Specific Targeting of Plant and Apicomplexa Parasite Tubulin through Differential Screening Using In Silico and Assay-Based Approaches
Abstract
1. Introduction
2. Results
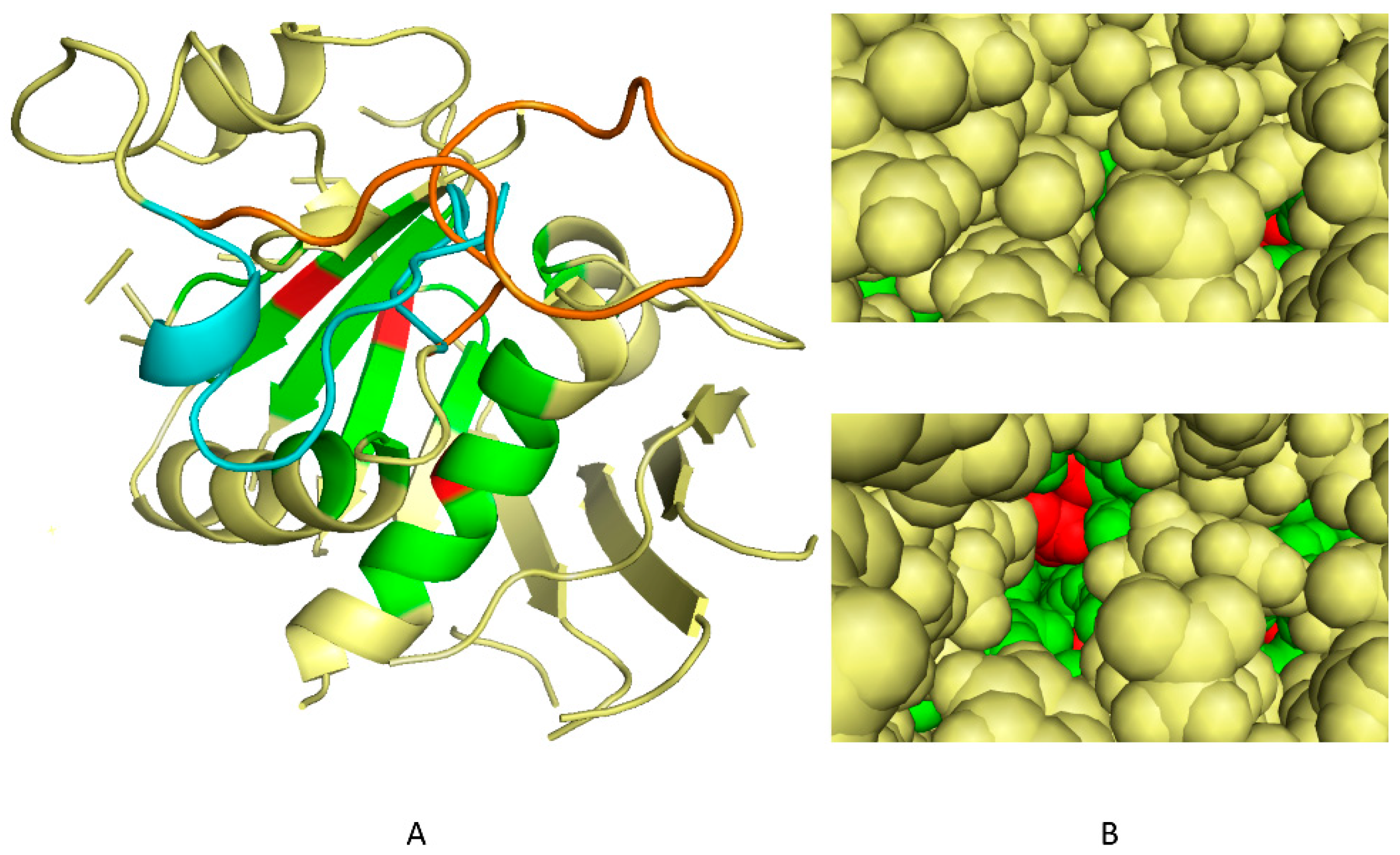
2.1. Determination of 3D Discriminating Conformations of P. falciparum α-Tubulin for In Silico Screening
2.2. In Silico Selection of Compounds that Bind to the Dinitroaniline Site of α-Tubulin from the Photosynthetic Lineage
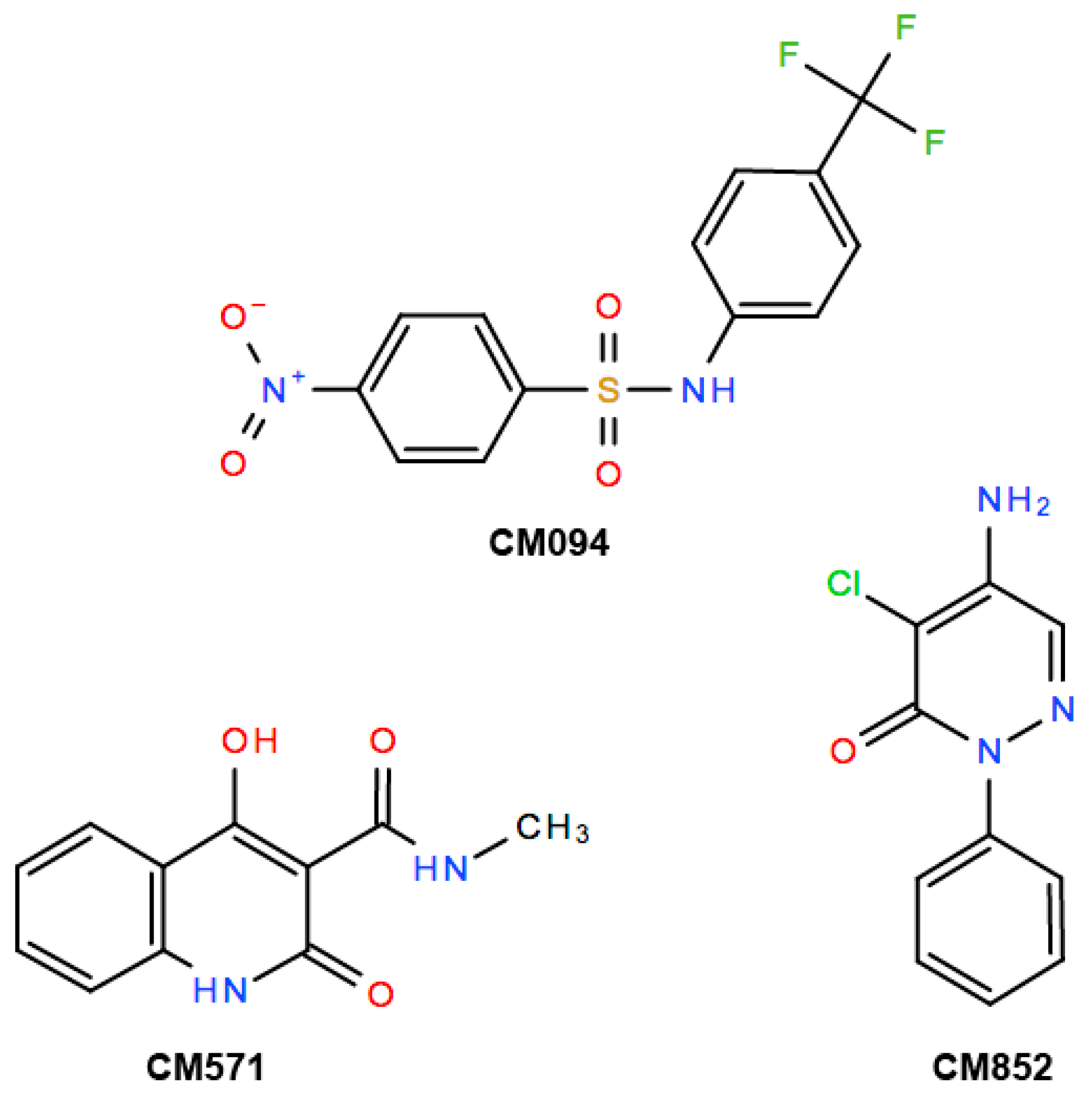
2.3. Selection of Compounds Active only on Plant Cell Microtubules
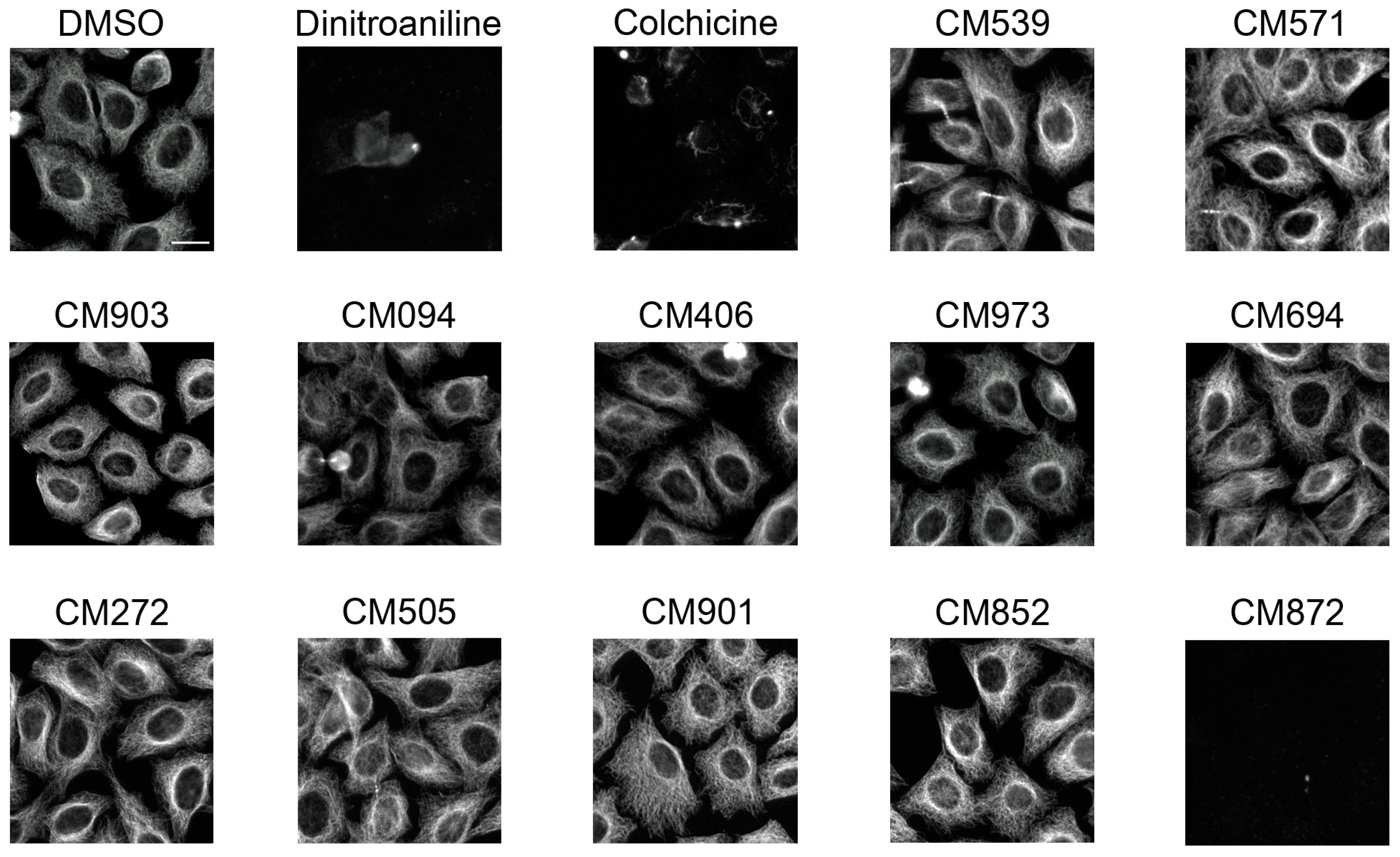
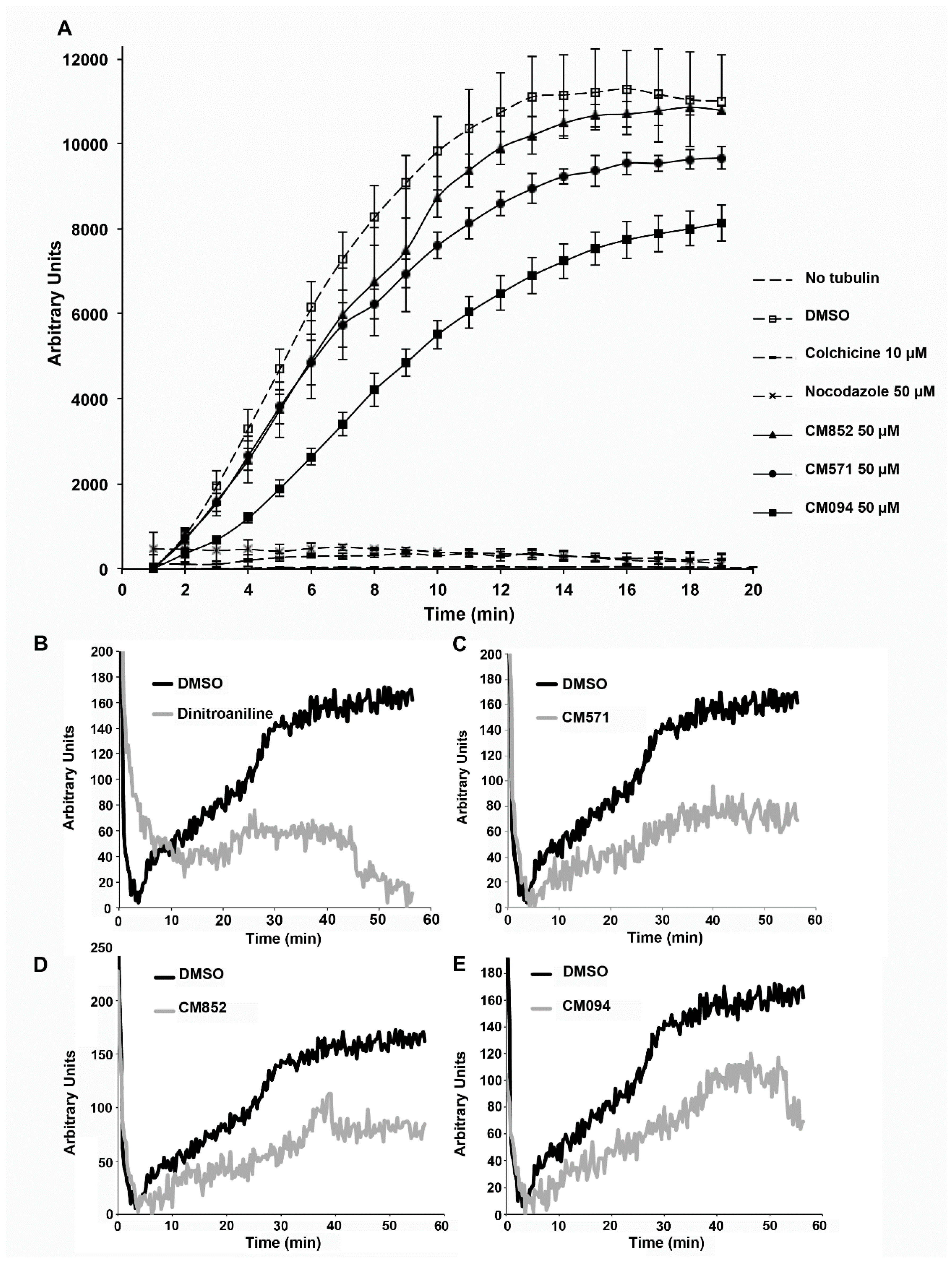
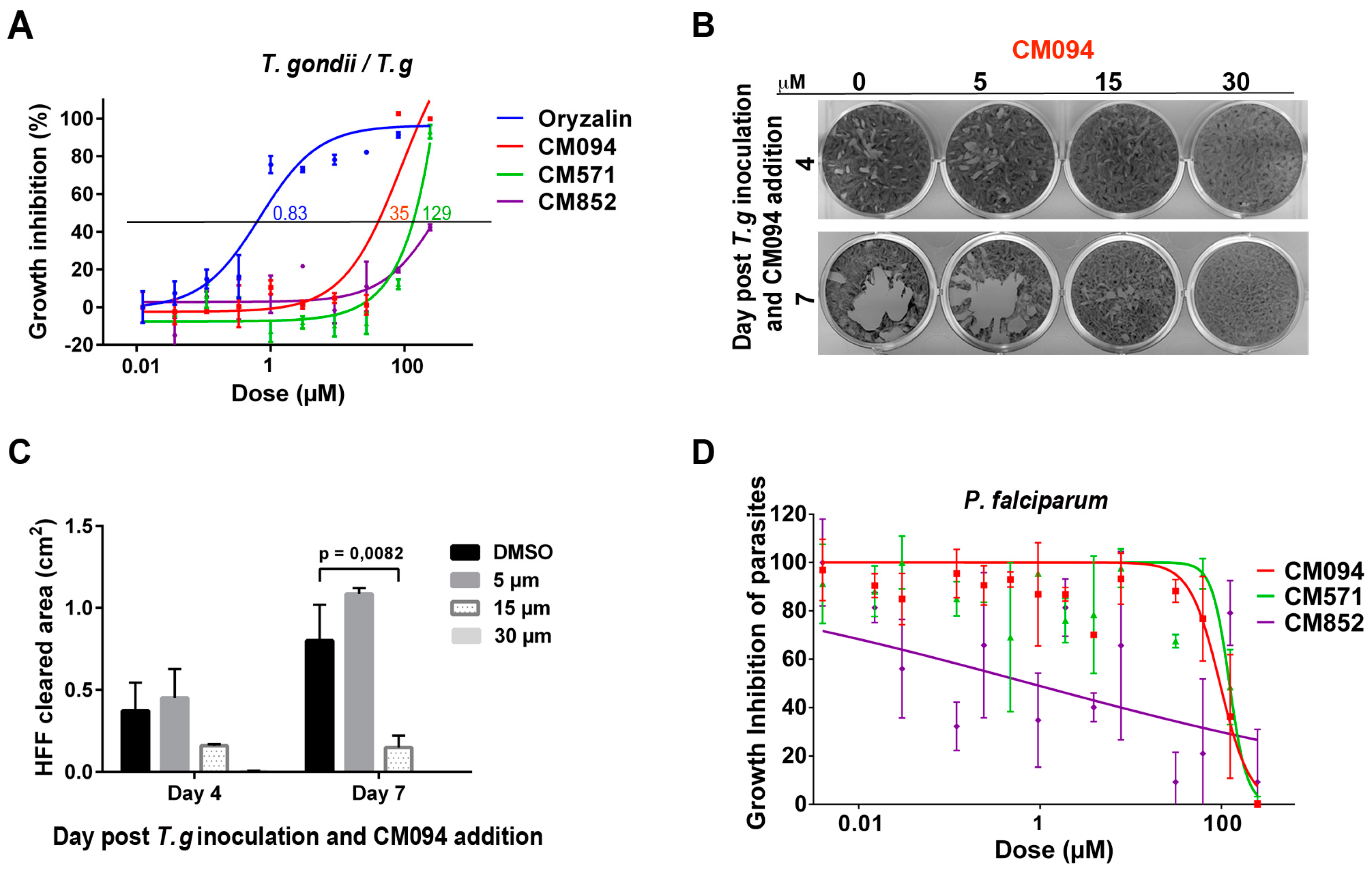
2.4. Selected Compounds Target Plant but not Mammalian Tubulin
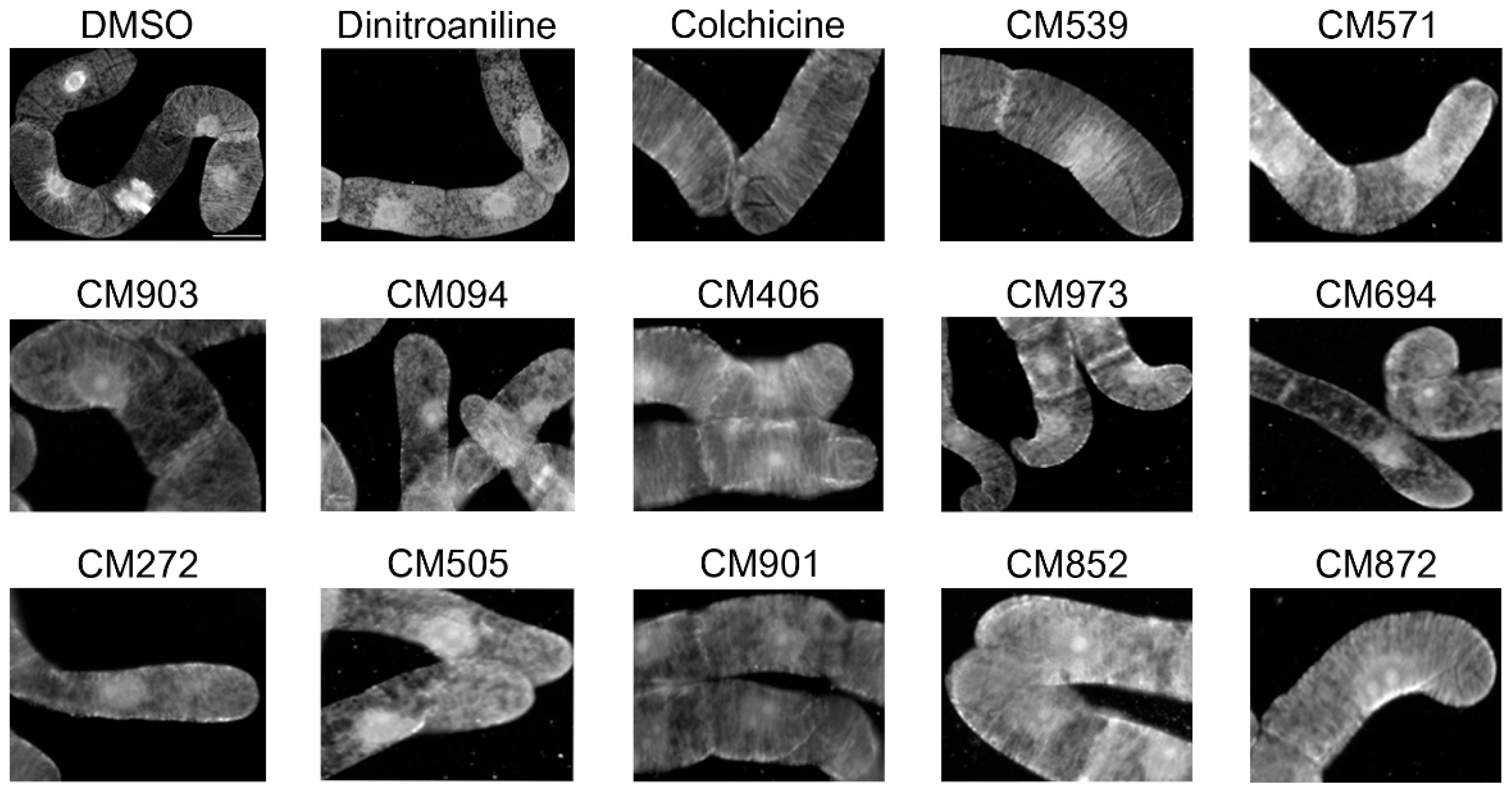
2.5. Analysis of the Effects of the Compounds on Apicomplexan Parasites
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents, Recombinant and Purified Protein
4.2. Antibodies
4.3. Mammalian, Plant and Apicomplexan Cell Lines
4.4. In Silico Screening on Alpha-Tubulin 3D-Model: Virtual Library of Compounds, Molecular Docking and Processing Methods
4.5. Microtubule-Interference Assay in BY-2 Tobacco Cells
4.6. Microtubule-Interference Assay in Hela Cells
