Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities?
Abstract
1. Introduction
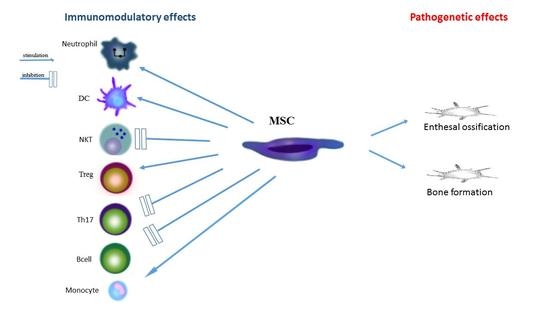
2. The Role of Mesenchymal Stromal Cells in the Inflammatory Process and in the Pathogenesis of Spondyloarthropathies
2.1. Origin of Stromal Cells
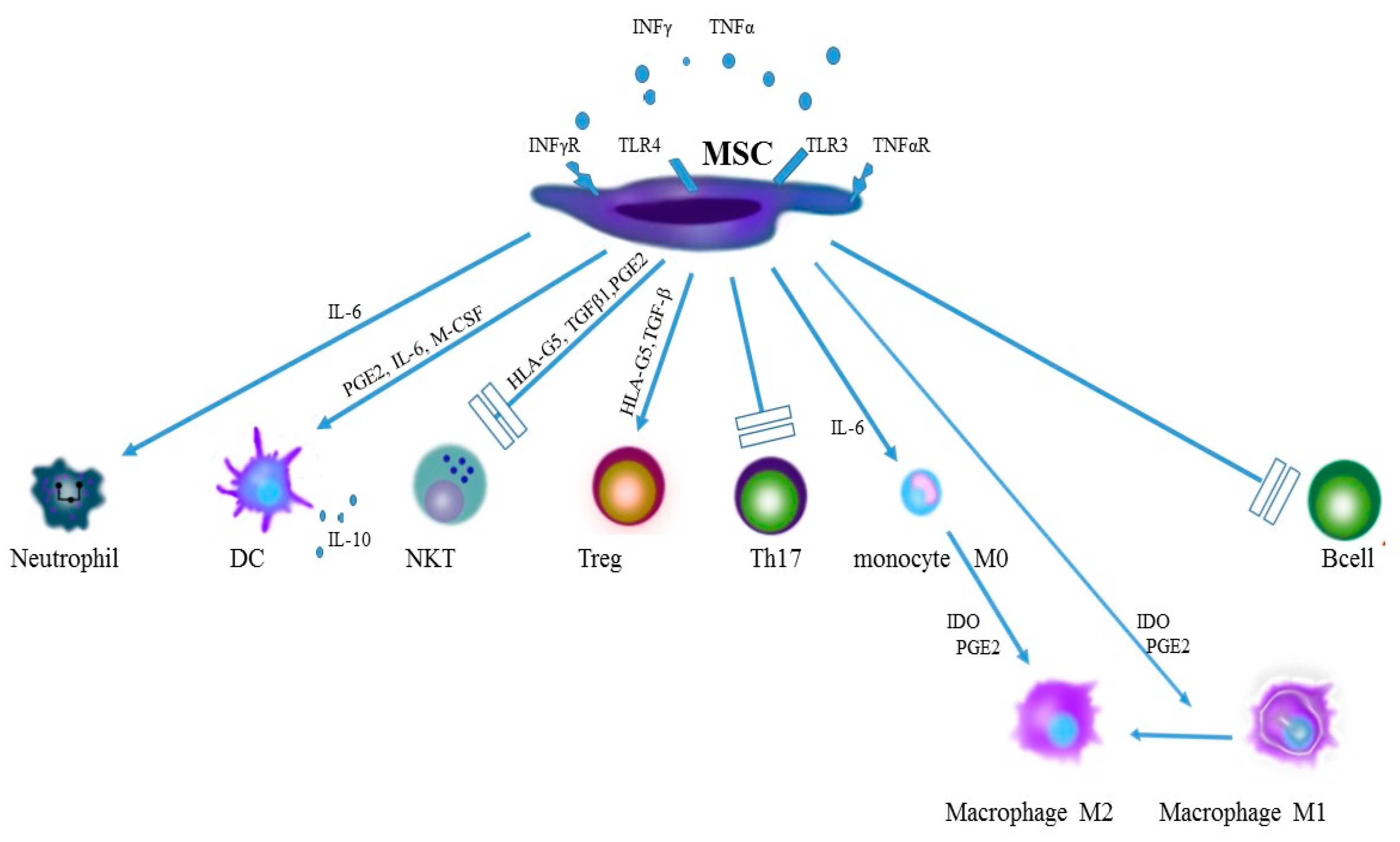
2.2. The Role of Toll-Like Receptors in Activity of Stem Cells
2.3. Stem Cells at an Early Phase of Inflammation
2.4. Monocytes and Macrophages
2.5. Dendritic Cells
2.6. Neutrophils
2.7. NK Cells
2.8. T Cells
2.9. B Cells
3. The Role of Stem Cells of Irregular Ossification in Spondyloarthropathy
4. The Role of MSC in the Treatment of Spondyloarthropathies
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rutwaleit, M. New approaches to diagnosis and classification of axial and peripheral spondyloarthritis. Curr. Opin. Rheumatol. 2010, 22, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Callhoff, J.; Sieper, J.; Weiß, A.; Zink, A.; Listing, J. Efficacy of TNF-α blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: A meta-analysis. Ann. Rheum. Dis. 2015, 74, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Hermann, K.G.; Callhoff, J.; Listing, J.; Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: Results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 2014, 73, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Baraliakos, X.; Braun, J.; Sieper, J.; Emery, P.; van der Heijde, D.; McInnes, I.; van Laar, J.M.; Landewé, R.; Wordsworth, P.; et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: A randomised, double-blind, placebocontrolled trial. Lancet 2013, 382, 1705–1713. [Google Scholar] [CrossRef]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Colter, D.C.; Sekiya, I.; Prockop, D.J. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc. Natl. Acad. Sci. USA 2001, 98, 7841–7845. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Kurtz, A.; Barutcu, N.; Bodo, J.; Thiel, A.; Dong, J. Concerted regulation of CD34 and CD105 accompanies mesenchymal stromal cell derivation from human adventitial stromal cell. Stem Cells Dev. 2013, 22, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.D.; Wagner, W.; Franke, W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy 2008, 10, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Girlovanu, M.; Susman, S.; Soritau, O.; Rus-Ciuca, D.; Melincovici, C.; Constantin, A.M.; Mihu, C.M. Stem cells—Biological update and cell therapy progress. Clujul Med. 2015, 88, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J. Biomed. Mater. Res. A 2017, 105, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.S.; Pinho, S.; Lucas, D.; Scheiermann, C. Mesenchymal stem cell: Keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu. Rev. Immunol. 2013, 31, 285–316. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Concise review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 2013, 31, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Delarosa, O.; Dalemans, W.; Lombardo, E. Toll-like receptors as modulators of mesenchymal stem cells. Front. Immunol. 2012, 3, 182. [Google Scholar] [CrossRef] [PubMed]
- Raicevic, G.; Rouas, R.; Najar, M.; Stordeur, P.; Boufker, H.I.; Bron, D.; Martiat, P.; Goldman, M.; Nevessignsky, M.T.; Lagneaux, L. Inflammation modifies the pattern and the function of Toll-like receptors expressed by human mesenchymal stromal cells. Hum. Immunol. 2010, 71, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mo, I.F.; Yip, K.H.; Chan, W.K.; Law, H.K.; Lau, Y.L.; Chan, G.C. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biol. 2008, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef] [PubMed]
