Stereoselective Synthesis, Synthetic and Pharmacological Application of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles
2.2. Application of the Prepared Catalysts 9–11 and 14
2.3. Antiproliferative Activities
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. General Procedure for the Preparation of 3, 4 and 5
4.3. General Procedure for the Preparation of 6, 7 and 8
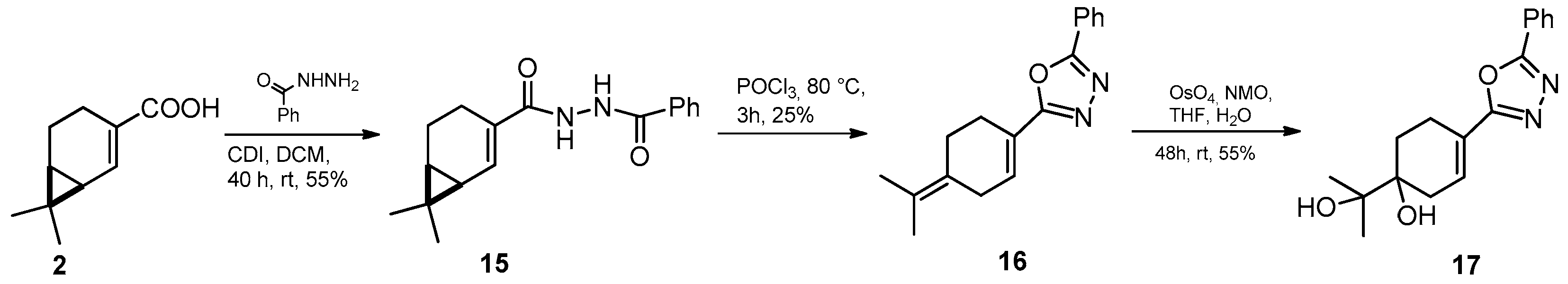
4.4. General Procedure for the Preparation of 12 and 15
4.5. General Procedure for the Preparation of 13 and 16
4.6. General Procedure for Dihydroxylation
4.7. Antiproliferative Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DCM | dichloromethane |
| CDI | 1,1′-carbonyldiimidazol |
| TBAF | tetrabutylammonium fluoride |
| NMO | 4-methylmorpholine N-oxide |
References
- Dalko, P.I. Enantioselective Organocatalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Caprio, V.; Williams, J.M.J. Catalysis in Asymmetric Synthesis; John Wiley & Sons: Oxford, UK, 2009. [Google Scholar]
- Carreira, E.M.; Yamamoto, H. Comprehensive Chirality; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Szakonyi, Z.; Fülöp, F. Monoterpene-based chiral β-amino acid derivatives prepared from natural sources: Syntheses and applications. Amino Acids 2011, 41, 597–608. [Google Scholar] [CrossRef] [PubMed]
- El Alami, M.S.I.; El Amrani, M.A.; Agbossou-Niedercorn, F.; Suisse, I.; Mortreux, A. Chiral ligands derived from monoterpenes: Application in the synthesis of optically pure secondary alcohols via asymmetric catalysis. Chem. Eur. J. 2015, 21, 1398–1413. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Rewucki, P.; Walenczak, S. Sulfur-containing derivatives from (1R)-(−)-myrtenal designed as chiral ligands. Tetrahedron 2016, 72, 3851–3857. [Google Scholar] [CrossRef]
- Makaev, F.Z.; Vlad, L.A.; Bets, L.P.; Malinovskii, S.T.; Gavrilov, K.N.; Gdanets, M. Addition products of chlorosulfonylisocyanate to (+)-3-carene and α-pinene enantiomers. Chem. Nat. Compd. 2010, 46, 528–533. [Google Scholar] [CrossRef]
- Roy, C.D.; Brown, H.C. A study of transesterification of chiral (−)-pinanediol methylboronic ester with various structurally modified diols. Mon. Für Chem. Chem. Mon. 2007, 138, 747–753. [Google Scholar] [CrossRef]
- Philipova, I.; Dimitrov, V.; Simova, S. Synthesis of new enantiopure aminodiols and their use as ligands for the addition of diethylzinc to benzaldehyde. Tetrahedron Asymmetry 1999, 10, 1381–1391. [Google Scholar] [CrossRef]
