Exploring the Mechanisms of Electroacupuncture-Induced Analgesia through RNA Sequencing of the Periaqueductal Gray
Abstract
1. Introduction
2. Results
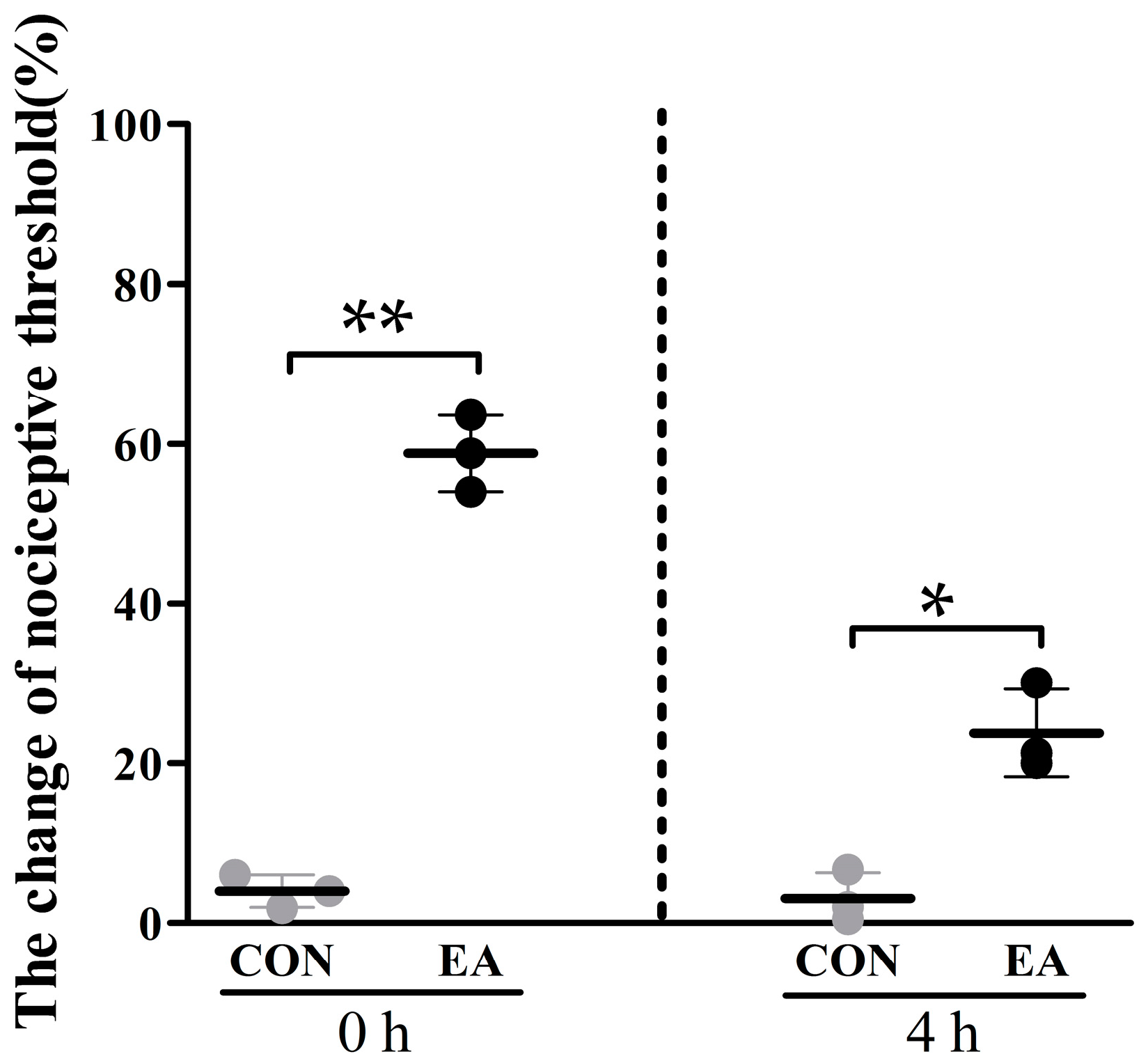
2.1. Effects of Electroacupuncture (EA) on Nociceptive Threshold
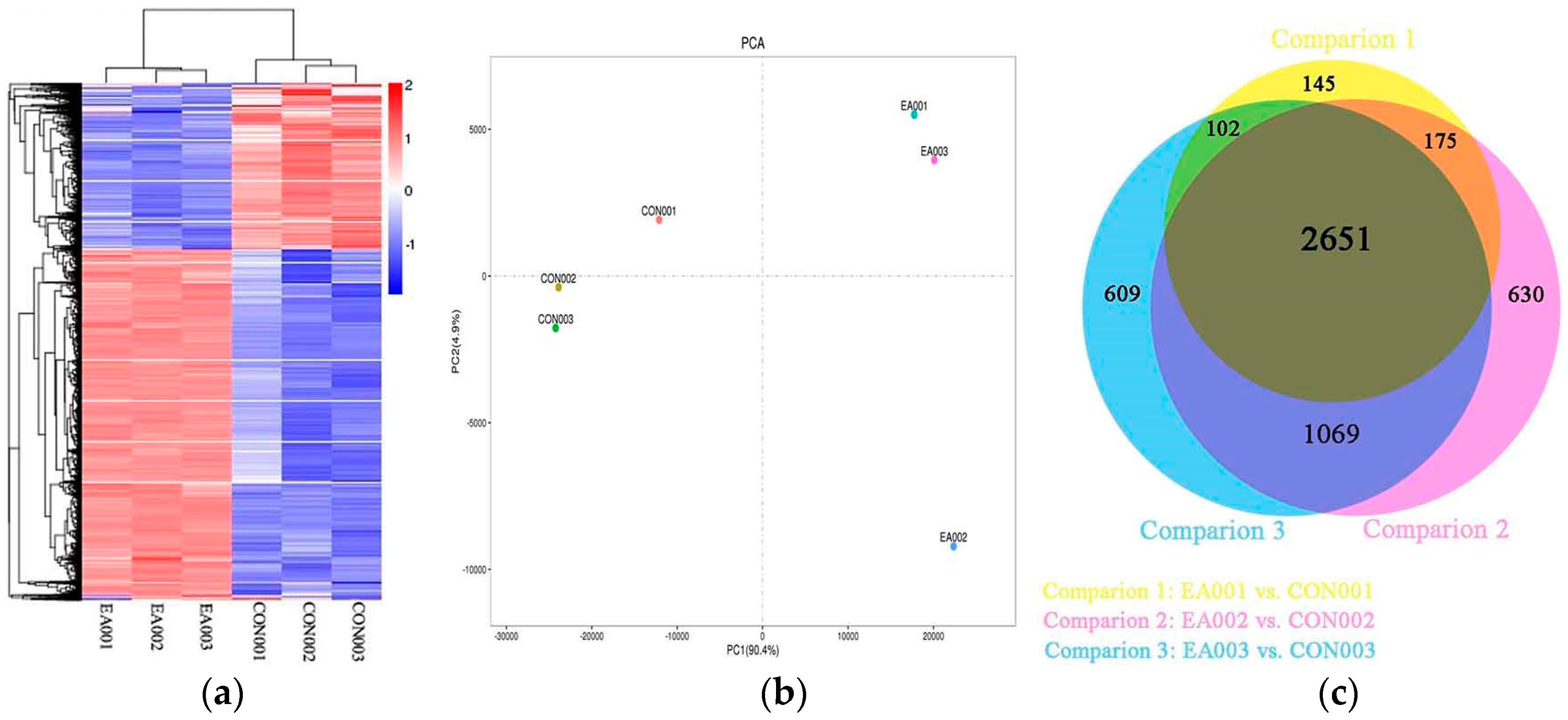
2.2. Global Analysis of Transcriptome
2.3. Differentially Expressed Genes between EA and Control Group
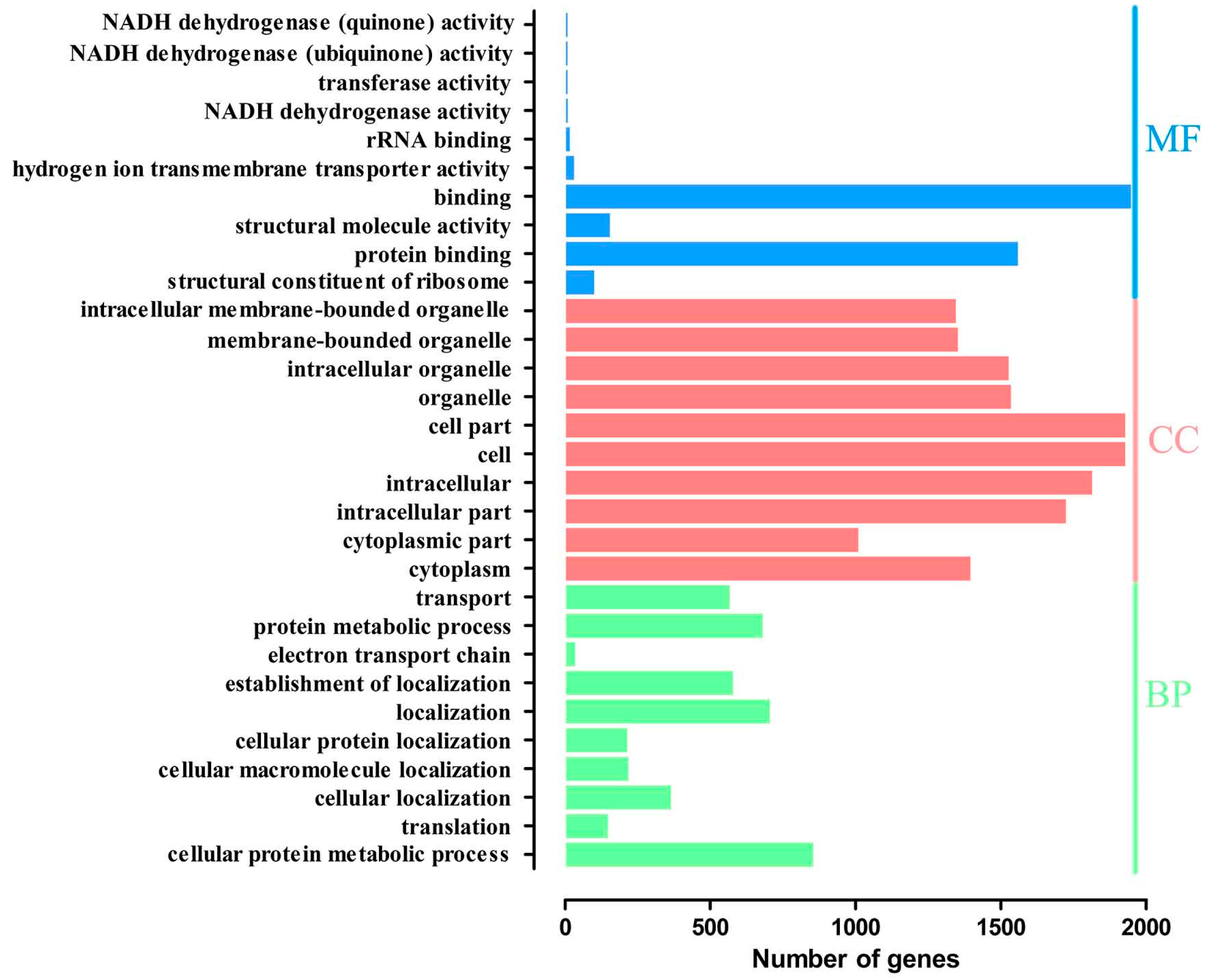
2.4. Gene Ontology Overrepresentation Analysis
2.5. Kyoto Encyclopedia of Genes and Genomes Pathway Analysis
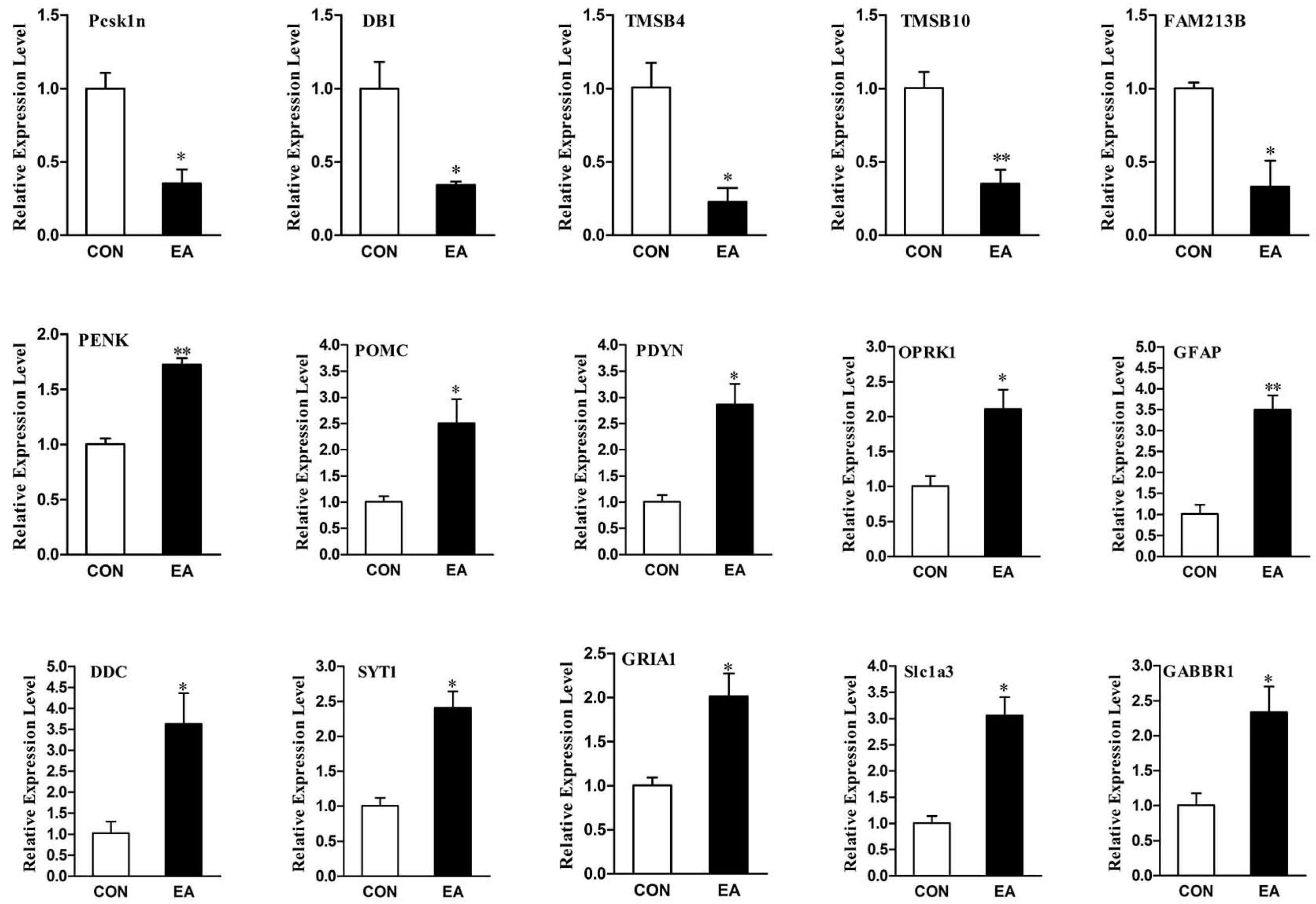
2.6. Differential Expression Genes Analysis and qPCR Validation
3. Discussion
4. Materials and Methods
4.1. Animal Preparation
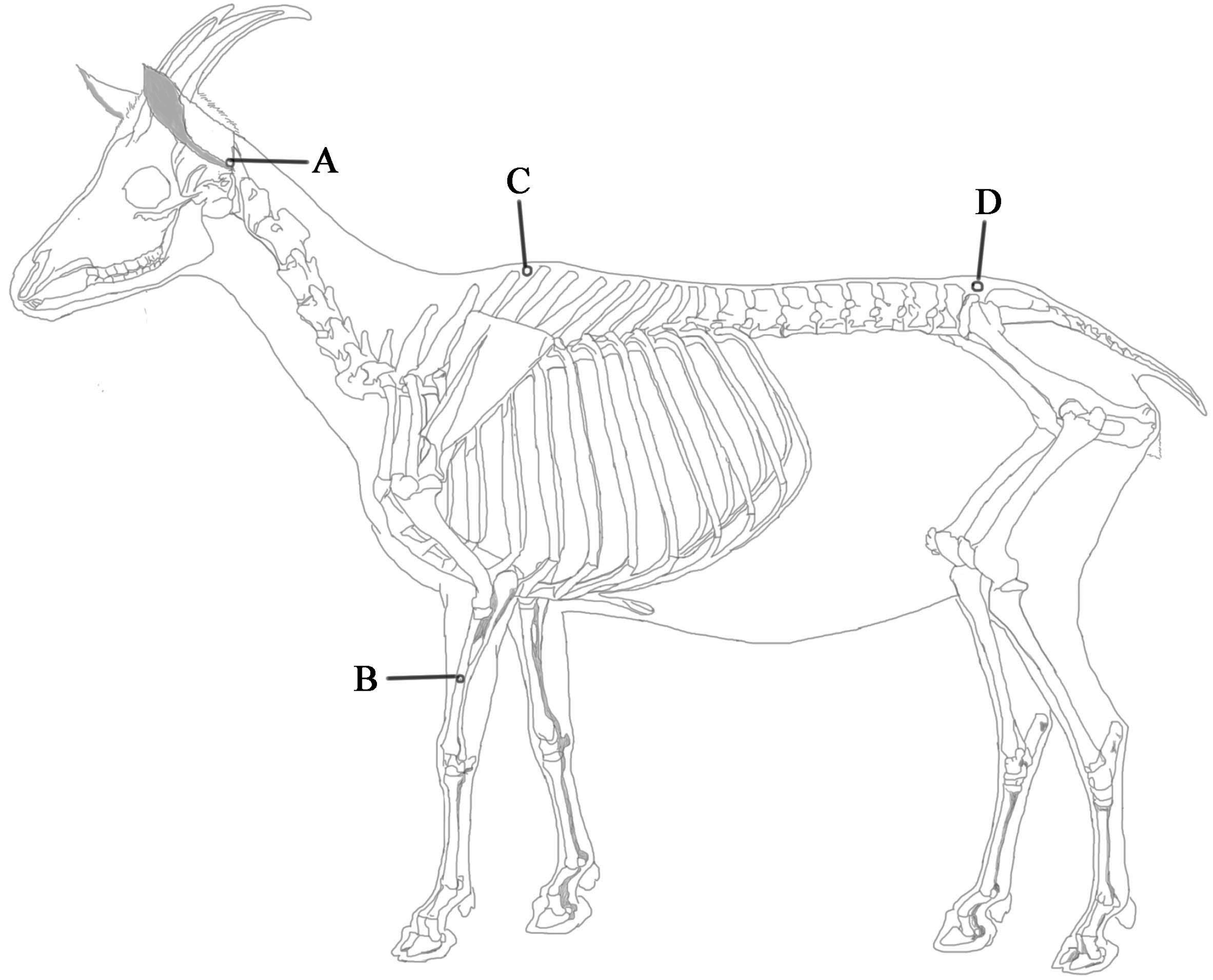
4.2. EA Application
4.3. Determination of Nociceptive Threshold.
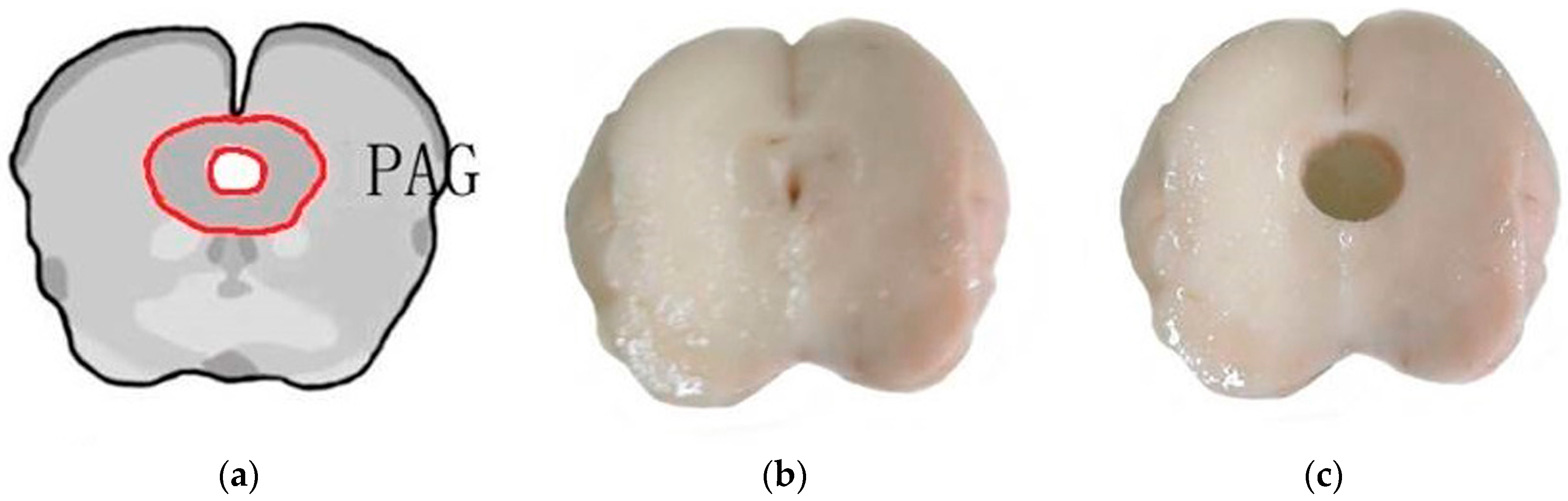
4.4. Extraction of RNA and Sequencing Using RNA-Seq Technology
4.5. Quality Control for Raw Sequencing Data
4.6. Transcriptome Analysis for mRNA Data
4.7. Validation of Differential Expression Genes
4.8. Statistical Analysis.
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EA | electroacupuncture |
| EAA | EA-induced analgesia |
| CNS | central nerve system |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinase |
| PAG | periaqueductal gray |
| PCA | principal component analysis |
| SDH | spinal dorsal horn |
| PENK | proenkephalin |
| POMC | proopiomelanocortin |
| PDYN | preprodynorphin |
| DBI | diazepam-binding inhibitor |
| Pcsk1n | proprotein convertase 1 inhibitor |
| RPKM | Reads Per Kilobase of exon model per Million mapped reads |
| DEGs | differentially expressed genes |
| OPRK1 | opioid receptor kappa 1 |
| GO | gene ontology |
| KEGG | kyoto Encyclopedia of Genes and Genomes |
| GRIA1 | glutamate receptor 1 |
| Slc1a3 | excitatory amino acid transporter |
| SYT1 | synaptotagmin-1 |
| GABBR1 | γ-aminobutyric acid type B receptor subunit 1 |
| GABA | γ-aminobutyric acid |
| GABAa | γ-aminobutyric acid type A |
| GABAb | γ-aminobutyric acid type B |
| TMSB4 | thymosin β4 (gene) |
| Tβ4 | thymosin β4 (protein) |
| NMDA | N-Methyl-d-aspartate |
References
- Coutaux, A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Jt. Bone Spine Rev. Rhum. 2017, 84, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, H.; Wu, W.; Yu, W.; Li, Y.; Bai, J.; Luo, B.; Li, S. Acupuncture for pain management in cancer: A systematic review and meta-analysis. Evid.-Based Complement. Altern. Med. 2016, 2016, 1720239. [Google Scholar] [CrossRef] [PubMed]