- Heuschen, G.; Leowardi, C.; Hinz, U.; Autschbach, F.; Stallmach, A.; Herfarth, C.; Heuschen, U.A. Differential expression of toll-like receptor 3 and 5 in ileal pouch mucosa of ulcerative colitis patients. Int. J. Colorectal Dis. 2007, 22, 293–301. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, L.; Vandooren, B.; Kruithof, E.; De Keyser, F.; Veys, E.M.; Baeten, D. Tumor necrosis factor alpha blockade treatment down-modulates the increased systemic and local expression of Toll-like receptor 2 and Toll-like receptor 4 in spondylarthropathy. Arthritis Rheum. 2005, 52, 2146–2158. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Liang, Y.; Zhu, Y.; Li, C.; Zhang, L.Z.; Zeng, X.M.; Zhong, R.Q. Increased expression of Toll-like receptor 4 in peripheral blood leucocytes and serum levels of some cytokines in patients with ankylosing spondylitis. Clin. Exp. Immunol. 2007, 149, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Candia, L.; Marquez, J.; Hernandez, C.; Zea, A.H.; Espinoza, L.R. Toll-like receptor-2 expression is upregulated in antigen-presenting cells from patients with psoriatic arthritis: A pathogenic role for innate immunity. J. Rheumatol. 2007, 34, 374–379. [Google Scholar] [PubMed]
- Myles, A.; Aggarwal, A. Expression of Toll-like receptors 2 and 4 is increased in peripheral blood and synovial fluid monocytes of patients with enthesitis-related arthritis subtype of juvenile idiopathic arthritis. Rheumatology 2011, 50, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Brandau, S.; Jakob, M.; Hemeda, H.; Bruderek, K.; Janeschik, S.; Bootz, F.; Lang, S. Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J. Leukoc. Biol. 2010, 88, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Cassatella, M.A.; Mosna, F.; Micheletti, A.; Lisi, V.; Tamassia, N.; Cont, C.; Calzetti, F.; Pelletier, M.; Pizzolo, G.; Krampera, M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells 2011, 29, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal stem cells: A double-edged sword in regulating immune responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Jia, T.; Mendez-Ferrer, S.; Hohl, T.M.; Serbina, N.V.; Lipuma, L.; Leiner, I.; Li, M.O.; Frenette, P.S.; Pamer, E.G. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 2011, 34, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; Abomaray, F.M.; Fatani, A.S.; Chamley, L.W.; Knawy, B.A. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev. 2013, 9, 620–641. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23812784 (accessed on 25 November 2017). [CrossRef] [PubMed]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef] [PubMed]
- Dayan, V.; Yannarelli, G.; Billia, F.; Filomeno, P.; Wang, X.H.; Davies, J.E.; Keating, A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 2011, 106, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Eggenhofer, E.; Hoogduijn, M.J. Mesenchymal stem cell-educated macrophages. Transp. Res. 2012, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, W.; Tao, C.; Sun, P.; Yang, Z.; Xu, W. M2 polarization of monocytes in ankylosing spondylitis and relationship with inflammation and structural damage. APMIS 2017. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, F.; Alessandro, R.; Rizzo, A.; Accardo-Palumbo, A.; Raimondo, S.; Raiata, F.; Guggino, G.; Giardina, A.; De Leo, G.; Sireci, G.; et al. Macrophage phenotype in the subclinical gut inflammation of patients with ankylosing spondylitis. Rheumatology 2014, 53, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Van Kuijk, A.W.; Reinders-Blankert, P.; Smeets, T.J.; Dijkmans, B.A.; Tak, P.P. Detailed analysis of the cell infiltrate and the expression of mediators of synovial inflammation and joint destruction in the synovium of patients with psoriatic arthritis: Implications for treatment. Ann. Rheum Dis. 2006, 65, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.Y.; Ju, J.H.; Park, S.H.; Kim, H.Y. The paradoxical effects of TNF inhibitors on bone mineral density and radiographic progression in patients with ankylosing spondylitis. Rheumatology 2013, 52, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of Osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.M.; Rogers, L.M.; Howe, R.; Hostetler, L.A.; Buhrman, J.; McCarter, M.D.; Wilson, C.C. Human intestinal lamina propria CD1c+ dendritic cells display an activated phenotype at steady state and produce IL-23 in response to TLR7/8 stimulation. J. Immunol. 2010, 184, 6612–6621. [Google Scholar] [CrossRef] [PubMed]
