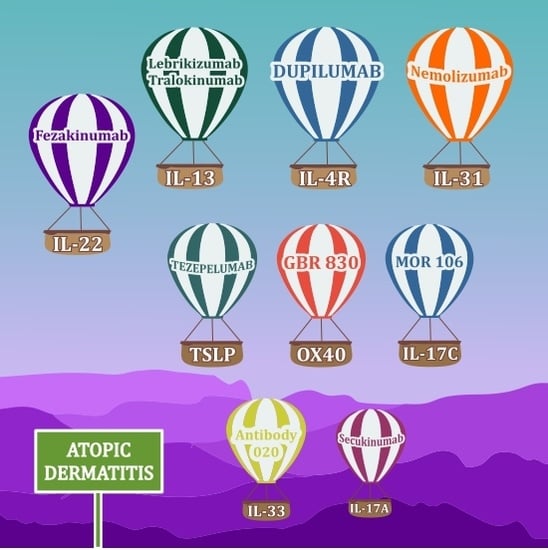
New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets
Abstract
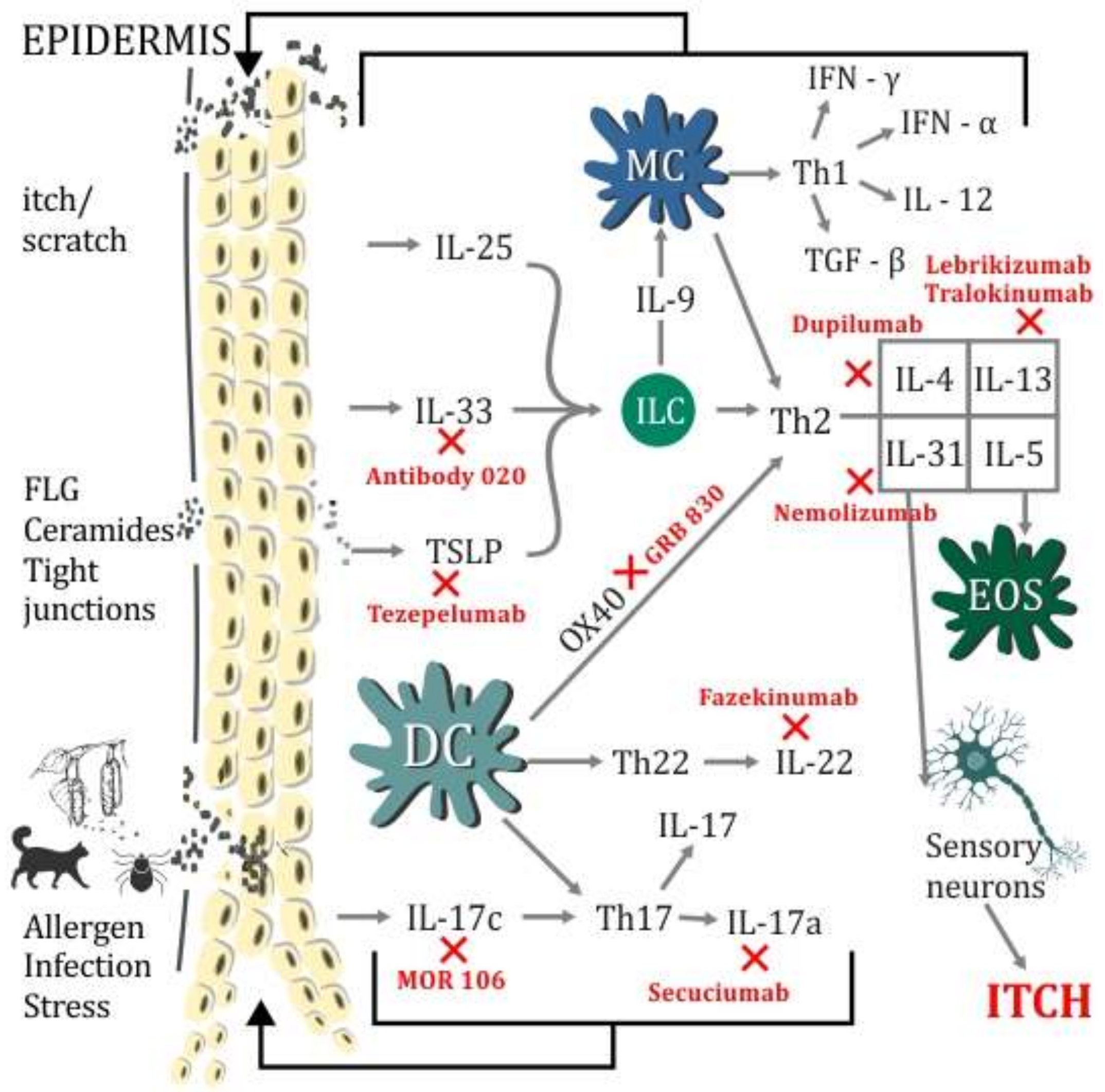
1. Introduction
2. TSLP—Thymic Stromal Lymphopoietin
3. TSLP in AD (Atopic Dermatitis)
4. Anti-TSLP Therapy, Anti OX40
5. Interleukin-33
6. IL-33 in AD
7. Interleukin IL-17
7.1. IL-17 in AD
7.2. Anti-IL-17 Therapy
7.2.1. Secukinumab
7.2.2. MOR106
8. Interleukin-19
9. Conclusions
Funding
Conflicts of Interest
References
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry crosssectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Carroll, C.L.; Balkrishnan, R.; Feldman, S.R.; Fleischer, A.B., Jr.; Manuel, J.C. The burden of atopic dermatitis: Impact on the patient, family, and society. Pediatr. Dermatol. 2005, 22, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Krueger, J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017, 48, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gittler, J.K.; Shemer, A.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Gulewicz, K.J.; Wang, C.Q.; Mitsui, H.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012, 130, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.A.; Kim, S.J.; de Guzman Strong, C.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Czarnowicki, T.; Krueger, J.G.; Guttman-Yassky, E. Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J. Allergy Clin. Immunol. 2017, 139, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Roan, F.; Ziegler, S.F. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Lemanske, R.F., Jr.; Castells, M.; Torres, M.J.; Khan, D.; Simon, H.U.; Bindslev-Jensen, C.; Burks, W.; Poulsen, L.K.; Sampson, H.A.; et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis—PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2016, 137, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Rochman, Y.; Kashyapa, M.; Robinson, G.W.; Sakamoto, K.; Gomez-Rodriguez, J.; Wagner, K.U.; Leonard, W.J. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signalling. Proc. Natl. Acad. Sci. USA 2010, 107, 19455–19460. [Google Scholar] [CrossRef] [PubMed]
- Quentmeier, H.; Drexler, H.G.; Fleckenstein, D.; Zaborski, M.; Armstrong, A.; Sims, J.E.; Lyman, S.D. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia 2001, 15, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Artis, D. Sensing the outside world: TSLP regulates barrier immunity. Nat. Immunol. 2010, 11, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; Krauss, R.H.; Sun, A.C.; Takei, F. Lung natural helper cells are a critical source of Th2 celltype cytokines in protease allergen-induced airway inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.H.; Duramad, O.; Hori, T.; Delespesse, G.J.; Watanabe, N.; Qin, F.X.; Yao, Z.; Cao, W.; Liu, Y.J. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005, 202, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Hu, S.; Cheung, P.F.; Lam, C.W. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2010, 43, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Mjosberg, J.; Bernink, J.; Golebski, K.; Karrich, J.J.; Peters, C.P.; Blom, B.; te Velde, A.A.; Fokkens, W.J.; van Drunen, C.M.; Spits, H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 2012, 37, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Bautista, D.M. Role of transient receptor potential channels in acute and chronic itch. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2014; ISBN 13-978-1-4665-0543-8. [Google Scholar]
- Demehri, S.; Morimoto, M.; Holtzman, M.J.; Kopan, R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009, 7, e1000067. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP produced by keratinocytes promotes allergen sensitization through skin and thereby triggers atopic march in mice. J. Investig. Dermatol. 2013, 133, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hener, P.; Li, J.; Li, M. Skin thymic stromal lymphopoietin promotes airway sensitization to inhalant house dust mites leading to allergic asthma in mice. Allergy 2012, 67, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hener, P.; Frossard, N.; Kato, S.; Metzger, D.; Li, M.; Chambon, P. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc. Natl. Acad. Sci. USA 2009, 106, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Xu, W.; Headley, M.B.; Jessup, H.K.; Lee, K.S.; Omori, M.; Comeau, M.R.; Marshak-Rothstein, A.; Ziegler, S.F. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal. Immunol. 2012, 5, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, A.E.; Sattentau, Q.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Hvid, M.; Johansen, C.; Buchner, M.; Fölster-Holst, R.; Deleuran, M.; Vestergaard, C. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.B.; Kim, K.W.; Hong, J.Y.; Jee, H.M.; Sohn, M.H.; Kim, K.-E. Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr. Allergy Immunol. 2010, 21, e457–e460. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Masuda, K.; Tamagawa-Mineoka, R.; Matsunaka, H.; Murakami, Y.; Yamashita, R.; Morita, E.; Katoh, N. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin. Exp. Immunol. 2013, 171, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.T.; Baba, T.; Chen, X.; Le, T.A.; Kinoshita, H.; Xie, Y.; Kamijo, S.; Hiramatsu, K.; Ikeda, S.; Ogawa, H.; et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J. Allergy Clin. Immunol. 