4.7. Drug Effect on Toxoplasma gondii and Plasmodium falciparum Parasite In Vitro
4.8. Automated Imaging
4.9. Arabidopsis Thaliana Growth Assay
4.10. Tubulin Polymerization Assay
4.11. Analysis of Cell Viability Using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Bartolini, F.; Gundersen, G.G. Generation of noncentrosomal microtubule arrays. J. Cell Sci. 2006, 119, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Wasteneys, G.O.; Ambrose, J.C. Spatial organization of plant cortical microtubules: Close encounters of the 2D kind. Trends Cell Biol. 2009, 19, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Portran, D.; Zoccoler, M.; Gaillard, J.; Stoppin-Mellet, V.; Neumann, E.; Arnal, I.; Martiel, J.L.; Vantard, M. MAP65/Ase1 promote microtubule flexibility. Mol. Biol. Cell 2013, 24, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Botté, C.Y.; Yamaryo-Botté, Y.; Janouskovec, J.; Rupasinghe, T.; Keeling, P.J.; Crellin, P.; Coppel, R.L.; Maréchal, E.; McConville, M.J.; McFadden, G.I. Identification of plant-like galactolipids in Chromera velia, a photosynthetic relative of malaria parasites. J. Biol. Chem. 2011, 286, 29893–29903. [Google Scholar] [CrossRef] [PubMed]