- DeLay, M.L.; Turner, M.J.; Klenk, E.I.; Smith, J.A.; Sowders, D.P.; Colbert, R.A. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009, 60, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Utriainen, L.; Firmin, D.; Wright, P.; Cerovic, V.; Breban, M.; McInnes, I.; Milling, S. Expression of HLA-B27 causes loss of migratory dendritic cells in a rat model of spondyloarthritis. Arthritis Rheum. 2012, 64, 3199–3209. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, J.P.; Joyce-Shaikh, B.; Turner, S.P.; Chao, C.C.; Sathe, M.; Grein, J.; Gorman, D.M.; Bowman, E.P.; McClanahan, T.K.; Yearley, J.H.; et al. IL-23 induces spondyloarthropathy by acting on ROR-γt(+)CD3(+)CD4(-)CD8(-) entheseal resident T cells. Nat. Med. 2012, 18, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Kruisselbrink, A.B.; Lurvink, E.; Willemze, R.; Fibbe, W.E. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J. Immunol. 2006, 177, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ge, W.; Li, C.; You, S.; Liao, L.; Han, Q.; Deng, W.; Zhao, R.C. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004, 13, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, S.; Morbelli, S.; Morando, S.; Massollo, M.; Marini, C.; Bertoni, A.; Frassoni, F.; Bartolomé, S.T.; Sambuceti, G.; Traggiai, E.; et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc. Natl. Acad. Sci. USA 2011, 108, 17384–17389. [Google Scholar] [CrossRef] [PubMed]
- Favaro, E.; Carpanetto, A.; Caorsi, C.; Giovarelli, M.; Angelini, C.; Cavallo-Perin, P.; Tetta, C.; Camussi, G.; Zanone, M.M. Human mesenchymal stem cells and derived extracellular vesicles induce regulatory dendritic cells in type 1 diabetic patients. Diabetologia 2016, 59, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.; Maier, R.; Wu, P.; Scheer, R.; Hempfing, A.; Kayser, R.; Thiel, A.; Radbruch, A.; Loddenkemper, C.; Sieper, J. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 2011, 13, R95. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Vidyadaran, S.; George, E.; Ramasamy, R. Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biol. Int. 2011, 35, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Zvyagin, I.V.; Mamedov, I.Z.; Britanova, O.V.; Staroverov, D.B.; Nasonov, E.L.; Bochkova, A.G.; Chkalina, A.V.; Kotlobay, A.A.; Korostin, D.O.; Rebrikov, D.V.; et al. Contribution of functional KIR3DL1 to ankylosing spondylitis. Cell. Mol. Immunol. 2010, 7, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Perez, S.A.; Gritzapis, A.D.; Baxevanis, C.N.; Papamichail, M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Mougiakakos, D. Multipotent mesenchymal stromal cells and the innate immune system. Nat. Rev. Immunol. 2012, 12, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Prigione, I.; Benvenuto, F.; Bocca, P.; Battistini, L.; Uccelli, A.; Pistoia, V. Reciprocal interactions between human mesenchymal stem cells and gammadelta T cells or invariant natural killer T cells. Stem Cells 2009, 27, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Galipeau, J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr. Mol. Med. 2013, 13, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.; Romieu-Mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.; Pene, J.; Torcy-Moquet, G.; Jorgensen, C.; Yssel, H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010, 185, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, S.; Qu, X.; Ge, C.; Cheng, K.; Zhao, R.C. Mesenchymal stem cells inhibit Th17 cells differentiation via IFN-γ-mediated SOCS3 activation. Immunol. Res. 2015, 61, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rafei, M.; Campeau, P.M.; Aguilar-Mahecha, A.; Buchanan, M.; Williams, P.; Birman, E.; Yuan, S.; Young, Y.K.; Boivin, M.N.; Forner, K.; et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J. Immunol. 