2010, 126, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Dajnoki, Z.; Béke, G.; Mócsai, G.; Kapitány, A.; Gáspár, K.; Hajdu, K.; Emri, G.; Nagy, B.; Kovács, I.; Beke, L.; et al. Immune-mediated skin inflammation is similar in severe atopic dermatitis patients with or without filaggrin mutation. Acta Derm. Venereol. 2016, 96, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Angelova-Fischer, I.; Fernandez, I.M.; Donnadieu, M.H.; Bulfone-Paus, S.; Zillikens, D.; Fischer, T.W.; Soumelis, V. Injury to the stratum corneum induces in vivo expression of human thymic stromal lymphopoietin in the epidermis. J. Investig. Dermatol. 2010, 130, 2505–2507. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Wu, L.S.; Lockett, G.A.; Karmaus, W.J. TSLP polymorphisms, allergen exposures, and the risk of atopic disorders in children. Ann. Allergy Asthma Immunol. 2016, 116, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.S.; Rafaels, N.M.; Mu, D.; Hand, T.; Murray, T.; Boguniewicz, M.; Hata, T.; Schneider, L.; Hanifin, J.M.; Gallo, R.L.; et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J. Allergy Clin. Immunol. 2010, 125, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.; Kim, B.; Apter, A.; Gupta, J.; Hoffstad, O.; Papadopoulos, M.; Mitra, N. Thymic Stromal Lymphopoietin Variation, Filaggrin Loss of Function, and the Persistence of Atopic Dermatitis. JAMA Dermatol. 2014, 150, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Hitsumoto, S.; Tanaka, K.; Arakawa, M. Association between TSLP Polymorphisms and Eczema in Japanese Women: The Kyushu Okinawa Maternal and Child Health Study. Inflammation 2015, 38, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.C.; Yu, A.; Heroux, D.; Akhabir, L.; Sandford, A.J.; Neighbour, H.; Denburg, J.A. Thymic stromal lymphopoietin (TSLP) secretion from human nasal epithelium is a function of TSLP genotype. Mucosal. Immunol. 2015, 8, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Hirota, T.; Jodo, A.I.; Hitomi, Y.; Sakashita, M.; Tsunoda, T.; Miyagawa, T.; Doi, S.; Kameda, M.; Fujita, K.; et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Vestergaard, C.; Deleuran, M. Emerging Treatment Options in Atopic Dermatitis: Systemic Therapies. Dermatology 2017, 233, 344–357. [Google Scholar] [CrossRef] [PubMed]
- A Study of Intravenous MK-8226 in Participants with Moderate-to-Severe Atopic Dermatitis (MK-8226-003). ClinicalTrials.gov Identifier:NCT01732510. Available online: https://clinicaltrials.gov/ct2/show/NCT01732510 (accessed on 3 October 2018).