- Ulett, G.A.; Han, S.; Han, J.S. Electroacupuncture: Mechanisms and clinical application. Biol. Psychiatry 1998, 44, 129–138. [Google Scholar] [CrossRef]
- Research-Group, A.A. The role of some neurotransmitters of brain in finger-acupuncture analgesia. Sci. China Ser. A 1974, 1, 114–132. [Google Scholar]
- Han, J.S.; Terenius, L. Neurochemical basis of acupuncture analgesia. Ann. Rev. Pharmacol. Toxicol. 1982, 22, 193–220. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.J.; Price, D.D.; Rafii, A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977, 121, 368–372. [Google Scholar] [CrossRef]
- Sjölund, B.H.; Eriksson, M.B.E. The influence of naloxone on analgesia produced by peripheral conditioning stimuli. Brain Res. 1979, 173, 295–301. [Google Scholar] [CrossRef]
- Jiang, Z.; Ye, Q.; Tang, S. Effects of naloxone on experimental acupuncture analgesia evaluated by sensory decision theory. Acta Zool. Sin. 1978, 24, 1–10. [Google Scholar]
- Zhao, Z.Q. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 2008, 85, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Hu, Z.S.Z.; Li, H.T.; Han, J.S.; Wan, Y. CCKB receptor antagonist L365,260 potentiates the efficacy to and reverses chronic tolerance to electroacupuncture-induced analgesia in mice. Brain Res. Bull. 2007, 71, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.S.; Kim, S.K.; Kim, J.T.; Lee, G.; Han, J.B.; Rho, S.W.; Hong, M.C.; Bae, H.; Min, B.I. The difference in mRNA expressions of hypothalamic CCK and CCK-A and -B receptors between responder and non-responder rats to high frequency electroacupuncture analgesia. Peptides 2006, 27, 1841–1845. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Geller, E.B.; Adler, M.W. CCK B receptors in the periaqueductal grey are involved in electroacupuncture antinociception in the rat cold water tail-flick test. Neuropharmacology 1998, 37, 751–757. [Google Scholar] [CrossRef]
- Yonehara, N.; Sawada, T.; Matsuura, H.; Inoki, R. Influence of electro-acupuncture on the release of substance P and the potential evoked by tooth pulp stimulation in the trigeminal nucleus caudalis of the rabbit. Neurosci. Lett. 1992, 142, 53–56. [Google Scholar] [CrossRef]
- Zhu, L.X.; Zhao, F.Y.; Cui, R.L. Effect of acupuncture on release of substance P. Ann. N. Y. Acad. Sci. 1991, 632, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Shen, S.; Li, J.; Wang, X.M.; Han, J.S. Angiotensin II release and anti-electroacupuncture analgesia in spinal cord. Sheng Li Xue Bao 1996, 48, 543–550. [Google Scholar] [PubMed]
- NeuroPep. Available online: http://www.neuropeptides.nl/tabel%20neuropeptides%20linked.htm (accessed on 23 November 2017).