2009, 182, 5994–6002. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.F.; Zhu, J.; Lu, S.H.; Zhang, J.L.; Chen, X.; Du, L.X.; Yang, Z.G.; Song, Y.K.; Wu, D.Y.; Liu, B.; et al. Inhibitory effect of human umbilical cord-derived mesenchymal stem cells on interleukin-17 production in peripheral blood T cells from spondyloarthritis patients. Zhongguo Shi Yan Xue Ye Za Zhi 2013, 21, 455–459. [Google Scholar] [CrossRef]
- Shen, H.; Goodall, J.C.; Hill Gaston, J.S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009, 60, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Limón-Camacho, L.; Vargas-Rojas, M.I.; Vázquez-Mellado, J.; Casasola-Vargas, J.; Moctezuma, J.F.; Burgos-Vargas, R.; Llorente, L. In vivo peripheral blood proinflammatory T cells in patients with ankylosing spondylitis. J. Rheumatol. 2012, 39, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res. Ther. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Obermajer, N.; Popp, F.C.; Soeder, Y.; Haarer, J.; Geissler, E.K.; Schlitt, H.J.; Dahlke, M.H. Conversion of Th17 into IL-17A(neg) regulatory T cells: A novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J. Immunol. 2014, 193, 4988–4999. [Google Scholar] [CrossRef] [PubMed]
- Xueyi, L.; Lina, C.; Zhenbiao, W.; Qing, H.; Qiang, L.; Zhu, P. Levels of circulating Th17 cells and regulatory T cells in ankylosing spondylitis patients with an inadequate response to anti-TNF-alpha therapy. J. Clin. Immunol. 2013, 33, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.; Wu, P.; Scheer, R.; Kedor, C.; Sawitzki, B.; Thiel, A.; Radbruch, A.; Sieper, J.; Syrbe, U. Synovial and peripheral blood CD4+FoxP3+ T cells in spondyloarthritis. J. Rheumatol. 2011, 38, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zheng, M.; Zhang, K.; Yang, F.; Zhang, X.; Han, Q.; Chen, Z.N.; Zhu, P. Functional defects in CD4+ CD25high FoxP3+ regulatory cells in ankylosing spondylitis. Sci. Rep. 2016, 6, 37559. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, M.; Yang, R.; Liang, X.; Ma, Y.; Tang, Y.; Huang, L.; Ye, J.; Chen, K.; Wanget, P.; et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res. Ther. 2011, 13, R29. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25Highforkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Schrama, C.L.M.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes towards anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- LeMaoult, J.; Caumartin, J.; Daouya, M.; Favier, B.; Le Rond, S.; Gonzalez, A.; Carosella, E.D. Immune regulation by pretenders: Cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood 2007, 109, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2008, 26, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Lund, F.E.; Randall, T.D. Effector and regulatory B cells: Modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010, 10, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Nova-Lamperti, E.; Fanelli, G.; Becker, P.D.; Chana, P.; Elgueta, R.; Dodd, P.C.; Lord, G.M.; Lombardi, G.; Hernandez-Fuentesa, M.P. IL-10-produced by human transitional B-cells down-regulates CD86 expression on B-cells leading to inhibition of CD4(+)T-cell responses. Sci. Rep. 2016, 6, 20044. [Google Scholar] [CrossRef] [PubMed]