- Tidwell, J.; Fowler, F., Jr. T-cell inhibitors for atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, S67–S70. [Google Scholar] [CrossRef] [PubMed]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Pichery, M.; Mirey, E.; Mercier, P.; Lefrancais, E.; Dujardin, A.; Ortega, N.; Girard, J.P. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: In situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012, 188, 3488–3495. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Pitman, N.; McInnes, I. Disease-associated functions of IL-33: The new kid in the IL-1 family. Nat. Rev. Immunol. 2010, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, S.; Kawanokuchi, J.; Parajuli, B.; Jin, S.; Doi, Y.; Noda, M.; Sonobe, Y.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Production and functions of IL-33 in the central nervous system. Brain Res. 2011, 1385, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Miller, A. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Tago, K.; Hayakawa, M.; Tominaga, S.; Ohshio, T.; Sonoda, Y.; Kasahara, T. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell. Signal. 2008, 20, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Carriere, V.; Roussel, L.; Ortega, N.; Lacorre, D.A.; Americh, L.; Aguilar, L.; Bouche, G.; Girard, J.P. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Ketelaar, M.; Hawro, T.; Church, M.K.; Maurer, M.; Nawijn, M.C. The role of the IL-33/IL-1R axis in mast cell and basophil activation in allergic disorders. Mol. Immunol. 2015, 63, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Hawro, T.; Eberle, J.; Church, M.K.; Maurer, M. Interleukin-33 promotes the proliferation of mouse mast cells through ST2/MyD88 and p38 MAPK-dependent and Kit-independent pathways. J. Biol. Regul. Homeost. Agents 2014, 28, 575–585. [Google Scholar] [PubMed]
- Schneider, E.; Petit-Bertron, A.; Bricard, R.; Levasseur, M.; Ramadan, A.; Girard, J.P.; Herbelin, A.; Dy, M. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J. Immunol. 2009, 183, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Suto, H.; Kajiwara, N.; Oboki, K.; Ohno, T.; Okayama, Y.; Saito, H.; Galli, S.J.; Nakae, S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab. Invest. 2007, 87, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, H.; Kewin, P.; Li, Y.; Mu, R.; Fraser, A.R.; Pitman, N.; Kurowska-Stolarska, M.; McKenzie, A.N.; McInnes, I.B.; et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc. Natl. Acad. Sci. USA 2008, 105, 10913–10918. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, G.A.; Costa, R.S.; Alcantara-Neves, N.M.; Nunes de Oliveira Costa, G.; Barreto, M.L.; Carneiro, V.L.; Figueiredo, C.A. IL33 and IL1RL1 variants are associated with asthma and atopy in a Brazilian population. Int. J. Immunogenet. 2017, 44, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Hu, H.; Jin, Y.; Xue, M. Polymorphisms of RAD50, IL33 and IL1RL1 are associated with atopic asthma in Chinese population. Tissue Antigens 2015, 86, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Schröder, P.C.; Casaca, V.I.; Illi, S.; Schieck, M.; Michel, S.; Böck, A.; Roduit, C.; Frei, R.; Lluis, A.; Genuneit, J.; et al. IL-33 polymorphisms are associated with increased risk of hay fever and reduced regulatory T cells in a birth cohort. Pediatr. Allergy Immunol. 2016, 27, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Yasuda, K.; Sakaguchi, Y.; Haneda, T.; Mizutani, H.; Yoshimoto, T.; Nakanishi, K.; Yamanishi, K. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 13921–13926. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gupta, K.; Dwivedi, P.D. Pathophysiology of IL-33 and IL-17 in allergic disorders. Cytokine Growth Factor Rev. 2017, 38, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimäki, S.; Karisola, P.; Reunala, T.; Wolff, H.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Smith, D. IL-33: A tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin. Exp. Allergy 2010, 40, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Barlow, J.; Saunders, S.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Ogg, G. Proof-of-Concept Phase-2a Clinical Trial of ANB020 (Anti-IL-33 Antibody) in the Treatment of Moderate-to-Severe Adult Atopic Dermatitis. Professor Graham Ogg University of Oxford United Kingdom European Academy of Allergy and Clinical Immunology Congress. 29 May 2018. Available online: https://www2.anaptysbio.com/wp-content/uploads/ANB020-Graham-Ogg-EAACI-052918.pdf (accessed on 3 October 2018).
- Infante-Duarte, C.; Horton, H.; Byrne, M.; Kamradt, T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000, 165, 6107–6115. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.; Yang, X.; Chang, S.H.; Nurieva, R.; Wang, Y.H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Veldhoen, M.; Hocking, R.; Atkins, C.; Locksley, R.M.; Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17 producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D. Flu strikes the hygiene hypothesis. Nat. Med. 2004, 10, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Moseley, T.; Haudenschild, D.; Rose, L.; Reddi, A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef]
- Rouvier, E.; Luciani, M.; Mattei, M.; Denizot, F.; Golstein, P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993, 150, 5445–5456. [Google Scholar] [PubMed]
- Hu, Y.; Shen, F.; Crellin, N.K.; Ouyang, W. The IL-17 pathway as a major therapeutic target in autoimmune diseases. Ann. N. Y. Acad. Sci. 2011, 1217, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Littman, D. Transcriptional regulatory networks in Th17 cell differentiation. Curr. Opin. Immunol. 2009, 21, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Krueger, J.G. IL-17C: A Unique Epithelial Cytokine with Potential for Targeting across the Spectrum of Atopic Dermatitis and Psoriasis. J. Investig. Dermatol. 2018, 138, 1467–1469. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Kitoh, A.; Egawa, G.; Natsuaki, Y.; Nakamizo, S.; Moniaga, C.S.; Otsuka, A.; Honda, T.; Hanakawa, S.; Amano, W.; et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J. Investig. Dermatol. 2014, 134, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Doreau, A.; Belot, A.; Bastid, J.; Riche, B.; Trescol-Biemont, M.C.; Ranchin, B.; Fabien, N.; Cochat, P.; Pouteil-Noble, C.; Trolliet, P. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 2009, 10, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, M.; Drozdenko, G.; Weise, C.; Babina, M.; Worm, M. Interleukin-17A promotes IgE production in human B cells. J. Investig. Dermatol. 2010, 130, 2621–2628. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Scarponi, C.; Cavani, A.; Federici, M.; Nasorri, F.; Girolomoni, G. Interleukin-17 is Produced by Both Th1 and Th2 Lymphocytes, and Modulates Interferon-γ- and Interleukin-4-Induced activation of Human Keratinocytes. J. Investig. Dermatol. 2000, 115, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.; Suárez-Fariñas, M.; Fuentes-Duculan, J. Attenuated neutrophil axis in atopic dermatitis compared to psoriasis reflects T pathway differences between these diseases. J. Allergy Clin. Immunol. 2013, 132, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Gutowska-Owsiak, G.; Schaupp, A.; Salimi, M.; Selvakumar, T.A.; McPherson, T.; Taylor, S.; Ogg, G.S. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp. Dermatol. 2012, 21, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef] [PubMed]
