; Liu, L. An ovarian cell microcapsule system simulating follicle structure for providing endogenous female hormones. Int. J. Pharm. 2013, 455, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Zhou, J.-L.; Xu, Q.; Lu, X.; Liang, Y.-J.; Weng, J.; Shi, X.-L. Prevention of osteoporosis in mice after ovariectomy via allograft of microencapsulated ovarian cells. Anat. Rec. 2010, 293, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Merchenthaler, I.; Lane, M.; Sabnis, G.; Brodie, A.; Nguyen, V.; Prokai, L.; Prokai-Tatrai, K. Treatment with an orally bioavailable prodrug of 17β-estradiol alleviates hot flushes without hormonal effects in the periphery. Sci. Rep. 2016, 6, 30721. [Google Scholar] [CrossRef] [PubMed]
- Prokai, L.; Nguyen, V.; Szarka, S.; Garg, P.; Sabnis, G.; Bimonte-Nelson, H.A.; McLaughlin, K.J.; Talboom, J.S.; Conrad, C.D.; et al. The prodrug DHED selectively delivers 17β-estradiol to the brain for treating estrogen-responsive disorders. Sci. Transl. Med. 2015, 7, 297ra113. [Google Scholar] [CrossRef] [PubMed]
- Cargill, S.L.; Carey, J.R.; Muller, H.-G.; Anderson, G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell 2003, 2, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B.; Cargill, S.L.; Anderson, G.B.; Carey, J.R. Transplantation of young ovaries to old mice increased life span in transplant recipients. J. Gerontol. Ser. A 2009, 64, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Shikanov, A.; Zhang, Z.; Xu, M.; Smith, R.M.; Rajan, A.; Woodruff, T.K.; Shea, L.D. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. Part A 2011, 17, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng. 2014, 16, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.J. Ovary cryopreservation and transplantation for fertility preservation. Mol. Hum. Reprod. 2012, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Turkcuoglu, I.; Rodriguez-Wallberg, K.A. Four spontaneous pregnancies and three live births following subcutaneous transplantation of frozen banked ovarian tissue: What is the explanation? Fertil. Steril. 2011, 95, 804.e7–804.e10. [Google Scholar] [CrossRef] [PubMed]
| Hormone | Preparation |
|---|---|
| Oral Oestrogen | Conjugated oestrogen Ethinyloestradiol Esterified oestrogens 17β-oestradiol |
| Transdermal Oestrogen | 17β-oestradiol patch 17β-oestradiol gel 17β-oestradiol emulsion 17β-oestradiol spray |
| Vaginal Oestrogen | 17β-oestradiol cream Conjugated oestrogen cream 17β-oestradiol ring 17β-oestradiol tablet |
| Oral Progestogen | Medroxyprogesterone acetate Norethindrone acetate Megestrol acetate Drosperinone Micronised progesterone |
| Transdermal Progestogen | Norethindrone acetate Levonorgoestrel |
| Progestin: Intrauterine System | Levonorgoestrel IUS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, S.; Alzahrani, F.A.; Ahmed, A. Hormone Replacement Therapy: Would it be Possible to Replicate a Functional Ovary? Int. J. Mol. Sci. 2018, 19, 3160. https://doi.org/10.3390/ijms19103160
Agarwal S, Alzahrani FA, Ahmed A. Hormone Replacement Therapy: Would it be Possible to Replicate a Functional Ovary? International Journal of Molecular Sciences. 2018; 19(10):3160. https://doi.org/10.3390/ijms19103160
Chicago/Turabian StyleAgarwal, Swati, Faisal A Alzahrani, and Asif Ahmed. 2018. "Hormone Replacement Therapy: Would it be Possible to Replicate a Functional Ovary?" International Journal of Molecular Sciences 19, no. 10: 3160. https://doi.org/10.3390/ijms19103160
APA StyleAgarwal, S., Alzahrani, F. A., & Ahmed, A. (2018). Hormone Replacement Therapy: Would it be Possible to Replicate a Functional Ovary? International Journal of Molecular Sciences, 19(10), 3160. https://doi.org/10.3390/ijms19103160