- Morrissette, N. Targeting Toxoplasma Tubules: Tubulin, Microtubules, and Associated Proteins in a Human Pathogen. Eukaryot. Cell 2015, 14, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, M.; Carter, R.; Ito, Y.; Nijhout, M.M. New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum. J. Protozool. 1984, 31, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Morejohn, L.C.; Fosket, D.E. The biochemistry of compounds with anti-microtubule activity in plant cells. Pharmacol. Ther. 1991, 51, 217–230. [Google Scholar] [CrossRef]
- Lyons-Abbott, S.; Sackett, D.L.; Wloga, D.; Gaertig, J.; Morgan, R.E.; Werbovetz, K.A.; Morrissette, N.S. α-Tubulin Mutations Alter Oryzalin Affinity and Microtubule Assembly Properties to Confer Dinitroaniline Resistance. Eukaryot. Cell 2010, 9, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Fennell, B.; Naughton, J.; Barlow, J.; Brennan, G.; Fairweather, I.; Hoey, E.; McFerran, N.; Trudgett, A.; Bell, A. Microtubules as antiparasitic drug targets. Expert Opin. Drug Discov. 2008, 3, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Morejohn, L.C.; Bureau, T.E.; Molé-Bajer, J.; Bajer, A.S.; Fosket, D.E. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 1987, 172, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, J.D.; Morejohn, L.C. Rapid and Reversible High-Affinity Binding of the Dinitroaniline Herbicide Oryzalin to Tubulin from Zea mays L. Plant Physiol. 1993, 102, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Stokkermans, T.J.W.; Schwartzman, J.D.; Keenan, K.; Morrissette, N.S.; Tilney, L.G.; Roos, D.S. Inhibition of Toxoplasma gondii Replication by Dinitroaniline Herbicides. Exp. Parasitol. 1996, 84, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Sept, D. Binding and Interaction of Dinitroanilines with Apicomplexan and Kinetoplastid α-Tubulin. J. Med. Chem. 2006, 49, 5226–5231. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Prudent, R.; Vassal-Stermann, É.; Nguyen, C.-H.; Mollaret, M.; Viallet, J.; Desroches-Castan, A.; Martinez, A.; Barette, C.; Pillet, C.; Valdameri, G.; et al. Azaindole derivatives are inhibitors of microtubule dynamics, with anti-cancer and anti-angiogenic activities. Br. J. Pharmacol. 2013, 168, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Soleilhac, E.; Barette, C.; Prudent, R.; Gozzi, G.J.; Vassal-Stermann, E.; Pillet, C.; di Pietro, A.; Fauvarque, M.-O.; Lafanechère, L.; et al. Novel Synthetic Pharmacophores Inducing a Stabilization of Cellular Microtubules. Curr. Cancer Drug Targets 2014, 15, 2–13. [Google Scholar] [CrossRef]
