Could Aspirin and Diets High in Fiber Act Synergistically to Reduce the Risk of Colon Cancer in Humans?
Abstract
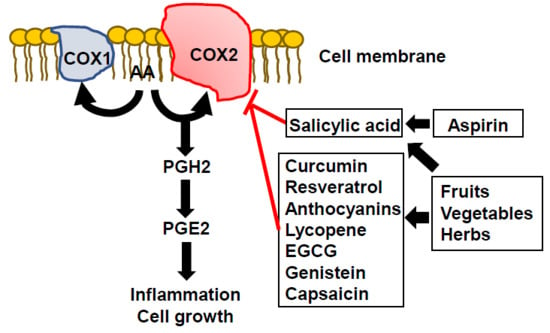
:1. Introduction
2. COX2 Inhibitors
3. Phytochemicals as Natural Sources of Anti-COX2 Agents in Humans
4. Is There a “Right” dose of Phytochemicals for Cancer Chemoprevention?
5. Less is More When Phytochemicals are Used for Cancer Chemoprevention
6. Interactions Between Aspirin and High Fiber Diets in Colon Adenoma and Colon Cancer Incidence in Humans
7. Conclusions
Reference
Acknowledgments
Conflict of interest
References
- Rothwell, P.M.; Fowkes, F.G.R.; Belch, J.F.F.; Ogawa, H.; Warlow, C.P.; Meade, T.W. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. The Lancet 2011, 377, 31–41. [Google Scholar] [CrossRef]
- Rothwell, P.M.; Wilson, M.; Elwin, C.-E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. The Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef]
- Paterson, J.R.; Lawrence, J.R. Salicylic acid: A link between aspirin, diet and the prevention of colorectal cancer. QJM Int. J. Med. 2001, 94, 445–448. [Google Scholar] [CrossRef]
- Patrignani, P.; Patrono, C. Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.; Baxter, G.; Lawrence, J.; Duthie, G. Is there a role for dietary salicylates in health? Proc. Nutr. Soc. 2006, 65, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.R.; Peter, R.; Baxter, G.J.; Robson, J.; Graham, A.B.; Paterson, J.R. Urinary excretion of salicyluric and salicylic acids by non-vegetarians, vegetarians, and patients taking low dose aspirin. J. Clin. Pathol. 2003, 56, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Blacklock, C.J.; Lawrence, J.R.; Wiles, D.; Malcolm, E.A.; Gibson, I.H.; Kelly, C.J.; Paterson, J.R. Salicylic acid in the serum of subjects not taking aspirin. Comparison of salicylic acid concentrations in the serum of vegetarians, non-vegetarians, and patients taking low dose aspirin. J. Clin. Pathol. 2001, 54, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.; Dutton, S.; Truswell, A. Salicylates in foods. J. Am. Diet. Assoc. 1985, 85, 950–960. [Google Scholar] [PubMed]
- Samadi, A.K.; Bilsland, A.; Georgakilas, A.G.; Amedei, A.; Amin, A.; Bishayee, A.; Azmi, A.S.; Lokeshwar, B.L.; Grue, B.; Panis, C.; et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin. Cancer Biol. 2015, 35, S151–S184. [Google Scholar] [CrossRef] [PubMed]
- Madka, V.; Rao, C.V. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr. Cancer Drug Targets 2013, 13, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.M.; Piazza, G.A. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G59–G70. [Google Scholar] [CrossRef] [PubMed]
- Temraz, S.; Mukherji, D.; Shamseddine, A. Potential Targets for Colorectal Cancer Prevention. Int. J. Mol. Sci. 2013, 14, 17279–17303. [Google Scholar] [CrossRef] [PubMed]
- Mayank, B.; Lalit Singh, C. The Ambidextrous Cyclooxygenase: An Enduring Target. Inflamm. Allergy Drug Targets (Discontinued) 2014, 13, 387–392. [Google Scholar] [CrossRef]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Fujii, G.; Takahashi, M.; Iigo, M.; Mutoh, M. Prevention and Intervention Trials for Colorectal Cancer. Jpn. J. Clin. Oncol. 2013, 43, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Smartt, H.J.M.; Greenhough, A.; Ordóñez-Morán, P.; Talero, E.; Cherry, C.A.; Wallam, C.A.; Parry, L.; Al Kharusi, M.; Roberts, H.R.; Mariadason, J.M.; et al. β-catenin represses expression of the tumour suppressor 15-prostaglandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut 2012, 61, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.S.