- Cantaert, T.; Doorenspleet, M.E.; Francosalinas, G.; Paramarta, J.E.; Klarenbeek, P.L.; Tiersma, Y.; van der Loos, C.M.; De Vries, N.; Tak, P.P.; Baeten, D.L. Increased numbers of CD5+ B lymphocytes with a regulatory phenotype in spondylarthritis. Arthritis Rheum. 2012, 64, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Caro, M.B.; de Miguel, E.; Peiteado, D.; Plasencia-Rodríguez, C.; Villalba, A.; Monjo-Henry, I.; Puig-Kröger, A.; Sánchez-Mateos, P.; Martín-Mola, E.; Miranda-Carús, M.E. Increased frequency of circulating CD19+CD24hiCD38hi B cells with regulatory capacity in patients with Ankylosing spondylitis (AS) naïve for biological agents. PLoS ONE 2017, 12, e0180726. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Yi, T.G.; Lee, H.J.; Kim, S.N.; Park, S.; Jeon, M.S.; Song, S.U. Mesenchymal stem cells infected with Mycoplasma arginini secrete complement C3 to regulate immunoglobulin production in b lymphocytes. Cell Death Dis. 2014, 5, e1192. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, M.; Yuan, Y.; Wang, Z.Z.; Jiang, H.; Chen, T. Priming of Toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem. Biophys. Res. Commun. 2014, 448, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, C.; Quade-Lyssy, P.; Radeke, H.H.; Henschler, R.; Konigs, C.; Kohl, U.; Seifried, E.; Schüttrumpf, J. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014, 23, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.R.; Yang, Z.X.; Han, Z.B.; Meng, L.; Liang, L.; Feng, X.M.; Yang, S.G.; Chi, Y.; Chen, D.D.; Wang, Y.W.; et al. Mesenchymal stem cells support proliferation and terminal differentiation of B cells. Cell Physiol. Biochem. 2012, 30, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ghali, O.; Lencel, P.; Broux, O.; Chauveau, C.; Devedjian, J.C.; Hardouin, P.; Magne, D. TNFα and IL1β inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci. 2009, 84, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Briolay, A.; Lencel, P.; Bessueille, L.; Caverzasio, J.; Buchet, R.; Magne, D. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-α in human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2013, 430, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Kurth, T.B.; Augello, A. Mesenchymal stem cells from development to postnatal joint homeostasis, aging, and disease. Birth Defects Res. C. Embryo Today 2010, 90, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Nourissat, G.; Diop, A.; Maurel, N.; Salvat, C.; Dumont, S.; Pigenet, A.; Gosset, M.; Houard, X.; Berenbaum, F. Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS ONE 2010, 5, e12248. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.F.; Lui, P.P.; Ni, M.; Chan, L.S.; Lee, Y.W.; Chan, K.M. Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. J. Orthop. Res. 2011, 29, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Guerra, G. Ca2+ Signalling in endothelial progenitor cells: Friend or foe? J. Cell Physiol. 2016, 231, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Ronco, V.; Potenza, D.M.; Denti, F.; Vullo, S.; Gagliano, G.; Tognolina, M.; Guerra, G.; Pinton, P.; Genazzani, A.A.; Mapelli, L.; et al. A novel Ca2+-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca2+ signaling. Cell Calcium 2015, 57, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Sun, J.; Lu, S.; Qi, Y.X.; Wang, Y. Prolonged mechanical stretch initiates intracellular calcium oscillations in human mesenchymal stem cells. PLoS ONE 2014, 9, e109378. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Joo, C.; Seong, J.; Vafabakhsh, R.; Botvinick, E.L.; Berns, M.W.; Palmer, A.E.; Wang, N.; Ha, T.; Jakobsson, E.; et al. Distinct mechanisms regulating mechanical force-induced Ca2+ signals at the plasma membrane and the ER in human MSCs. eLife 2015, 4, e04876. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, P.; Li, Y.; Deng, W.; Zhang, X.; Su, H.; Li, D.; Wu, Y.; Shen, H. Imbalance between BMP2 and Noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 2016, 68, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Bassi, E.J.; Moraes-Vieira, P.M.; Moreira-Sa, C.S.; Almeida, D.C.; Vieira, L.M.; Cunha, C.S.; Hiyane, M.I.; Basso, A.S.; Pacheco-Silva, A.; Câmara, N.O. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 2012, 61, 2534–2545. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Souza-Moreira, L.; Morell, M.; Caro, M.; O’Valle, F.; Gonzalez-Rey, E.; Delgado, M. Adipose-derived mesenchymal stromal cells induce mmunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013, 62, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, R.H.; Yu, J.M.; Ko, J.H.; Lee, H.J.; Ko, A.Y.; Roddy, G.W.; Prockop, D.J. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol. Ther. 2012, 20, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Zappia, E.; Casazza, S.; Pedemonte, E.; Benvenuto, F.; Bonanni, I.; Gerdoni, E.; Giunti, D.; Ceravolo, A.; Cazzanti, F.; Frassoni, F.; et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005, 106, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Mathias, L.J.; Khong, S.M.; Spyroglou, L.; Payne, N.L.; Siatskas, C.; Thorburn, A.N.; Boyd, R.L.; Heng, T.S. Alveolar macrophages are critical for the inhibition of allergic asthma by mesenchymal stromal cells. J. Immunol. 2013, 191, 5914–5924. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, L.; Sarukhan, A.; Dander, E.; Castor, M.; Cibella, J.; Soldani, C.; Trovato, A.E.; Ploia, C.; Luca, G.; Calvitti, M.; et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia 2013, 27, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Swart, J.F.; Wulffraat, N.M. Mesenchymal stromal cells for treatment of arthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Wyles, C.C.; Houdek, M.T.; Behfar, A.; Sierra, R.S. Mesenchymal stem cell therapy for osteoarthritis: Current perspectives. Stem Cells Cloning 2015, 8, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hinterberger, W.; Hinterberger-Fischer, M.; Marmont, A. Clinically demonstrable anti-autoimmunity mediated by allogeneic immune cells favorably affects outcome after stem cell transplantation in human autoimmune diseases. Bone Marrow Transplant. 2002, 30, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Slavin, S.; Nagler, A.; Varadi, G.; Or, R. Graft vs autoimmunity following allogeneic non-myeloablative blood stem cell transplantation in a patient with chronic myelogenous leukaemia and severe systemic psoriasis and psoriatic polyarthritis. Exp. Hematol. 2000, 28, 853–857. [Google Scholar] [CrossRef]
- Woods, A.C.; Mant, M.J. Amelioration of severe psoriasis with psoriatic arthritis for 20 years after allogeneic haematopoietic stem cell transplantation. Ann. Rheum. Dis. 2006, 65, 697. [Google Scholar] [CrossRef] [PubMed]
- Braiteh, F.; Hymes, S.R.; Giralt, S.A.; Jones, R. Complete remission of psoriasis after autologous hematopoietic stem-cell transplantation for multiple myeloma. J. Clin. Oncol. 2008, 26, 4511–4513. [Google Scholar] [CrossRef] [PubMed]
- Jantumen, E.; Myllykangas-Luosujärvi, R.; Kaipiainen-Seppänen, O.; Nousiainen, T. Autologous stem cell transplantation in a lymphoma patient with a long history of ankylosing spondylitis. Rheumatology 2000, 39, 563–564. [Google Scholar] [CrossRef]
- Yang, H.K.; Moon, S.J.; Shin, J.H.; Kwok, S.K.; Park, K.S.; Park, S.H.; Kim, H.Y.; Ju, J.H. Regression of syndesmophyte after bone marrow transplantation for acute myeloid leukemia in a patient with ankylosing spondylitis: A case report. J. Med. Case Rep. 2012, 6, 250. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3459693/ (accessed on 25 November 2017). [CrossRef] [PubMed]