- Xu, K.D.; Liang, T.; Wang, K.; Tian, D.A. Effect of pre-electroacupuncture on p38 and c-Fos expression in the spinal dorsal horn of rats suffering from visceral pain. Chin. Med. J. 2010, 123, 1176–1181. [Google Scholar] [PubMed]
- Hsu, S.F.; Zeng, Y.J.; Tsai, S.Y.; Chen, K.B.; Chen, J.Y.; Chang, J.H.; Wen, Y.R. Spinal p38 activity and analgesic effect after low- and high-intensity electroacupuncture stimulation in a plantar incision rat model. Life Sci. 2015, 128, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.C.; Lee, J.Y.; Lim, E.J.; Baik, H.H.; Oh, T.H.; Yune, T.Y. Inhibition of ROS-induced p38MAPK and ERK activation in microglia by acupuncture relieves neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2012, 236, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Samineni, V.K.; Grajalesreyes, J.G.; Copits, B.A.; O’Brien, D.E.; Trigg, S.L.; Gomez, A.M.; Bruchas, M.R.; Th, G.R. Divergent Modulation of Nociception by Glutamatergic and GABAergic Neuronal Subpopulations in the Periaqueductal Gray. Eneuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Torres-Reverón, A.; Palermo, K.; Hernández-López, A.; Hernández, S.; Cruz, M.L.; Thompson, K.J.; Flores, I.; Appleyard, C.B. Endometriosis is associated with a shift in MU opioid and NMDA receptor expression in the brain periaqueductal gray. Reprod. Sci. 2016, 23, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Leon, A.; Fernández-Guasti, A.; González-Trujano, M.E. Rutin antinociception involves opioidergic mechanism and descending modulation of ventrolateral periaqueductal grey matter in rats. Eur. J. Pain 2016, 20, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Tjen-A-Looi, S.C.; Li, P.; Longhurst, J.C. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: Roles of endocannabinoids and GABA. J. Appl. Physiol. 2009, 106, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Jin, Z.; Wang, Y.; Li, K.; Kong, J.; Nixon, E.E.; Zeng, Y.; Ren, Y.; Tong, H.; Wang, Y. The salient characteristics of the central effects of acupuncture needling: Limbic-paralimbic-neocortical network modulation. Hum. Brain Mapp. 2009, 30, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fang, J.; Nixon, E.; Liu, J.; Papadimitriou, G.; Marina, O.; Cavanagh, G.; Napadow, V.; Kwong, K.; Vangel, M. Dynamic response of the human brain to acupuncture at LV3 as monitored by fMRI Evidence of limbic system modulation. NeuroImage 2006, 31, S161. [Google Scholar]
- Napadow, V.; Kettner, N.; Liu, J.; Li, M.; Kwong, K.; Vangel, M.; Makris, N.; Audette, J.; Hui, K. Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain 2007, 130, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Ann. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef]
- Shah, Z.; Hu, M.L.; Qiu, Z.Y.; Zhou, F.Y.; Zeng, J.; Wan, J.; Wang, S.W.; Zhang, W.; Ding, M.X. Physiologic and biochemical effects of electroacupuncture combined with intramuscular administration of dexmedetomidine to provide analgesia in goats. Am. J. Vet. Res. 2016, 77, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Zhou, Z.Y.; Ding, Y.; Chen, J.G.; Hu, C.M.; Chen, X.; Ding, M.X. Physiologic effects of electroacupuncture combined with intramuscular administration of xylazine to provide analgesia in goats. Am. J. Vet. Res. 2009, 70, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Gui-Lian, Q.U.; Zhuang, X.L.; Guo-Hui, X.U.; Yang, L.F.; Wang, Z.F.; Chen, S.L.; Wang, S.R.; Da, X.U.; Xie, T. Clincal observation on combined anesthetics-acupuncture anesthesia in 50 patients undergoing renal transplantation. Chin. J. Pain Med. 1996, 2, 72–77. [Google Scholar]
- Han, J.S. Acupuncture Anesthesia(AA)versus acupuncture-assisted Anesthesia(AAA). Zhen Ci Yan Jiu 1997, 7, 16–18. [Google Scholar]
- Cheng, L.L.; Ding, M.X.; Xiong, C.; Zhou, M.Y.; Qiu, Z.Y.; Wang, Q. Effects of electroacupuncture of different frequencies on the release profile of endogenous opioid peptides in the central nerve system of goats. Evid.-Based Complement. Altern. Med. eCAM 2012, 2012, 476457. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.L.; Ding, M.X.; Wei, J.; Wu, Y.Q.; Qiu, Z.Y.; Chen, J.G.; Liu, D.M.; Hu, C.M.; Hu, M.L.; Shah, Z. Electroacupuncture-induced dynamic processes of gene expression levels of endogenous opioid peptide precursors and opioid receptors in the CNS of goats. Evid.-Based Complement. Altern. Med. eCAM 2013, 2013, 257682. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Han, J.S. Acupuncture analgesia: Areas of consensus and controversy. Pain 2011, 152, 1439. [Google Scholar] [CrossRef] [PubMed]
- Staud, R.; Price, D.D. Mechanisms of acupuncture analgesia for clinical and experimental pain. Expert Rev. Neurother. 2006, 6, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xiang, X.H.; Qiao, N.; Qi, J.Y.; Lin, L.B.; Zhang, R.; Shou, X.J.; Ping, X.J.; Han, J.S.; Han, J.D. Genomewide analysis of rat periaqueductal gray-dorsal horn reveals time-, region- and frequency-specific mRNA expression changes in response to electroacupuncture stimulation. Sci. Rep. 2014, 4, 6713. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Ding, Y.; Feng, Y.; Chen, S.H.; Xu, Y.Q.; Li, M.; Hu, M.L.; Qiu, Z.Y.; Ding, M.X. MiRNAs are involved in chronic electroacupuncture tolerance in the rat hypothalamus. Mol. Neurobiol. 2017, 54, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.H.; Xie, B.N.; Wang, W.J.; Jing-Ming, L.I.; Jing, H.U. Variety of opioid peptides gene expression of inflamed location in different time after electro-acupuncture in adjuvant arthritis rats. China J. Basic Med. Tradit. Chin. Med. 2005, 6, 440–442. [Google Scholar]
