An In Vitro Model of Angiogenesis during Wound Healing Provides Insights into the Complex Role of Cells and Factors in the Inflammatory and Proliferation Phase
Abstract
1. Introduction
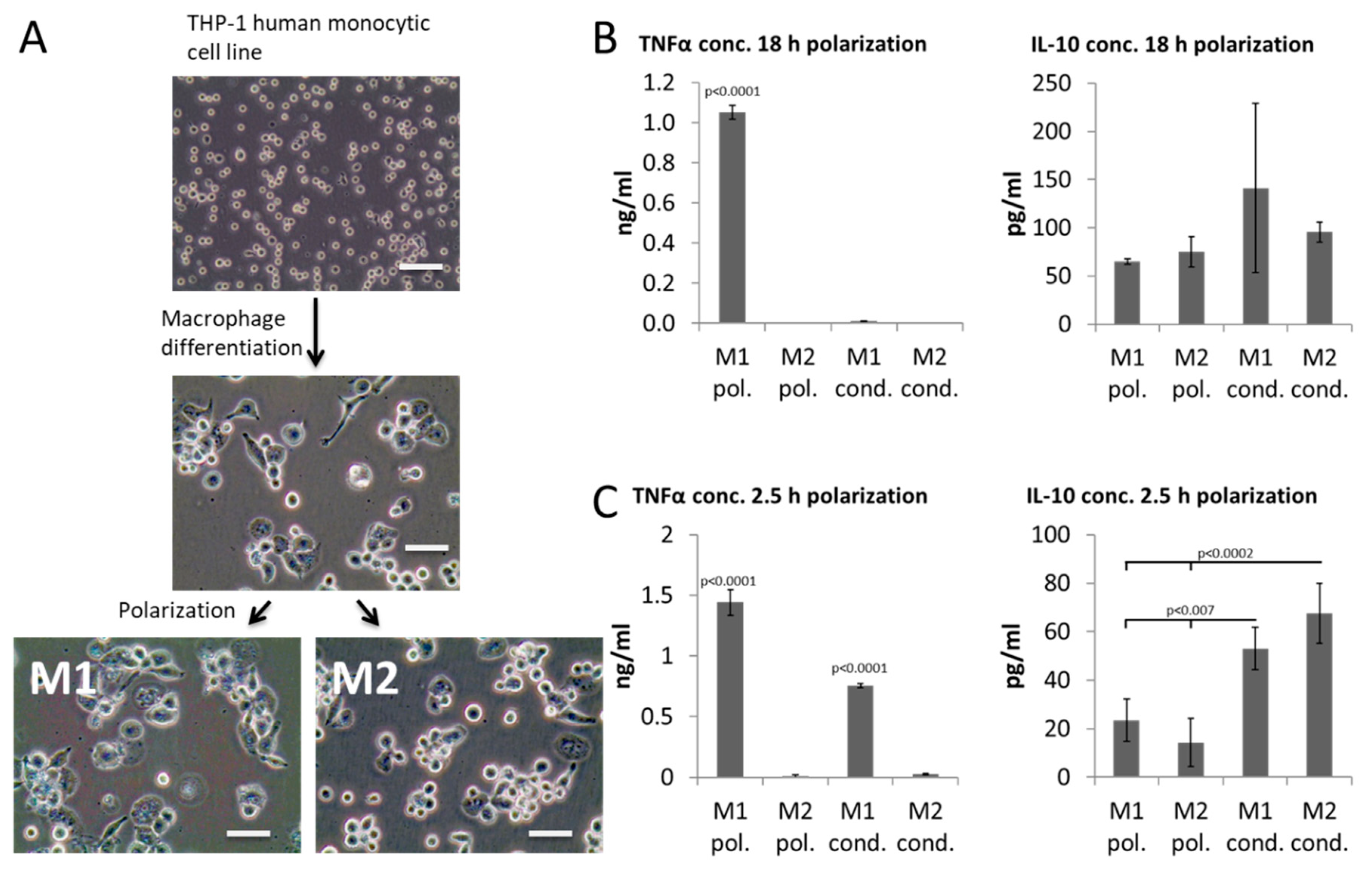
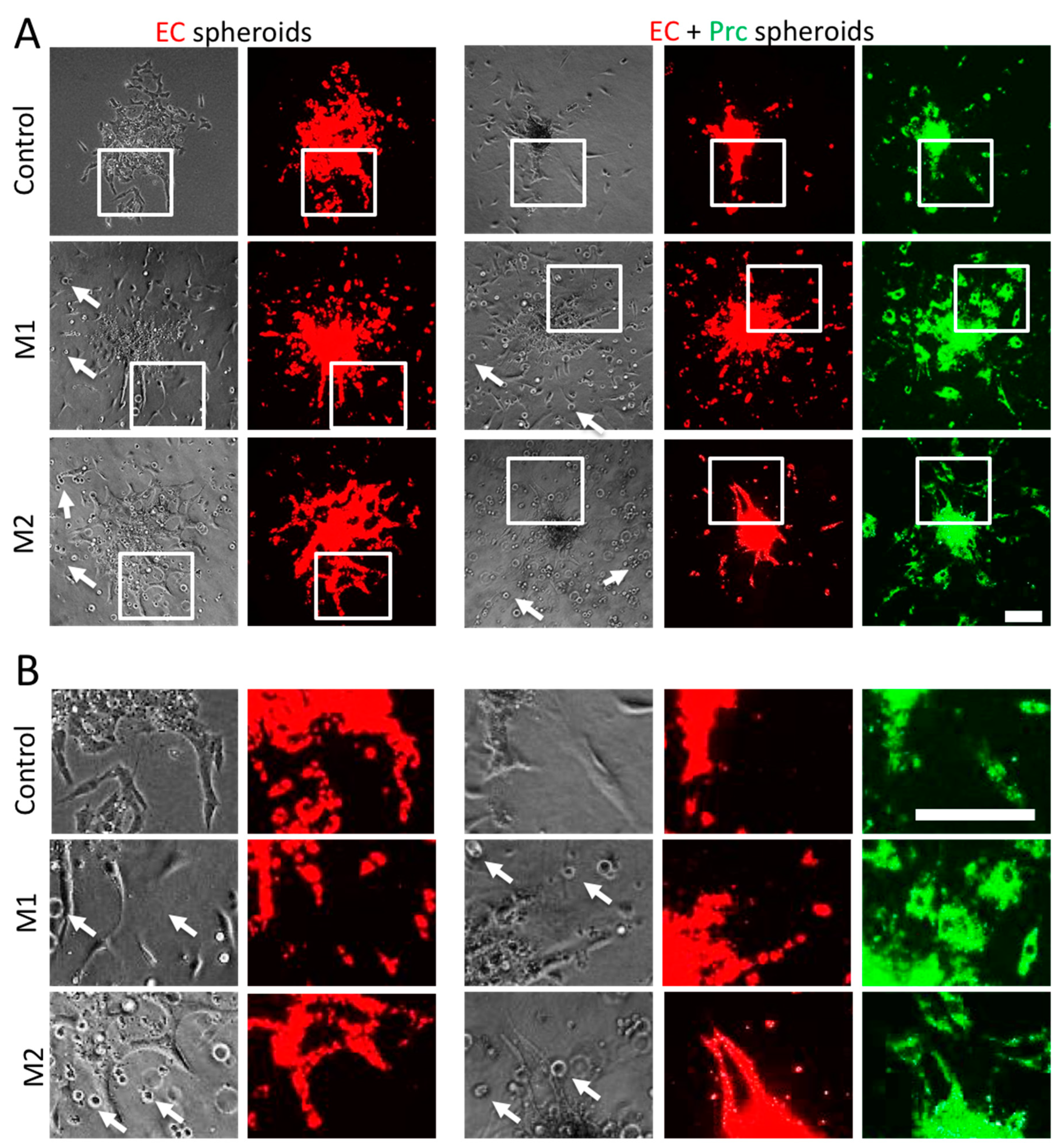
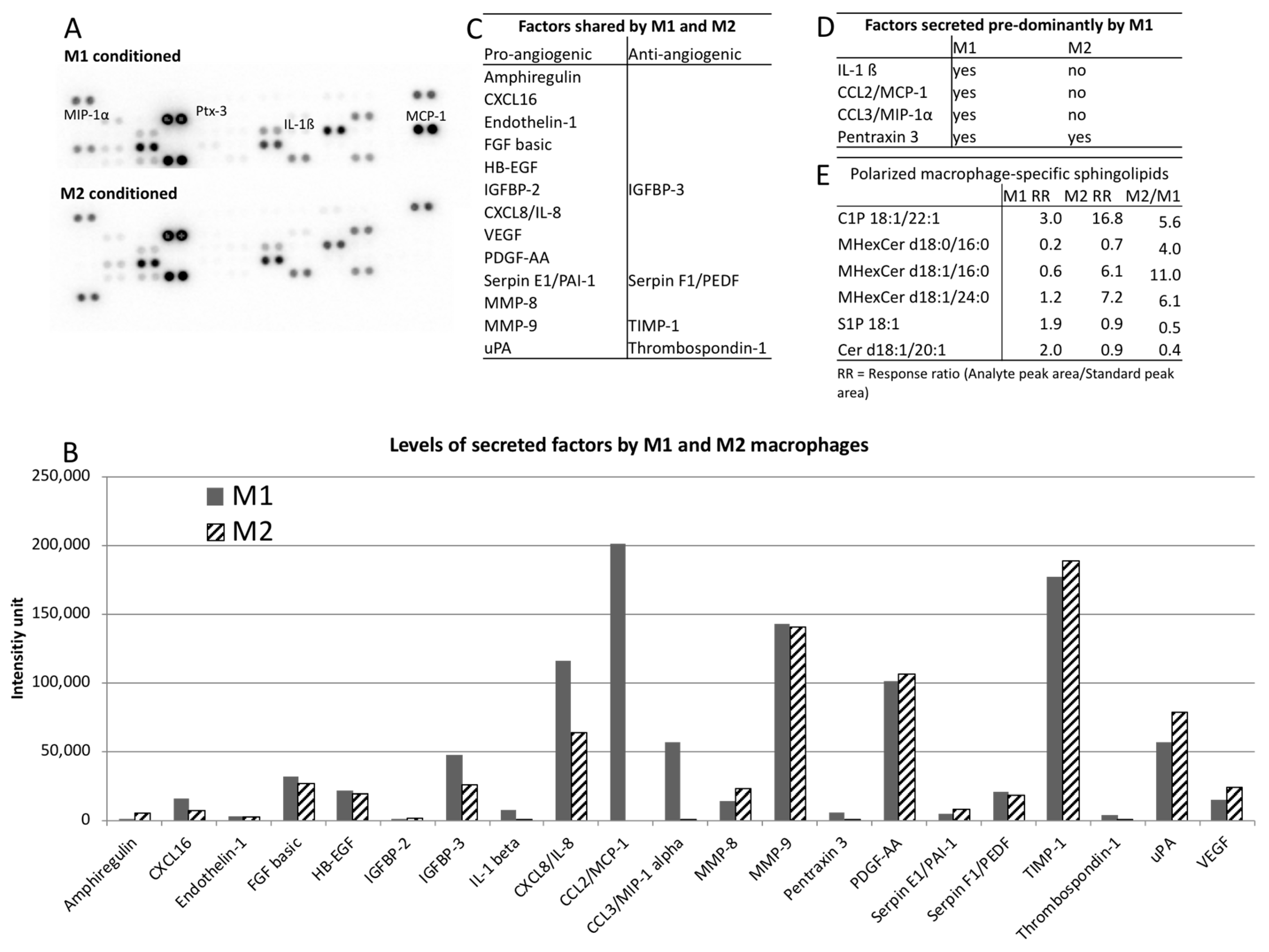
2. Results
3. Discussion
4. Materials and Methods
4.1. THP-1 Culture
4.2. Generation of Conditioned Media
4.3. ELISA
4.4. Spheroid Sprouting Assay
4.5. Angiogenesis Proteome Array
4.6. Mass Spectrometry-Based Sphingolipidomics
4.7. Statistical Analysis
5. Conclusions
Supplementary Material
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Irvin, M.W.; Zijlstra, A.; Wikswo, J.P.; Pozzi, A. Techniques and assays for the study of angiogenesis. Exp. Biol. Med. 2014, 239, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, U.A.; DiPietro, L. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2018, 18, E1419. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Chen, D.; Shen, C.; Shen, J.; Zhao, H.; He, Y. Platelet-rich plasma-loaded poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide) hydrogel dressing promotes full-thickness skin wound healing in a rodent model. Int. J. Mol. Sci. 2016, 17, E1001. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J. Chemokine Regulation of Angiogenesis During Wound Healing. Adv. Wound Care 2015, 4, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Wietecha, M.S.S.; DiPietro, L.A.A. Therapeutic Approaches to the Regulation of Wound Angiogenesis. Adv. Wound Care 2013, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Aplin, A.C.; Ligresti, G.; Fogel, E.; Zorzi, P.; Smith, K.; Nicosia, R.F. Regulation of angiogenesis, mural cell recruitment and adventitial macrophage behavior by Toll-like receptors. Angiogenesis 2014, 17, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Olingy, C.E.; San Emeterio, C.L.; Ogle, M.E.; Krieger, J.R.; Bruce, A.C.; Pfau, D.D.; Jordan, B.T.; Peirce, S.M. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci. Rep. 2017, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zajac, E.; Schweighofer, B.; Kupriyanova, T.A.; Juncker-Jensen, A.; Minder, P.; Quigley, J.P.; Deryugina, E.I. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 2013, 122, 4054–4067. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, A.J.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Blocki, A.; Wang, Y.; Koch, M.; Goralczyk, A.; Beyer, S.; Agarwal, N.; Lee, M.; Moonshi, S.; Dewavrin, J.Y.; Peh, P.; et al. Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications. Mol. Ther. 2015, 23, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Blocki, A.; Beyer, S.; Jung, F.; Raghunath, M. The controversial origin of pericytes during angiogenesis—Implications for cell-based therapeutic angiogenesis and cell-based therapies. Clin. Hemorheol. Microcirc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Cowin, A.J.; Mills, S.J. The Importance of Pericytes in Healing: Wounds and other Pathologies. Int. J. Mol. Sci. 2017, 18, E1129. [Google Scholar] [CrossRef] [PubMed]
- Blocki, A.; Wang, Y.; Koch, M.; Peh, P.; Beyer, S.; Law, P.; Hui, J.; Raghunath, M. Not all MSCs can act as pericytes: Functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev. 2013, 22, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci. 2017, 18, E1545. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kim, C.; Aref, A.R.; Kamm, R.D.; Raghunath, M. Complementary effects of ciclopirox olamine, a prolyl hydroxylase inhibitor and sphingosine 1-phosphate on fibroblasts and endothelial cells in driving capillary sprouting. Integr. Biol. 2013, 5, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, L.; Korff, T. Spheroid-based in vitro angiogenesis model Methods. Mol. Biol. 2016, 167–177. [Google Scholar]
- Spiller, K.L.; Wrona, E.A.; Romero-Torres, S.; Pallotta, I.; Graney, P.L.; Witherel, C.E.; Panicker, L.M.; Feldman, R.A.; Urbanska, A.M.; Santambrogio, L.; et al. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp. Cell. Res. 2016, 347, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.B.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; De Majo, F.; Kim, J.; Trenti, A.; Trevisi, L.; Fadini, G.P.; Bolego, C.; Zandstra, P.W.; Cignarella, A.; Vitiello, L. Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying in Vitro Polarization? Front Pharmacol. 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Simons, M. Fibroblast growth factor regulation of neovascularization. Curr. Opin. Hematol. 2008, 15, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hui, Q.; Jin, Z.; Li, X.; Liu, C.; Wang, X. FGF Family: From Drug Development to Clinical Application. Int. J. Mol. Sci. 2018, 19, 1875. [Google Scholar] [CrossRef] [PubMed]
- Carrero, R.; Cerrada, I.; Lledó, E.; Dopazo, J.; García-García, F.; Rubio, M.P.; Trigueros, C.; Dorronsoro, A.; Ruiz-Sauri, A.; Montero, J.A.; et al. IL1β induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-κB. Stem Cell Rev. 2012, 8, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Bacon, K.; Camp, R.D.; Kaspari, J.W.; Goeddel, D.V. Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J. Exp. Med. 1993, 177, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Carmi, Y.; Apte, R.N. The role IL-1 in tumor-mediated angiogenesis. Front Physiol. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Q.; Fei, T.; Han, J.-D.J.; Chen, Y.-G. MCP-1 mediates TGF-beta-induced angiogenesis by stimulating vascular smooth muscle cell migration. Blood 2007, 109, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Billich, A.; Bornancin, F.; Mechtcheriakova, D.; Natt, F.; Huesken, D.; Baumruker, T. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: Function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal 2005, 17, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Abuhusain, H.J.; Matin, A.; Qiao, Q.; Shen, H.; Kain, N.; Day, B.W.; Stringer, B.W.; Daniels, B.; Laaksonen, M.A.; Teo, C.; et al. A metabolic shift favoring sphingosine 1-phosphate at the expense of ceramide controls glioblastoma angiogenesis. J. Biol. Chem. 2013, 288, 37355–37364. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Mok, H.J.; Lee, D.Y.; Park, S.C.; Kim, G.S.; Lee, S.E.; Lee, Y.S.; Kim, K.P.; Kim, H.D. UPLC-QqQ/MS-Based Lipidomics Approach To Characterize Lipid Alterations in Inflammatory Macrophages. J. Proteome Res. 2017, 16, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Wang, F.; Wang, Z.; Lu, Y.; Xu, Y.; Wang, Y.; Shen, H.; Yang, P.; Li, S.; et al. Quantitative profiling of glycerophospholipids during mouse and human macrophage differentiation using targeted mass spectrometry. Sci. Rep. 2017, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Barbay, V.; Houssari, M.; Mekki, M.; Banquet, S.; Edwards-Lévy, F.; Henry, J.P.; Dumesnil, A.; Adriouch, S.; Thuillez, C.; Richard, V.; et al. Role of M2-like macrophage recruitment during angiogenic growth factor therapy. Angiogenesis 2015, 18, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; DiPietro, L.A. Apoptosis and angiogenesis: An evolving mechanism for fibrosis. FASEB J. 2013, 27, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Tan, C.W.; Venkatratnam, A.; Tan, C.S.; Cui, L.; Loh, S.F.; Griffith, L.; Tannenbaum, S.R.; Chan, J.K. Dysregulated sphingolipid metabolism in endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E1913–E1921. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyer, S.; Koch, M.; Lee, Y.H.; Jung, F.; Blocki, A. An In Vitro Model of Angiogenesis during Wound Healing Provides Insights into the Complex Role of Cells and Factors in the Inflammatory and Proliferation Phase. Int. J. Mol. Sci. 2018, 19, 2913. https://doi.org/10.3390/ijms19102913
Beyer S, Koch M, Lee YH, Jung F, Blocki A. An In Vitro Model of Angiogenesis during Wound Healing Provides Insights into the Complex Role of Cells and Factors in the Inflammatory and Proliferation Phase. International Journal of Molecular Sciences. 2018; 19(10):2913. https://doi.org/10.3390/ijms19102913
Chicago/Turabian StyleBeyer, Sebastian, Maria Koch, Yie Hou Lee, Friedrich Jung, and Anna Blocki. 2018. "An In Vitro Model of Angiogenesis during Wound Healing Provides Insights into the Complex Role of Cells and Factors in the Inflammatory and Proliferation Phase" International Journal of Molecular Sciences 19, no. 10: 2913. https://doi.org/10.3390/ijms19102913
APA StyleBeyer, S., Koch, M., Lee, Y. H., Jung, F., & Blocki, A. (2018). An In Vitro Model of Angiogenesis during Wound Healing Provides Insights into the Complex Role of Cells and Factors in the Inflammatory and Proliferation Phase. International Journal of Molecular Sciences, 19(10), 2913. https://doi.org/10.3390/ijms19102913