- Cherng, Y.-J.; Fang, J.-M.; Lu, T.-J. Pinane-type tridentate reagents for enantioselective reactions: Reduction of ketones and addition of diethylzinc to aldehydes. J. Org. Chem. 1999, 64, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Szakonyi, Z.; Csillag, K.; Fülöp, F. Stereoselective synthesis of carane-based aminodiols as chiral ligands for the catalytic addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2011, 22, 1021–1027. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Csőr, Á.; Haukka, M.; Fülöp, F. Stereoselective synthesis of carane-based chiral β- and γ-amino acid derivatives via conjugate addition. Tetrahedron 2015, 71, 4846–4852. [Google Scholar] [CrossRef]
- Frąckowiak, B.; Ochalik, K.; Białońska, A.; Ciunik, Z.; Wawrzeńczyk, C.; Lochyński, S. Stereochemistry of terpene derivatives. Part 5: Synthesis of chiral lactones fused to a carane system—Insect feeding deterrents. Tetrahedron Asymmetry 2006, 17, 124–129. [Google Scholar] [CrossRef]
- Sánchez-Carnerero, E.M.; Sandoval-Torrientes, R.; Urieta-Mora, J.; Moreno, F.; Maroto, B.L.; de la Moya, S. Speeding up heterogeneous catalysis with an improved highly reusable catalyst for the preparation of enantioenriched secondary alcohols. React. Funct. Polym. 2017, 113, 23–30. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Fülöp, F. Carbocyclic nucleosides from enantiomeric, α-pinane-based aminodiols. Tetrahedron Asymmetry 2010, 21, 831–836. [Google Scholar] [CrossRef]
- Geramipour, A.; Kohajda, Z.; Corici, C.; Prorok, J.; Szakonyi, Z.; Oravecz, K.; Márton, Z.; Nagy, N.; Tóth, A.; Acsai, K.; et al. The investigation of the cellular electrophysiological and antiarrhythmic effects of a novel selective sodium–calcium exchanger inhibitor, GYKB-6635, in canine and guinea-pig hearts. Can. J. Physiol. Pharmacol. 2016, 94, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.A.; Brandt, T.A. Enantioselective allylic substitution using a novel (phosphino-1,3-oxazine)palladium catalyst. Tetrahedron Lett. 1996, 37, 9143–9146. [Google Scholar] [CrossRef]
- Evans, P.A.; Brandt, T.A. Enantioselective palladium-catalyzed allylic alkylation using e- and z-vinylogous sulfonates. Org. Lett. 1999, 1, 1563–1565. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Hetényi, A.; Fülöp, F. Synthesis of enantiomeric spirooxazolines and spirooxazolidines by the regioselective ring closure of (−)-α-pinene-based aminodiols. Arkivoc 2007, 2008, 33. [Google Scholar] [CrossRef]
- Szakonyi, Z.; Csőr, Á.; Csámpai, A.; Fülöp, F. Stereoselective synthesis and modelling-driven optimisation of carane-based aminodiols and 1,3-oxazines as catalysts for the enantioselective addition of diethylzinc to benzaldehyde. Chem. Eur. J. 2016, 22, 7163–7173. [Google Scholar] [CrossRef] [PubMed]
- Csillag, K.; Németh, L.; Martinek, T.A.; Szakonyi, Z.; Fülöp, F. Stereoselective synthesis of pinane-type tridentate aminodiols and their application in the enantioselective addition of diethylzinc to benzaldehyde. Tetrahedron Asymmetry 2012, 23, 144–150. [Google Scholar] [CrossRef]