- Cui, L.Y.; Ding, Y.; Zeng, J.; Feng, Y.; Li, M.; Ding, M.X. Spinal glutamate transporters are involved in the development of electroacupuncture tolerance. Int. J. Mol. Sci. 2016, 17, 357. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, Y.; Ohta, M.; Ito, A.; Takamatsu, K.; Sugano, A.; Funakoshi, K.; Takaoka, N.; Sato, N.; Yokozaki, H.; Arizono, N. Electroacupuncture suppresses myostatin gene expression: Cell proliferative reaction in mouse skeletal muscle. Physiol. Genom. 2007, 30, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Tian, J.; Wang, X.; Fang, Y.; Hou, Y.; Han, J. Brain substrates activated by electroacupuncture of different frequencies (I): Comparative study on the expression of oncogene c-fos and genes coding for three opioid peptides. Brain Res. Mol. Brain Res. 1996, 43, 157–166. [Google Scholar] [CrossRef]
- Pei, P.; Liu, L.; Zhao, L.; Cui, Y.; Qu, Z.; Wang, L. Effect of electroacupuncture pretreatment at GB20 on behaviour and the descending pain modulatory system in a rat model of migraine. Acupunct. Med. 2016, 34, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Qi, D.; Li, W. Downregulation of the spinal NMDA receptor NR2B subunit during electro-acupuncture relief of chronic visceral hyperalgesia. J. Physiol. Sci. 2016, 67, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Qiu, Z.Y.; Hu, K.; Ding, M.X. Analgesic neural circuits are activated by electroacupuncture at two sets of acupoints. Evid.-Based Complement. Altern. Med. 2016, 2016, 3840202. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.Y.; Ding, Y.; Cui, L.Y.; Hu, M.L.; Ding, M.X. The expression patterns of c-Fos and c-Jun induced by different frequencies of electroacupuncture in the brain. Evid.-Based Complement. Altern. Med. eCAM 2015, 2015, 343682. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, J. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: Another cross-tolerance study. Behav. Brain Res. 1992, 47, 143–149. [Google Scholar] [CrossRef]
- Gao, P.; Gao, X.I.; Fu, T.; Xu, D.; Wen, Q. Acupuncture: Emerging evidence for its use as an analgesic (Review). Exp. Ther. Med. 2015, 9, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.; Chu, J.; Wang, G.; Xu, H.; Liu, W.Y.; Wang, C.H.; Lin, B.C. Endogenous opiate peptides in the spinal cord are involved in the analgesia of hypothalamic paraventricular nucleus in the rat. Peptides 2009, 30, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, W.Y.; Song, C.Y.; Lin, B.C. Through central arginine vasopressin, not oxytocin and endogenous opiate peptides, glutamate sodium induces hypothalamic paraventricular nucleus enhancing acupuncture analgesia in the rat. Neurosci. Res. 2006, 54, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, W.; Cao, Y.; Han, J.S. Effects of synchronous or asynchronous electroacupuncture stimulation with low versus high frequency on spinal opioid release and tail flick nociception. Exp. Neurol. 2005, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.F.; Wang, X.M.; Tian, J.H.; Huo, Y.P.; Han, J.S. 2 Hz and 100 Hz electroacupuncture accelerate the expression of genes encoding three opioid peptides in the rat brain. Acta Physiol. Sin. 1997, 49, 121–127. [Google Scholar]
- Chrétien, M.; Mbikay, M. 60 Years of POMC: From the prohormone theory to pro-opiomelanocortin and to proprotein convertases (PCSK1 to PCSK9). J. Mol. Endocrinol. 2016, 56, T49–T62. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Liu, J.; Jansen, E.J.; Ozawa, A.; Panula, P.; Martens, G.J.; Lindberg, I. Identification of proSAAS homologs in lower vertebrates: Conservation of hydrophobic helices and convertase-inhibiting sequences. Endocrinology 2009, 150, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Guidotti, A. Diazepam binding inhibitor (DBI): A peptide with multiple biological actions. Life Sci. 1991, 49, 325–344. [Google Scholar] [CrossRef]
- Guidotti, A.; Forchetti, C.M.; Corda, M.G.; Konkel, D.; Bennett, C.D.; Costa, E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc. Natl. Acad. Sci. USA 1983, 80, 3531–3535. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Han, J.B.; Kim, S.K.; Park, J.H.; Go, D.H.; Sun, B.; Min, B.I. Spinal GABA receptors mediate the suppressive effect of electroacupuncture on cold allodynia in rats. Brain Res. 2010, 1322, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, B.; Nguyen, P. Intrathecal diazepam suppresses nociceptive reflexes and potentiates electroacupuncture effects in pentobarbital-anesthetized rats. Neurosci. Lett. 1987, 77, 316–320. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Y.; Mahmood, A.; Meng, Y.; Zhang, Z.G.; Morris, D.C.; Chopp, M. Neuroprotective and neurorestorative effects of thymosin β4 treatment initiated 6 hours after traumatic brain injury in rats. J. Neurosurg. 2012, 116, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Dathe, V.; Brand-Saberi, B. Expression of thymosin β4 during chick development. Anat. Embryol. 2004, 208, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Márquez, J.; Anadón, R. The β-thymosins, small actin-binding peptides widely expressed in the developing and adult cerebellum. Cerebellum 2002, 1, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Santra, M.; Chopp, M.; Zhang, Z.G.; Lu, M.; Santra, S.; Nalani, A.; Santra, S.; Morris, D.C. Thymosin β4 mediates oligodendrocyte differentiation by upregulating p38 MAPK. Glia 2012, 60, 1826–1838. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.Y.; Song, X.X.; Zheng, H.; Zhao, Y.B.; Fu, G.S. Thymosin β4 Induces endothelial progenitor cell migration via PI3K/Akt/eNOS signal transduction pathway. J. Cardiovasc. Pharmacol. 2009, 53, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Chen, S.P.; Gao, Y.H.; Qiao, L.N.; Zhang, J.L.; Liu, J.L. Effect of repeated electroacupuncture intervention on hippocampal ERK and p38MAPK signaling in neuropathic pain rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 641286. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Du, J.Y.; Qiu, Y.J.; Fang, J.F.; Liu, J.; Fang, J.Q. Electroacupuncture attenuates spinal nerve ligation-induced microglial activation mediated by p38 mitogen-activated protein kinase. Chin. J. Integr. Med. 2016, 22, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology 2014, 120, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Q.; Wang, H.C.; Wan, Y.; Jing, Z.; Luo, F.; Han, J.S.; Wang, Y. Suppression of neuropathic pain by peripheral electrical stimulation in rats: Mu-opioid receptor and NMDA receptor implicated. Exp. Neurol. 