- Britanova, O.V.; Bochkova, A.G.; Staroverov, D.B.; Feforenko, D.A.; Bolotin, D.A.; Memedove, I.Z.; Turchaaninova, M.A.; Putintseva, E.V.; Kotlobay, A.A.; Lukyanov, S.; et al. First autologous hematopoietic SCT for ankylosing spondylitis: A case report and clues to understanding the therapy. Bone Marrow Transplant. 2012, 47, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Huang, L.; Yang, J.; Yang, R.; Deng, W.; Liang, B.; Dai, L.; Meng, Q.; Gao, L.; et al. Effects and safety of allogenic mesenchymal stem cells intravenous infusion in active ankylosing spondylitis patients who failed NSAIDs: A 20 week clinical trial. Cell Transplant. 2014, 23, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Safety and Efficacy Study of Umbilical Cord/Placenta-Derived Mesenchymal Stem Cells to Treat Ankylosing Spondylitis. ClinicalTrials.gov Identifier: NCT01420432. Available online: www.clinicaltrials.gov (accessed on 22 October 2017).
- ClinicalTrials.gov. A Molecule Basic Study of Early Warning of New Pathogenic Risk of Ankylosing Spondylitis. ClinicalTrial.gov Identifier: NCT01709656. Available online: www.clinicaltrials.gov (accessed on 22 October 2017).
- ClinicalTrials.gov. A Pilot Study of MSCs Infusion and Etanercept to Treat Ankylosing Spondylitis. ClinicalTrial.gov Identifier: NCT02809781. Available online: www.clinicaltrials.gov (accessed on 22 October 2017).
- Chinese Clinical Trial Registry. Clinical Study of Mesenchymal Stem Cells Transplantation in Ankylosing Spondylitis. Registration Number: ChiCTR-TRC-11001417. Available online: http://www.chictr.org.cn/showprojen.aspx?proj=8122 (accessed on 22 October 2017).

| Elements of Pathogenesis of Spondyloarthropathy | Results of Stem Cell Action |
|---|---|
| Dysregulation of TLR. Increase in expression of TLR2 and TLR 4 on mononuclear cells of peripheral blood and in articular synovial membrane [21,22,23,24]. | Acquisition of the pro-inflammatory phenotype by MSC following stimulation by TLR4 and the anti-inflammatory phenotype following stimulation by TLR3 [18,19,20]. |
| Increased production of pro-inflammatory TNF-α and IFN-γ by activated monocytes and macrophages. | Activation of MSC with TNF-α and IFN-γ boosts expression of iNOS, COX2 and IDO and favours polarisation of monocytes and macrophages to the anti-inflammatory M2 phenotype M2 [34,35,36]. |
| Increase in production of inflammatory cytokines, e.g., IL-12, IL-23, IL-6 by dendritic cells [42,43]. | Inhibition of differentiation of precursors of CD40CD1a into DC, inhibition of the ability to present antigen by DC, induction of the loss of maturity features by DC [46,48,49]. |
| Increase in local production of IL-17 in joints by neutrophils [52]. | Inhibition of apoptosis and stimulation of activity of activity of neutrophils by IL-6, IL-8 IFN-β and GM-CSF [28,54]. |
| A link between expression of activating KIR receptors on NK cells with the disease activity. Recognising of HLA B27 antigen by the KIR3DL1 receptor [55]. | Inhibition of proliferation, cytokine secretion and cytotoxicity of NK cells [56,57,58,59]. |
| The key role of Th17 cells in development of SpA [67,68] | Ability of mature Th17 to convert into Treg [69,70]. |
| Decrease in the amount of Treg. Upsetting the Treg/Th17 balance. Functional defects of CD4+CD25+FOXP3 [71,72,73,74]. | Induction of Treg proliferation. Stimulation of differentiation of CD4 towards CD4+CD25+FOXP3 [75]. |
| Ossification of entheses, formation of new bone tissue on marginal surfaces of joints [1]. | Regulation of ossification with TNAP. Increased bone formation by activation of Wnt/β-catenin pathway with Wnt5a. Ossification of entheses following stimulation of calcium channels in MSC by mechanical stimuli [89,90,97]. |
| SpA | Stem Cells | Description | Reference |
|---|---|---|---|