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The Effect of Celecoxib, a Cyclooxygenase-2 Inhibitor, in Familial Adenomatous Polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.T.; Arber, N.; Burn, J.; Chia, J.W.-K.; Elwood, P.; Hull, M.A.; Logan, R.F.; Rothwell, P.M.; Schrör, K.; Baron, J.A. Aspirin in the Chemoprevention of Colorectal Neoplasia: An Overview. Cancer Prev. Res. 2012, 5, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.F.; Logan, R.F.; Halabi, S.; Benamouzig, R.; Sandler, R.S.; Grainge, M.J.; Chaussade, S.; Baron, J.A. Aspirin for the Chemoprevention of Colorectal Adenomas: Meta-analysis of the Randomized Trials. J. Natl. Cancer Inst. 2009, 101, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Dachineni, R.; Ai, G.; Kumar, D.R.; Sadhu, S.S.; Tummala, H.; Bhat, G.J. Cyclin A2 and CDK2 as Novel Targets of Aspirin and Salicylic Acid: A Potential Role in Cancer Prevention. Mol. Cancer Res. (MCR) 2016, 14, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Lochhead, P.; Nishihara, R.; Morikawa, T.; Kuchiba, A.; Yamauchi, M.; Imamura, Y.; Qian, Z.R.; Baba, Y.; Shima, K.; et al. Aspirin Use, Tumor PIK3CA Mutation, and Colorectal-Cancer Survival. N. Engl. J. Med. 2012, 367, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.R.; Kim, E.J.; Choi, H.J.; Park, J.J.; Kim, H.S.; Lee, Y.J.; Park, M.J.; Lee, M. Aspirin Targets SIRT1 and AMPK to Induce Senescence of Colorectal Carcinoma Cells. Mol. Pharmacol. 2015, 88, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C. The Multifaceted Clinical Readouts of Platelet Inhibition by Low-Dose Aspirin. J. Am. Coll. Cardiol. 2015, 66, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 1998, 396, 77. [Google Scholar] [CrossRef] [PubMed]
- Din, F.V.; Valanciute, A.; Houde, V.P.; Zibrova, D.; Green, K.A.; Sakamoto, K.; Alessi, D.R.; Dunlop, M.G. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 2012, 142, 1504–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Chang, D.K.; Ricciardiello, L.; Gasche, C.; Boland, C.R. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin. Cancer Res. 2003, 9, 383–390. [Google Scholar] [PubMed]
- Langley, R.E.; Rothwell, P.M. Potential biomarker for aspirin use in colorectal cancer therapy. Nat. Rev. Clin. Oncol. 2013, 10, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Singh Ranger, G. The role of aspirin in colorectal cancer chemoprevention. Crit. Rev. Oncol./Hematol. 2016, 104, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.W.; Luo, F.; Cheng, H.; Zhao, J.J.; Liu, P. Chemopreventive effects of aspirin at a glance. Biochim. Biophys. Acta 2015, 1855, 254–263. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. Minimizing the cancer-promotional activity of cox-2 as a central strategy in cancer prevention. Med. Hypotheses 2012, 78, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-Lopez, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules (Basel, Switz.) 2016, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcea, G.; Berry, D.P.; Jones, D.J.L.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum and their Pharmacodynamic Consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar] [PubMed]
- Bansal, S.S.; Vadhanam, M.V.; Gupta, R.C. Development and In Vitro-In Vivo Evaluation of Polymeric Implants for Continuous Systemic Delivery of Curcumin. Pharm. Res. 2011, 28, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Hougee, S.; Faber, J.; Sanders, A.; de Jong, R.B.; van den Berg, W.B.; Garssen, J.; Hoijer, M.A.; Smit, H.F. Selective COX-2 Inhibition by a Pterocarpus marsupium Extract Characterized by Pterostilbene, and its Activity in Healthy Human Volunteers. Planta Med. 2005, 71, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Rufini, A. New