- Jakopin, Z. Orthogonally protected oxadiazole-based building blocks: Synthesis and characterization. Curr. Org. Synth. 2015, 13, 126–131. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, W.-H.; Dou, W.; Tang, X.-L.; Liu, W.-S. Synthesis of a newC2-symmetric chiral catalyst and its application in the catalytic asymmetric borane reduction of prochiral ketones. Chirality 2008, 20, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Adib, M.; Kesheh, M.; Ansari, S.; Bijanzadeh, H. Reaction between n-isocyaniminotriphenylphosphorane, aldehydes, and carboxylic acids: A one-pot and three-component synthesis of 2-aryl-5-hydroxyalkyl-1,3,4-oxadiazoles. Synlett 2009, 2009, 1575–1578. [Google Scholar] [CrossRef]
- Kovács, D.; Mótyán, G.; Wölfling, J.; Kovács, I.; Zupkó, I.; Frank, É. A facile access to novel steroidal 17–2′-(1′,3′,4′)-oxadiazoles, and an evaluation of their cytotoxic activities in vitro. Bioorg. Med. Chem. Lett. 2014, 24, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Kovács, I.; Zupkó, I.; Frank, É. An efficient approach to novel 17–5′-(1′,2′,4′)-oxadiazolyl androstenes via the cyclodehydration of cytotoxic O-steroidacylamidoximes, and an evaluation of their inhibitory action on 17α-hydroxylase/C17,20-lyase. Eur. J. Med. Chem. 2013, 70, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Neves Filho, R.A.W.; da Silva-Alves, D.C.B.; dos Anjos, J.V.; Srivastava, R.M. One-step protection-free synthesis of 3-aryl-5-hydroxyalkyl-1,2,4-oxadiazoles as building blocks. Synth. Commun. 2013, 43, 2596–2602. [Google Scholar] [CrossRef]
- Scorzo, C.M.; Fascio, M.L.; D’Accorso, N.B.; Cabrera, M.G.; Saavedra, L.A. Synthesis and antiacetylcholinesterase activity of new D-glyceraldehyde heterocyclic derivatives. J. Braz. Chem. Soc. 2010, 21, 43–48. [Google Scholar] [CrossRef]
- Pitman, M.R.; Costabile, M.; Pitson, S.M. Recent advances in the development of sphingosine kinase inhibitors. Cell Signal. 2016, 28, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.D.; Kadam, K.S.; Kandre, S.; Guha, T.; Reddy, M.M.K.; Brahma, M.K.; Deshmukh, N.J.; Dixit, A.; Doshi, L.; Potdar, N.; et al. Synthesis and biological evaluation of isoxazole, oxazole, and oxadiazole containing heteroaryl analogs of biaryl ureas as DGAT1 inhibitors. Eur. J. Med. Chem. 2012, 54, 324–342. [Google Scholar] [CrossRef] [PubMed]
- Koryakova, A.G.; Ivanenkov, Y.A.; Ryzhova, E.A.; Bulanova, E.A.; Karapetian, R.N.; Mikitas, O.V.; Katrukha, E.A.; Kazey, V.I.; Okun, I.; Kravchenko, D.V.; et al. Novel aryl and heteroaryl substituted N-[3-(4-phenylpiperazin-1-yl)propyl]-1,2,4-oxadiazole-5-carboxamides as selective GSK-3 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3661–3666. [Google Scholar] [CrossRef] [PubMed]
- Huhtiniemi, T.; Suuronen, T.; Rinne, V.M.; Wittekindt, C.; Lahtela-Kakkonen, M.; Jarho, E.; Wallén, E.A.A.; Salminen, A.; Poso, A.; Leppänen, J. Oxadiazole-carbonylaminothioureas as SIRT1 and SIRT2 inhibitors. J. Med. Chem. 2008, 51, 4377–4380. [Google Scholar] [CrossRef] [PubMed]