2004, 187, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, H.T.; Shi, Y.S.; Han, J.S.; Wan, Y. Ketamine potentiates the effect of electroacupuncture on mechanical allodynia in a rat model of neuropathic pain. Neurosci. Lett. 2004, 368, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.T.; Lee, J.H.; Wan, Y.; Han, J.S. Involvement of ionotropic glutamate receptors in low frequency electroacupuncture analgesia in rats. Neurosci. Lett. 2005, 377, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.T.; Kang, J.; Jo, U.B. Effects of electroacupuncture with different frequencies on spinal ionotropic glutamate receptor expression in complete Freund’s adjuvant-injected rat. Acta Histochem. 2005, 107, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Wang, L.; Wang, X.; Ren, K.; Berman, B.M.; Lao, L. Electroacupuncture combined with MK-801 prolongs anti-hyperalgesia in rats with peripheral inflammation. Pharmacol. Biochem. Behav. 2005, 81, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, C.; Yang, B.; Ji, C.; Li, W. The effect of neonatal capsaicin on acupuncture analgesia—To evaluate the role of C fibers in acupuncture analgesia. Acupunct. Res. 1990, 15, 285–291. [Google Scholar]
- Zhu, L.; Yanyan, Y.E.; Xiaorong, M.O.; Changfu, J.I. The Important Role of Activation of GABA_B Receptors in Acupuncture Analgesia. Acupunct. Res. 2002, 27, 85–91. [Google Scholar]
- Zhu, L.; Mo, X.; Ye, Y.; Ji, C. Is GABA in Brain Involved in Acupuncture Analgesia? Acupunct. Res. 2001, 3, 199–200. [Google Scholar]
- Kim, H.N.; Kim, Y.R.; Jang, J.Y.; Shin, H.K.; Choi, B.T. Electroacupuncture Confers Antinociceptive Effects via Inhibition of Glutamate Transporter Downregulation in Complete Freund’s Adjuvant-Injected Rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 643973. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Cui, L.Y.; Feng, Y.; Ding, M.X. Electroacupuncture relieves neuropathic pain via upregulation of glutamate transporters in the spinal cord of rats. Neurosci. Lett. 2016, 620, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Nishiki, T.; Augustine, G.J. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 6127–6132. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Chacón, R.; Königstorfer, A.; Gerber, S.H.; García, J.; Matos, M.F.; Stevens, C.F.; Brose, N.; Rizo, J.; Rosenmund, C.; Südhof, T.C. Synaptotagmin I functions as a calcium regulator of release probability. Nature 2001, 410, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Edelmayer, R.M.; Brederson, J.-D.; Jarvis, M.F.; Bitner, R.S. Biochemical and pharmacological assessment of MAP-kinase signaling. Biochem. Pharmacol. 2014, 87, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Th, G.R.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Kawasaki, H.; Nishina, H. Diverse Roles of JNK and MKK Pathways in the Brain. J. Signal Transduct. 2012, 2012, 459265. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Q.; Du, J.Y.; Liang, Y.; Fang, J.F. Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1 pathway in treating inflammatory pain induced by CFA in rats. Mol. Pain 2013, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choi, D.C.; Oh, T.H.; Yune, T.Y. Analgesic effect Of acupuncture is mediated Via inhibition Of JNK activation in astrocytes after spinal cord injury. PLoS ONE 2013, 8, e73948. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, J.J.; Jeon, S.; Doo, A.R.; Kim, S.N.; Lee, H.; Chae, Y.; Maixner, W.; Lee, H.; Park, H.J. From peripheral to central: The role of ERK signaling pathway in acupuncture analgesia. J. Pain 2014, 15, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Liu, S.; Zhang, M.; Zhao, J.; Wang, Y.; Wu, G.; Mi, W. Inhibition of spinal interlukin-33/ST2 signaling and downstream ERK and JNK pathways in electroacupuncture analgesia in formalin mice. PLoS ONE 2015, 10, e0129576. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, M.A.; Canteras, N.S.; Suchecki, D.; Mello, L.E. Analgesia and c-Fos expression in the periaqueductal gray induced by electroacupuncture at the Zusanli point in rats. Brain Res. 2003, 973, 196–204. [Google Scholar] [CrossRef]
- At, G.D.F.; Lemonica, L.; De, F.J.; Pereira, S.; Bedoya Henao, M.D. Preemptive analgesia with acupuncture monitored by c-Fos expression in Rats. J. Acupunct. Meridian Stud. 2016, 9, 16–21. [Google Scholar]
- Yang, Z.; Tang, J.; Yuan, B.; Jia, H. The integrative action of thalamic nucleus submedius and ventrolateral orbital cortex in acupuncture modulation on nociceptive stimulation. Acupunct. Res. 2001, 26, 197–198. [Google Scholar]
- Liu, Y.; Li, Z.; Shi, X.; Liu, Y.; Li, W.; Duan, G.; Li, H.; Yang, X.; Zhang, C.; Zou, L. Neuroprotection of up-regulated carbon monoxide by electrical acupuncture on perinatal hypoxic-ischemic brain damage in rats. Neurochem. Res. 2014, 39, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.K.; Zhou, H.J.; Wu, J.; Tang, T.; Liang, Q.H. Electroacupuncture at Zusanli (ST36) accelerates intracerebral hemorrhage-induced angiogenesis in rats. Chin. J. Integr. Med. 2013, 19, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Dai, Q.F.; Chen, W.H.; Jiang, S.T.; Chen, S.X.; Zhang, Y.J.; Tang, C.Z.; Cheng, S.B. Effects of acupuncture on cortical expression of Wnt3a, β-catenin and Sox2 in a rat model of traumatic brain injury. Acupunct. Med. J. Br. Med. Acupunct. Soc. 2016, 34, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Tao, J.; Lin, Y.; Lin, R.; Liu, W.; Chen, L. Electro-acupuncture exerts beneficial effects against cerebral ischemia and promotes the proliferation of neural progenitor cells in the cortical peri-infarct area through the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2015, 36, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Ravid, T.; Hochstrasser, M. Degradation signal diversity in the ubiquitin-proteasome system. Nat. Rev. Mol. Cell Biol. 2008, 9, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Tindal, J.; Knaggs, G.; Turvey, A. The forebrain of the goat in stereotaxic coordinates. J. Anat. 1968, 103, 457–469. [Google Scholar] [PubMed]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Mccarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential Expression of RNA-Seq Data at the Gene Level—The DESeq Package; European Molecular Biology Laboratory: Heidelberg, Germany, 2012. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Control Group | Electroacupuncture (EA) Group | ||||