concepts and challenges in the clinical translation of cancer preventive therapies: The role of pharmacodynamic biomarkers. Ecancermedicalscience 2015, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.L.; Michos, E.D.; Post, W.; Astor, B. 25-hydroxyl Vitamin D Levels and the Risk of Mortality in the General Population. Arch. Int. Med. 2008, 168, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Gandini, S.; Puntoni, M.; Bonanni, B.; Gennari, A.; DeCensi, A. The science behind vitamins and natural compounds for breast cancer prevention. Getting the most prevention out of it. The Breast 2011, 20 (Suppl. 3), S36–S41. [Google Scholar] [CrossRef]
- Garland, C.; Garland, F.; Shaw, E.; Comstock, G.; Helsing, K.; Gorham, E. Serum 25-Hydroxyvitamin D and Colon Cancer: Eight-Year Prospective Study. The Lancet 1989, 334, 1176–1178. [Google Scholar] [CrossRef]
- Tuohimaa, P.; Tenkanen, L.; Ahonen, M.; Lumme, S.; Jellum, E.; Hallmans, G.; Stattin, P.; Harvei, S.; Hakulinen, T.; Luostarinen, T.; et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: A longitudinal, nested case-control study in the Nordic countries. Int. J. Cancer 2004, 108, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Kristal, A.R.; Darke, A.K.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline Selenium Status and Effects of Selenium and Vitamin E Supplementation on Prostate Cancer Risk. J. Natl. Cancer Inst. 2014, 106, djt456. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.; Cai, H.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton, R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D.; et al. Less is more for cancer chemoprevention: Evidence of a non-linear dose response for the protective effects of resveratrol in humans and mice. Sci. Transl. Med. 2015, 7, 298ra117. [Google Scholar] [CrossRef]
- Hartman, T.J.; Yu, B.; Albert, P.S.; Slattery, M.L.; Paskett, E.; Kikendall, J.W.; Iber, F.; Brewer, B.K.; Schatzkin, A.; Lanza, E. Does Nonsteroidal Anti-inflammatory Drug Use Modify the Effect of a Low-Fat, High-Fiber Diet on Recurrence of Colorectal Adenomas? Cancer Epidemiol. Biomark. Prev. 2005, 14, 2359–2365. [Google Scholar] [CrossRef] [PubMed]
- Tangrea, J.A.; Albert, P.S.; Lanza, E.; Woodson, K.; Corle, D.; Hasson, M.; Burt, R.; Caan, B.; Paskett, E.; Iber, F.; et al. Non-steroidal anti-inflammatory drug use is associated with reduction in recurrence of advanced and non-advanced colorectal adenomas (United States). Cancer Causes Control 2003, 14, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Willett, W.C.; Fuchs, C.S.; Colditz, G.A.; Giovannucci, E.L. Calcium Intake and Risk of Colon Cancer in Women and Men. J. Natl. Cancer Inst. 2002, 94, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Beach, M.; Mandel, J.S.; van Stolk, R.U.; Haile, R.W.; Sandler, R.S.; Rothstein, R.; Summers, R.W.; Snover, D.C.; Beck, G.J.; et al. Calcium Supplements for the Prevention of Colorectal Adenomas. N. Engl. J. Med. 1999, 340, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hauret, K.G.; Bostick, R.M.; Matthews, C.E.; Hussey, J.R.; Fina, M.F.; Geisinger, K.R.; Roufail, W.M. Physical Activity and Reduced Risk of Incident Sporadic Colorectal Adenomas: Observational Support for Mechanisms Involving Energy Balance and Inflammation Modulation. Am. J. Epidemiol. 2004, 159, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Beresford, S.A.; Johnson, K.C.; Ritenbaugh, C.; Lasser, N.L.; Snetselaar, L.G.; Black, H.R.; Anderson, G.L.; Assaf, A.R.; Bassford, T.; Bowen, D.; et al. Low-fat dietary pattern and risk of colorectal cancer: The women’s health initiative randomized controlled dietary modification trial. JAMA 2006, 295, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Lane, D.R.; Womack, C.; Bock, C.; Hou, L.H.; Lin, J.; Wu, C.; Beebe Dimmer, J.S.; Simon, M. Interaction between Nonsteroidal Anti-inflammatory Drugs and Low-fat Dietary Intervention on Colorectal Cancer Incidence; the Women’s Health Initiative (WHI) Dietary Modification Trial. J. Am. Coll. Nutr. 2017, 36, 462–469. [Google Scholar] [PubMed]