| Psoriatic arthritis | Allogenic blood stem cell transplantation (myeloablative) | Concomitant chronic myelogenous leukemia. Graft versus autoimmunity effect. | Slavin et al. [109] |
| Psoriatic arthritis | Allogenic hematopoetic stem cell transplantation | Concomitant aplastic anemia. Short remission with long chronic disability-free period | Woods et al. [110] |
| Psoriatic arthritis | Autologous hematopoetic stem cell transplantation (myeloablative) | Concomitant multiple myeloma. Complete remission of arthritis and skin lesions | Braiteh et al. [111] |
| Ankylosing spondylitis | Autologous hematopoetic stem cell transplantation | Concomitant lymphoma. The patient underwent chemotherapy. Clinical remission for both AS and lymphoma | Jantumen et al. [112] |
| Ankylosing spondylitis | Allogenic blood stem cell transplantation | Concomitant acute myeloid leukemia. The patient underwent chemotherapy and body irradiation. Clinical remission. Partial radiological regression of syndesophytes | Britanova et al. [114] |
| Ankylosing spondylitis | Autologus hematopoetic stem cell transplant | The first reported intentional stem cell transplant for AS. The patient underwent chemotherapy. Complete remission for AS for two-year follow up period | Yang et al. [113] |
| Ankylosing spondylitis | Allogenic mesenchymal stem cells intravenous infusion | Trial involving 31 AS patients. No adverse effects noted. Reduction of ASDAS-CRP from 3.6 ± 0.6 to 2.4 ± 0.5 at the 4th week. The percentage of ASAS 20 responders reached 77.4% | Wanga et al. [115] |
| Ankylosing spondylitis | Human umbilical cord-derived mesenchymal stem cells | Clinical trial. Phase 1. Human umbilical cord-derived MSCs at a dose of 1.0 × 106 MSC/kg, repeated after three months and DMARDs such as sulfasalazine, methotrexate, thalidomide for 12 months | Clinical Trials. gov Identifier: NCT01420432 [116] |
| Ankylosing spondylitis | Human mesenchymal stem cells | Clinical trial. human mesenchymal stem cells: 1.0 × 104-6 cells/kg, IV on day 1 of each 14–60 day cycle, 1–6 times treatment, plus NSAIDs. | ClinicalTrials.gov Identifier: NCT01709656 [117] |
| Ankylosing spondylitis | Human bone marrow-derived MSCs | Recruiting clinical trial. Phase 2. hBM-MSCs at a dose of 1.0 × 106 MSC/kg, receive infusion per week in the first 4 weeks and every two weeks in the second 8 weeks. Study Start Date: June 2016 Estimated Study Completion Date: December 2018 | ClinicalTrials.gov Identifier: NCT02809781 [118] |
| Ankylosing spondylitis | Mesenchymal stem cells | Clinical trial. Phase I/II. To observe the safety and clinical effect of MSC transplantation in AS | Clinical trial. Registration number: ChiCTR-TRC-11001417 [119] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Włodarczyk, M.; Owczarczyk-Saczonek, A.; Placek, W.; Osowski, A.; Engelgardt, P.; Wojtkiewicz, J. Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities? Int. J. Mol. Sci. 2018, 19, 80. https://doi.org/10.3390/ijms19010080
Krajewska-Włodarczyk M, Owczarczyk-Saczonek A, Placek W, Osowski A, Engelgardt P, Wojtkiewicz J. Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities? International Journal of Molecular Sciences. 2018; 19(1):80. https://doi.org/10.3390/ijms19010080
Chicago/Turabian StyleKrajewska-Włodarczyk, Magdalena, Agnieszka Owczarczyk-Saczonek, Waldemar Placek, Adam Osowski, Piotr Engelgardt, and Joanna Wojtkiewicz. 2018. "Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities?" International Journal of Molecular Sciences 19, no. 1: 80. https://doi.org/10.3390/ijms19010080
APA StyleKrajewska-Włodarczyk, M., Owczarczyk-Saczonek, A., Placek, W., Osowski, A., Engelgardt, P., & Wojtkiewicz, J. (2018). Role of Stem Cells in Pathophysiology and Therapy of Spondyloarthropathies—New Therapeutic Possibilities? International Journal of Molecular Sciences, 19(1), 80. https://doi.org/10.3390/ijms19010080