|---|---|---|---|---|---|---|
| CON001 | CON002 | CON003 | EA001 | EA002 | EA003 | |
| Raw reads | 63566836 | 63131918 | 58793220 | 60857264 | 57505218 | 59363680 |
| Clean reads (A) | 61436432 | 61325678 | 57038940 | 59053276 | 55818042 | 57636846 |
| Error rate (%) | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| Q30 (%) | 92.43 | 92.32 | 92.67 | 92.02 | 91.84 | 92.28 |
| GC content (%) | 48.07 | 49.28 | 48.03 | 48.56 | 49.20 | 49.07 |
| Total mapped (B) | 47483936 | 48420282 | 44162522 | 44152353 | 42426278 | 43177715 |
| Multiple mapped | 1824973 | 1827216 | 1778697 | 1357349 | 1654599 | 1290277 |
| Uniquely mapped (C) | 45658963 | 46593066 | 42383825 | 42795004 | 40771679 | 41887438 |
| Ratio of (B) and (A) | 77.29% | 78.96% | 77.43% | 74.77% | 76.01% | 74.91% |
| Ratio of (C) and (B) | 96.16% | 96.23% | 95.97% | 96.92% | 96.10% | 97.02% |
| Pathway | Pathway Id | Count | p-Value |
|---|---|---|---|
| Ribosome | bta03010 | 73 | 0 |
| Oxidative phosphorylation | bta00190 | 76 | 7.86 × 10−14 |
| Alzheimers’ disease | bta05010 | 82 | 6.12 × 10−10 |
| Parkinson’s disease | bta05012 | 67 | 2.14 × 10−9 |
| Huntington’s disease | bta05016 | 83 | 1.29 × 10−8 |
| Citrate cycle (TCA cycle) | bta00020 | 19 | 1.58 × 10−5 |
| Endocytosis | bta04144 | 76 | 2.66 × 10−5 |
| ErbB signaling pathway | bta04012 | 31 | 0.000163 |
| Long-term potentiation | bta04720 | 22 | 0.00085 |
| Phosphatidylinositol signaling system | bta04070 | 28 | 0.000888 |
| Proteasome | bta03050 | 18 | 0.001086 |
| GABAergic synapse | bta04727 | 23 | 0.001395 |
| Inositol phosphate metabolism | bta00562 | 26 | 0.001604 |
| Bacterial invasion of epithelial cells | bta05100 | 24 | 0.001823 |
| Adherens junction | bta04520 | 27 | 0.002634 |
| Fc gamma R-mediated phagocytosis | bta04666 | 34 | 0.003565 |
| HIF-1 signaling pathway | bta04066 | 38 | 0.004882 |
| Retrograde endocannabinoid signaling | bta04723 | 26 | 0.004904 |
| Tight junction | bta04530 | 44 | 0.005358 |
| Dopaminergic synapse | bta04728 | 30 | 0.007337 |
| Glutamatergic synapse | bta04724 | 25 | 0.009755 |
| Protein processing in endoplasmic reticulum | bta04141 | 54 | 0.016489 |
| Amphetamine addiction | bta05031 | 25 | 0.017283 |
| Renal cell carcinoma | bta05211 | 25 | 0.017283 |
| Cholinergic synapse | bta04725 | 22 | 0.020068 |
| Gap junction | bta04540 | 29 | 0.040443 |
| Neurotrophin signaling pathway | bta04722 | 32 | 0.041074 |
| Wnt signaling pathway | bta04310 | 47 | 0.04123 |
| Morphine addiction | bta05032 | 30 | 0.043044 |
| MAPK signaling pathway | bta04010 | 76 | 0.04338 |
| Pathways in KEGG | Genes |
|---|---|
| Glutamatergic synapse | GNAO1, GNB1, GNAI1, GNAI2, PRKACA, PRKCB, ADCY7, ADCY2, ITPR1, PLCB4, SLC38A2, GLUL, GRIA1, GRIA2, GRIA3, PPP3CB, PPP3CA, Slc1a3, SLC1A1, SLC17A6, GRIN1, ADRBK1, Ppp3r1, MAPK1, CHP1 |
| GABAergic synapse | GNAO1, GNB1, GNAI1, GNAI2, PRKACA, PRKCB, ADCY7, ADCY2, SLC38A2, GLUL, GABRA1, GABRA3, GABRA5, GABBR1, GABBR2, GABRG2, GABARAPL1, SLC6A1, Src, Slc32a1, GAD1, NSF, PLCL1 |
| Dopaminergic synapse | GNAO1, GNB1, GNAI1, GNAI2, PRKACA, PRKCB, AKT2, AKT3, Camk2g, Camk2d, CAMK2B, CAMK2A, ATF4, ITPR1, PLCB4, GRIA1, GRIA2, GRIA3, PPP3CB, PPP3CA, Ppp1cb, Ppp2r5a, PPP2R2C, PPP2CA, SCN1A, Mapk10, DDC, KIF5B, Kif5a, MAOB |
| Cholinergic synapse | GNAO1, GNB1, GNAI1, GNAI2, PRKACA, PRKCB, AKT2, AKT3, Camk2g, Camk2d, CAMK2B, CAMK2A, ATF4, ADCY7, ADCY2, ITPR1, PLCB4, PIK3R2, PIK3CB, PIK3R1, JAK2, GNA11 |
| Long-term potentiation | Ppp1cb, PPP3CA, GRIA2, RAP1B, PRKACA, Camk2g, CAMK2A, ITPR1, GRIA1, RPS6KA6, CHP1, Ppp3r1, MAPK1, CAMK2B, PLCB4, PPP3CB, Camk2d, GRIN1, MAP2K2, ATF4, PRKCB, PPP1R1A |
| Neurotrophin signaling pathway | CAMK2B, PRDM4, RAP1B, PIK3CB, Camk2g, Crk, CAMK2A, RPS6KA6, GAB1, MAPK1, SHC1, Camk2d, Mapk10, PIK3R2, Map2k7, AKT3, SOS2, Map3k5, YWHAE, PTPN11, RAPGEF1, RPS6KA5, PLCG1, PIK3R1, AKT2, NFKB1, ABL1, MAP2K2, ARHGD, ATF4, MATK, NGFRAP1 |
| Retrograde endocannabinoid signaling | Gng5, FAAH, ADCY2, GRIA3, GNB1, ABHD6, GNAI2, Slc32a1, Mapk10, PRKCB, PLCB4, MAPK1, MGLL, GNAO1, ADCY7, GRIA1, ITPR1, PRKACA, RIMS1, GRIA2, GABRA3, GABRA1, SLC17A6, GABRA5, GNAI1, GABRG2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.-L.; Zhu, H.-M.; Zhang, Q.-L.; Liu, J.-J.; Ding, Y.; Zhong, J.-M.; Vodyanoy, V.; Ding, M.-X. Exploring the Mechanisms of Electroacupuncture-Induced Analgesia through RNA Sequencing of the Periaqueductal Gray. Int. J. Mol. Sci. 2018, 19, 2. https://doi.org/10.3390/ijms19010002
Hu M-L, Zhu H-M, Zhang Q-L, Liu J-J, Ding Y, Zhong J-M, Vodyanoy V, Ding M-X. Exploring the Mechanisms of Electroacupuncture-Induced Analgesia through RNA Sequencing of the Periaqueductal Gray. International Journal of Molecular Sciences. 2018; 19(1):2. https://doi.org/10.3390/ijms19010002
Chicago/Turabian StyleHu, Man-Li, Hong-Mei Zhu, Qiu-Lin Zhang, Jing-Jing Liu, Yi Ding, Ju-Ming Zhong, Vitaly Vodyanoy, and Ming-Xing Ding. 2018. "Exploring the Mechanisms of Electroacupuncture-Induced Analgesia through RNA Sequencing of the Periaqueductal Gray" International Journal of Molecular Sciences 19, no. 1: 2. https://doi.org/10.3390/ijms19010002
APA StyleHu, M.-L., Zhu, H.-M., Zhang, Q.-L., Liu, J.-J., Ding, Y., Zhong, J.-M., Vodyanoy, V., & Ding, M.-X. (2018). Exploring the Mechanisms of Electroacupuncture-Induced Analgesia through RNA Sequencing of the Periaqueductal Gray. International Journal of Molecular Sciences, 19(1), 2. https://doi.org/10.3390/ijms19010002