- Burn, J.; Bishop, D.T.; Chapman, P.D.; Elliott, F.; Bertario, L.; Dunlop, M.G.; Eccles, D.; Ellis, A.; Evans, D.G.; Fodde, R.; et al. A Randomized Placebo-controlled Prevention Trial of Aspirin and/or Resistant Starch in Young People with Familial Adenomatous Polyposis. Cancer Prev. Res. (Philadelphia, Pa.) 2011, 4, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Burke, C.A.; Hasson, H.; Kuo, C.-T.; Molmenti, C.L.S.; Seguin, C.; Liu, P.; Huang, T.H.-M.; Frankel, W.L.; Stoner, G.D. A Phase Ib Study of the Effects of Black Raspberries on Rectal Polyps in Patients with Familial Adenomatous Polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef] [PubMed]
| Trial Name | Primary end Point | Duration | Participants | Diet Intervention | Findings | Ref. |
|---|---|---|---|---|---|---|
| The Polyp Prevention Trial | Colorectal adenoma recurrence | Intervention for 3 years | 1905 (intervention) | High-fiber (18 g/1000 kcal) | Interaction between intervention and aspirin (p = 0.03). Interaction between intervention and all NSAIDs (p = 0.008) | [48] |
| High fruit and vegetable (3.5 servings/1000 kcal) | ||||||
| Low-fat (20% energy) | ||||||
| The Women‘s Health Initiative Randomized Controlled Dietary Modification Trial | Invasive colorectal cancer incidence | Intervention for 8.1 years | 19,541 (intervention) 29,294 (comparison) | Fruit and vegetable, at least 5 servings daily | Interaction between intervention and aspirin (p = 0.01) | [54] |
| Grains, at least 6 servings daily | ||||||
| Fat: 20% energy | ||||||
| The Women’s Health Initiative Randomized Controlled Dietary Modification Trial | Invasive colorectal cancer incidence | Intervention for 8.1 years and followed for an additional 9.4 years | 19,541 (intervention) 29,294 (comparison) | Fruit and vegetable, at least 5 servings daily | Interaction between intervention and aspirin (p = 0.07). Interaction between intervention and all NSAIDs (p = 0.14) | [55] |
| Grains, at least 6 servings daily | ||||||
| Fat: 20% energy |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, P.; Huang, Y.-W.; Oshima, K.; Yearsley, M.; Zhang, J.; Yu, J.; Arnold, M.; Wang, L.-S. Could Aspirin and Diets High in Fiber Act Synergistically to Reduce the Risk of Colon Cancer in Humans? Int. J. Mol. Sci. 2018, 19, 166. https://doi.org/10.3390/ijms19010166
Pan P, Huang Y-W, Oshima K, Yearsley M, Zhang J, Yu J, Arnold M, Wang L-S. Could Aspirin and Diets High in Fiber Act Synergistically to Reduce the Risk of Colon Cancer in Humans? International Journal of Molecular Sciences. 2018; 19(1):166. https://doi.org/10.3390/ijms19010166
Chicago/Turabian StylePan, Pan, Yi-Wen Huang, Kiyoko Oshima, Martha Yearsley, Jianying Zhang, Jianhua Yu, Mark Arnold, and Li-Shu Wang. 2018. "Could Aspirin and Diets High in Fiber Act Synergistically to Reduce the Risk of Colon Cancer in Humans?" International Journal of Molecular Sciences 19, no. 1: 166. https://doi.org/10.3390/ijms19010166
APA StylePan, P., Huang, Y.-W., Oshima, K., Yearsley, M., Zhang, J., Yu, J., Arnold, M., & Wang, L.-S. (2018). Could Aspirin and Diets High in Fiber Act Synergistically to Reduce the Risk of Colon Cancer in Humans? International Journal of Molecular Sciences, 19(1), 166. https://doi.org/10.3390/ijms19010166