- Botté, C.Y.; Maréchal, E. Plastids with or without galactoglycerolipids. Trends Plant Sci. 2014, 19, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Botté, C.Y.; Dubar, F.; McFadden, G.I.; Maréchal, E.; Biot, C. Plasmodium falciparum Apicoplast Drugs: Targets or Off-Targets? Chem. Rev. 2012, 112, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, L.; Chira, C.; Marcou, G.; Varnek, A.; Horvath, D. S4MPLE—Sampler for Multiple Protein-Ligand Entities: Methodology and Rigid-Site Docking Benchmarking. Molecules 2015, 20, 8997–9028. [Google Scholar] [CrossRef] [PubMed]
- Tantar, A.-A.; Conilleau, S.; Parent, B.; Melab, N.; Brillet, L.; Roy, S.; Talbi, E.-G.; Horvath, D. Docking and Biomolecular Simulations on Computer Grids: Status and Trends. Curr. Comput. Aided-Drug Des. 2008, 4, 235–249. [Google Scholar] [CrossRef]
- Dauber-Osguthorpe, P.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and energetics of ligand binding to proteins: Escherichia coli dihydrofolate reductase-trimethoprim, a drug-receptor system. Proteins 1988, 4, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Marcou, G.; Rognan, D. Optimizing fragment and scaffold docking by use of molecular interaction fingerprints. J. Chem. Inf. Model. 