- Huguet, F.; Melet, A.; Alves de Sousa, R.; Lieutaud, A.; Chevalier, J.; Maigre, L.; Deschamps, P.; Tomas, A.; Leulliot, N.; Pages, J.-M.; et al. Hydroxamic acids as potent inhibitors of Fe II and Mn II E. coli methionine aminopeptidase: Biological activities and X-ray structures of oxazole hydroxamate- Ec MetAP-Mn Complexes. ChemMedChem 2012, 7, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Minorics, R.; Zupkó, I.; Frank, É. Efficient access to novel androsteno-17-(1′,3′,4′)-oxadiazoles and 17β-(1′,3′,4′)-thiadiazoles via N-substituted hydrazone and N,N′-disubstituted hydrazine intermediates, and their pharmacological evaluation in vitro. Eur. J. Med. Chem. 2015, 98, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ibrar, A.; Abbas, N. Oxadiazoles as privileged motifs for promising anticancer leads: Recent advances and future prospects. Arch. Pharm. 2014, 347, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Jain, S.; Jain, P.; Jain, P.; Tiwari, N.; Jain, R.; Jain, R.; Jain, A.K.; Agrawal, R.K. Synthesis and biological activities of oxadiazole derivatives: A review. Mini-Rev. Med. Chem. 2016, 16, 825–845. [Google Scholar] [CrossRef] [PubMed]
- Ragab, F.A.F.; Abou-Seri, S.M.; Abdel-Aziz, S.A.; Alfayomy, A.M.; Aboelmagd, M. Design, synthesis and anticancer activity of new monastrol analogues bearing 1,3,4-oxadiazole moiety. Eur. J. Med. Chem. 2017, 138, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Szakonyi, Z.; Balázs, Á.; Martinek, T.A.; Fülöp, F. Stereoselective synthesis of pinane-based β- and γ-amino acids via conjugate addition of lithium amides and nitromethane. Tetrahedron Asymmetry 2010, 21, 2498–2504. [Google Scholar] [CrossRef]
- Jimeno, C.; Pastó, M.; Riera, A.; Pericàs, M.A. Modular amino alcohol ligands containing bulky alkyl groups as chiral controllers for Et2Zn addition to aldehydes: Illustration of a design principle. J. Org. Chem. 2003, 68, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yasuda, Y.; Hayashi, M. New chiral schiff base as a tridentate ligand for catalytic enantioselective addition of diethylzinc to aldehydes. J. Org. Chem. 2006, 71, 7091–7093. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]


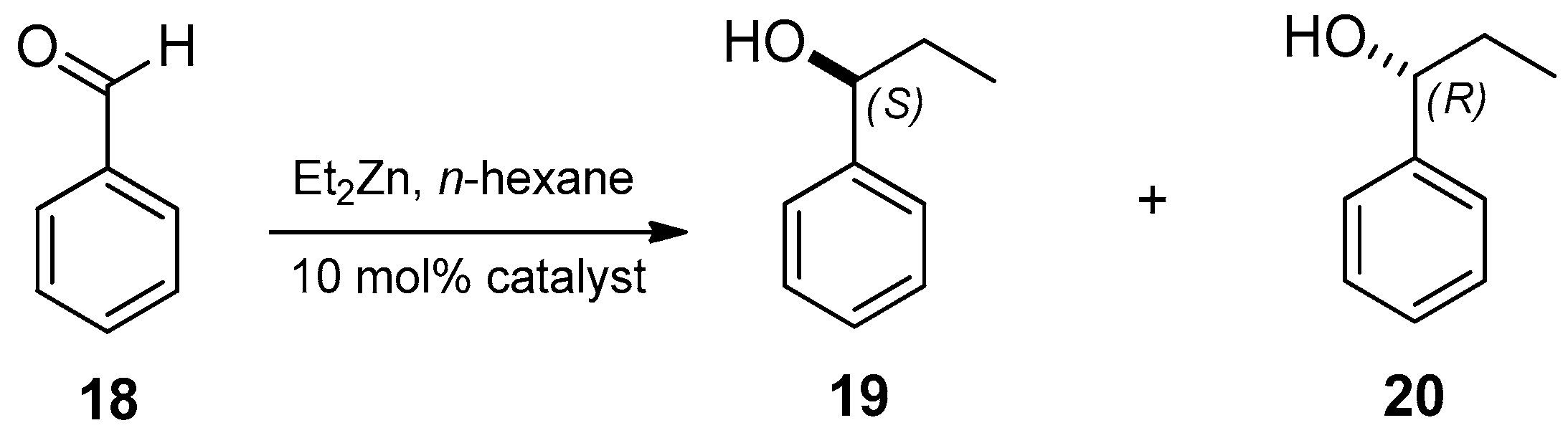
| Entry | Catalyst | Yield (%) a | ee (%) b | Configuration of the Major Enantiomer c |
|---|---|---|---|---|
| 1 | 9 | 87 | 74 | S |
| 2 | 10 | 85 | 62 | S |
| 3 | 11 | 86 | 50 | S |
| 4 | 14 | 83 | 70 | S |
| Analog | Conc. (µM) | Inhibition (%) ± SEM [Calculated IC50 Value (µM)] | |||
|---|---|---|---|---|---|
| HeLa | A2780 | MCF7 | MDA-MB-231 | ||
| 3 | 10 | 29.83 ± 1.65 | 96.66 ± 0.23 | 35.07 ± 2.64 | 19.06 ± 2.98 |