- Narbutt, J.; Wojtczak, M.; Zalińska, A.; Salinski, A.; Przybylowska-Sygut, K.; Kuna, P.; Majak, P.; Sysa-Jedrzejowska, A.; Lesiak, A. The A/A genotype of an interleukin-17A polymorphism predisposes to increased severity of atopic dermatitis and coexistence with asthma. Clin. Exp. Dermatol. 2015, 40, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Raeiszadeh Jahromi, S.; Mahesh, P.A.; Jayaraj, B.S.; Holla, A.D.; Vishweswaraiah, S.; Ramachandra, N.B. IL-10 and IL-17F Promoter Single Nucleotide Polymorphism and Asthma: A Case-Control Study in South India. Lung 2015, 193, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Vandeghinste, N.; Klattig, J.; Jagerschmidt, C.; Lavazais, S.; Marsais, F.; Haas, J.D.; Auberval, M.; Lauffer, F.; Moran, T.; Ongenaert, M. Neutralization of IL-17C Reduces Skin Inflammation in Mouse Models of Psoriasis and Atopic Dermatitis. J. Investig. Dermatol. 2018, 138, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.B.; Bojar, R.A.; Farrar, M.D.; Holland, K.T. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 2009, 290, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Farinas, M.; Dhingra, N.; Gittler, J.; Shemer, A.; Cardinale, I.; de Guzman Strong, C.; Krueger, J.G.; Guttman-Yassky, E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013, 132, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Thaci, D.; Constantin, M.M.; Rojkovich, B.; Timmis, H.; Klöpfer, P.; Härtle, S.; Vandeghinste, N.; Knebel, I.; Lindner, J.; Van Kaem, T.; et al. MOR106, an Anti-IL-17C mAb, a Potential New Approach for Treatment of Moderate-to-severe Atopic Dermatitis: Phase 1 Study. In Proceedings of the American Academy of Dermatology Annual Meeting, Orlando, FL, USA, 3–7 March 2017. [Google Scholar]
- Oka, T.; Sugaya, M.; Takahashi, N.; Nakajima, R.; Otobe, S.; Kabasawa, M.; Suga, H.; Miyagaki, T.; Asano, Y.; Sato, S. Increased Interleukin-19 Expression in Cutaneous T-cell Lymphoma and Atopic Dermatitis. Acta Derm. Venereol. 2017, 97, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Dembic, Z. The Cytokines of the Immune System. In The Role of Cytokines in Disease Related to Immune Response; Academic Press: Cambridge, MA, USA, 2015; pp. 99–122. ISBN 978-0-12-419998-9. [Google Scholar]
- Drysdale, S.B.; Milner, A.D.; Greenough, A. Respiratory syncytial virus infection and chronic respiratory morbidity—Is there a functional or genetic predisposition? Acta Paediatr. 2012, 101, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klonowska, J.; Gleń, J.; Nowicki, R.J.; Trzeciak, M. New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. Int. J. Mol. Sci. 2018, 19, 3086. https://doi.org/10.3390/ijms19103086
Klonowska J, Gleń J, Nowicki RJ, Trzeciak M. New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. International Journal of Molecular Sciences. 2018; 19(10):3086. https://doi.org/10.3390/ijms19103086
Chicago/Turabian StyleKlonowska, Jolanta, Jolanta Gleń, Roman J. Nowicki, and Magdalena Trzeciak. 2018. "New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets" International Journal of Molecular Sciences 19, no. 10: 3086. https://doi.org/10.3390/ijms19103086
APA StyleKlonowska, J., Gleń, J., Nowicki, R. J., & Trzeciak, M. (2018). New Cytokines in the Pathogenesis of Atopic Dermatitis—New Therapeutic Targets. International Journal of Molecular Sciences, 19(10), 3086. https://doi.org/10.3390/ijms19103086