2007, 47, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Mpamhanga, C.P.; Chen, B.; McLay, I.M.; Willett, P. Knowledge-based interaction fingerprint scoring: A simple method for improving the effectiveness of fast scoring functions. J. Chem. Inf. Model. 2006, 46, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chuaqui, C.; Singh, J. Structural Interaction Fingerprint (SIFt): A Novel Method for Analyzing Three-Dimensional Protein−Ligand Binding Interactions. J. Med. Chem. 2004, 47, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Perlman, Z.E.; Mitchison, T.J.; Mayer, T.U. High-content screening and profiling of drug activity in an automated centrosome-duplication assay. ChemBioChem 2005, 6, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Prudent, R.; Lafanechère, L. Institute for Advanced Biosciences (IAB), Team Regulation and Pharmacology of the Cytoskeleton, Grenoble, France. Analysis of the effect of selected molecules on the polymerization and depolymerization of tubulin, compared with dinitroaniline used as a positive control. Effects were studied at increasing doses of molecules up to 50 µM. Not intended for publication. 2011.
- Buttiglieri, G.; Peschka, M.; Frömel, T.; Müller, J.; Malpei, F.; Seel, P.; Knepper, T.P. Environmental occurrence and degradation of the herbicide n-chloridazon. Water Res. 2009, 43, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Pokora, W.; Tukaj, Z. Induction time of Fe-SOD synthesis and activity determine different tolerance of two Desmodesmus (green algae) strains to chloridazon: A study with synchronized cultures. Pestic. Biochem. Physiol. 2013, 107, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Touquet, B.; Tardieux, I. Institute for Advanced Biosciences (IAB), Team Membrane and Cell Dynamics of Host Parasite Interactions, Grenoble, France. Analysis of the effect of increasing doses of the selected molecules on the growth of human fibroblasts. Not intended for publication. 2017.
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Dass, S.; Botté, C. Institute for Advanced Biosciences (IAB), Team ApicoLipid, Grenoble, France, Analysis of the cytotoxic effect of the selected compounds on human erythrocytes. Not intended for publication. 2017.