| 30 | 98.46 ± 0.08 | 96.60 ± 0.37 | 93.00 ± 0.68 | 84.58 ± 1.87 | |
| [12.23] | [1.44] | [12.37] | [16.47] | ||
| 4 | 10 | 37.96 ± 2.20 | 96.28 ± 0.32 | 49.99 ± 0.80 | 33.33 ± 1.94 |
| 30 | 98.17 ± 0.19 | 96.82 ± 0.18 | 96.08 ± 0.52 | 89.95 ± 0.90 | |
| [11.46] | [1.91] | [10.02] | [13.02] | ||
| 5 | 10 | 21.94 ± 2.94 | 91.95 ± 0.26 | 28.62 ± 1.98 | 20.31 ± 1.45 |
| 30 | 96.31 ± 0.33 | 93.77 ± 0.27 | 85.27 ± 1.84 | 58.06 ± 1.75 | |
| [13.62] | [2.05] | [14.54] | [24.28] | ||
| 6 | 10 | – * | 56.80 ± 3.18 | 17.30 ± 1.74 | 19.61 ± 2.23 |
| 30 | 47.39 ± 2.99 | 93.34 ± 0.69 | 23.87 ± 2.37 | 29.68 ± 2.54 | |
| 7 | 10 | – | 15.35 ± 1.79 | 19.80 ± 1.99 | – |
| 30 | 24.18 ± 2.42 | 36.92 ± 1.39 | 23.44 ± 1.86 | 24.66 ± 1.38 | |
| 8 | 10 | – | 12.18 ± 1.70 | 14.62 ± 2.49 | 11.71 ± 0.74 |
| 30 | 12.31 ± 2.25 | 36.58 ± 1.38 | 35.75 ± 2.79 | 20.27 ± 2.62 | |
| 9 | 10 | – | – | – | 11.45 ± 2.75 |
| 30 | 10.83 ± 2.40 | 34.56 ± 1.83 | – | 21.57 ± 1.69 | |
| 10 | 10 | – | 17.93 ± 1.06 | – | 11.43 ± 1.68 |
| 30 | 26.01 ± 0.74 | 48.27 ± 0.64 | 44.00 ± 1.34 | 24.67 ± 2.13 | |
| 11 | 10 | 12.36 ± 1.23 | – | – | – |
| 30 | 13.41 ± 1.87 | 28.44 ± 0.92 | 25.32 ± 1.10 | 10.36 ± 2.14 | |
| 12 | 10 | – | – | – | 15.51 ± 2.77 |
| 30 | – | 31.13 ± 2.48 | – | 15.92 ± 2.69 | |
| 13 | 10 | – | 11.76 ± 0.76 | – | – |
| 30 | – | 48.66 ± 1.97 | – | 18.81 ± 2.59 | |
| 14 | 10 | – | – | – | – |
| 30 | – | 29.12 ± 2.32 | 14.46 ± 2.31 | 16.28 ± 1.85 | |
| cisplatin | 10 | 42.61 ± 2.33 | 83.57 ± 1.21 | 53.03 ± 2.29 | 67.51 ± 1.01 |
| 30 | 99.93 ± 0.26 | 95.02 ± 0.28 | 86.90 ± 1.24 | 87.75 ± 1.10 | |
| [12.43] | [1.30] | [5.78] | [3.74] | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonda, T.; Bérdi, P.; Zupkó, I.; Fülöp, F.; Szakonyi, Z. Stereoselective Synthesis, Synthetic and Pharmacological Application of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles. Int. J. Mol. Sci. 2018, 19, 81. https://doi.org/10.3390/ijms19010081
Gonda T, Bérdi P, Zupkó I, Fülöp F, Szakonyi Z. Stereoselective Synthesis, Synthetic and Pharmacological Application of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles. International Journal of Molecular Sciences. 2018; 19(1):81. https://doi.org/10.3390/ijms19010081
Chicago/Turabian StyleGonda, Tímea, Péter Bérdi, István Zupkó, Ferenc Fülöp, and Zsolt Szakonyi. 2018. "Stereoselective Synthesis, Synthetic and Pharmacological Application of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles" International Journal of Molecular Sciences 19, no. 1: 81. https://doi.org/10.3390/ijms19010081
APA StyleGonda, T., Bérdi, P., Zupkó, I., Fülöp, F., & Szakonyi, Z. (2018). Stereoselective Synthesis, Synthetic and Pharmacological Application of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles. International Journal of Molecular Sciences, 19(1), 81. https://doi.org/10.3390/ijms19010081