- Dejonghe, W.; Russinova, E. Plant Chemical Genetics: From Phenotype-Based Screens to Synthetic Biology. Plant Physiol. 2017, 174, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Prudent, R.; Vassal-Stermann, E.; Nguyen, C.-H.; Pillet, C.; Martinez, A.; Prunier, C.; Barette, C.; Soleilhac, E.; Filhol, O.; Beghin, A.; et al. Pharmacological inhibition of LIM kinase stabilizes microtubules and inhibits neoplastic growth. Cancer Res. 2012, 72, 4429–4439. [Google Scholar] [CrossRef] [PubMed]
- Langhans, M.; Niemes, S.; Pimpl, P.; Robinson, D.G. Oryzalin bodies: in addition to its anti-microtubule properties, the dinitroaniline herbicide oryzalin causes nodulation of the endoplasmic reticulum. Protoplasma 2009, 236, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Naughton, J.A.; Hughes, R.; Bray, P.; Bell, A. Accumulation of the antimalarial microtubule inhibitors trifluralin and vinblastine by Plasmodium falciparum. Biochem. Pharmacol. 2008, 75, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Endeshaw, M.M.; Li, C.; de Leon, J.; Yao, N.; Latibeaudiere, K.; Premalatha, K.; Morrissette, N.; Werbovetz, K.A. Synthesis and evaluation of oryzalin analogs against Toxoplasma gondii. Bioorg. Med. Chem. Lett. 2010, 20, 5179–5183. [Google Scholar] [CrossRef] [PubMed]
- Paturle-Lafanechere, L.; Edde, B.; Denoulet, P.; van Dorsselaer, A.; Mazarguil, H.; le Caer, J.P.; Wehland, J.; Job, D. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry 1991, 30, 10523–10528. [Google Scholar] [CrossRef] [PubMed]
- Peris, L.; Thery, M.; Faure, J.; Saoudi, Y.; Lafanechere, L.; Chilton, J.K.; Gordon-Weeks, P.; Galjart, N.; Bornens, M.; Wordeman, L.; et al. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J. Cell Biol. 2006, 174, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Nemoto, Y.; Hasezawa, S. Tobacco BY-2 Cell Line as the “HeLa” Cell in the Cell Biology of Higher Plants. Int. Rev. Cytol. 1992, 132, 1–30. [Google Scholar] [CrossRef]
- Roos, D.S.; Donald, R.G.; Morrissette, N.S.; Moulton, A.L. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994, 45, 27–63. [Google Scholar] [PubMed]
- Shears, M.J.; MacRae, J.I.; Mollard, V.; Goodman, C.D.; Sturm, A.; Orchard, L.M.; Llinás, M.; McConville, M.J.; Botté, C.Y.; McFadden, G.I. Characterization of the Plasmodium falciparum and P. berghei glycerol 3-phosphate acyltransferase involved in FASII fatty acid utilization in the malaria parasite apicoplast. Cell. Microbiol. 2017, 19, e12633. [Google Scholar] [CrossRef] [PubMed]
- Breton, V.; Jacq, N.; Kasam, V.; Hofmann-Apitius, M. Grid-Added Value to Address Malaria. IEEE Trans. Inf. Technol. Biomed. 2008, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Rarey, M.; Kramer, B.; Lengauer, T.; Klebe, G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996, 261, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleilhac, E.; Brillet-Guéguen, L.; Roussel, V.; Prudent, R.; Touquet, B.; Dass, S.; Aci-Sèche, S.; Kasam, V.; Barette, C.; Imberty, A.; et al. Specific Targeting of Plant and Apicomplexa Parasite Tubulin through Differential Screening Using In Silico and Assay-Based Approaches. Int. J. Mol. Sci. 2018, 19, 3085. https://doi.org/10.3390/ijms19103085
Soleilhac E, Brillet-Guéguen L, Roussel V, Prudent R, Touquet B, Dass S, Aci-Sèche S, Kasam V, Barette C, Imberty A, et al. Specific Targeting of Plant and Apicomplexa Parasite Tubulin through Differential Screening Using In Silico and Assay-Based Approaches. International Journal of Molecular Sciences. 2018; 19(10):3085. https://doi.org/10.3390/ijms19103085
Chicago/Turabian StyleSoleilhac, Emmanuelle, Loraine Brillet-Guéguen, Véronique Roussel, Renaud Prudent, Bastien Touquet, Sheena Dass, Samia Aci-Sèche, Vinod Kasam, Caroline Barette, Anne Imberty, and et al. 2018. "Specific Targeting of Plant and Apicomplexa Parasite Tubulin through Differential Screening Using In Silico and Assay-Based Approaches" International Journal of Molecular Sciences 19, no. 10: 3085. https://doi.org/10.3390/ijms19103085
APA StyleSoleilhac, E., Brillet-Guéguen, L., Roussel, V., Prudent, R., Touquet, B., Dass, S., Aci-Sèche, S., Kasam, V., Barette, C., Imberty, A., Breton, V., Vantard, M., Horvath, D., Botté, C., Tardieux, I., Roy, S., Maréchal, E., & Lafanechère, L. (2018). Specific Targeting of Plant and Apicomplexa Parasite Tubulin through Differential Screening Using In Silico and Assay-Based Approaches. International Journal of Molecular Sciences, 19(10), 3085. https://doi.org/10.3390/ijms19103085







