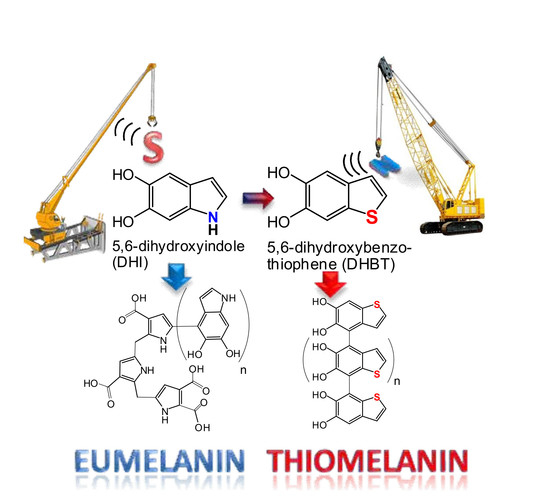
Replacing Nitrogen by Sulfur: From Structurally Disordered Eumelanins to Regioregular Thiomelanin Polymers
Abstract
1. Introduction
2. Results and Discussion
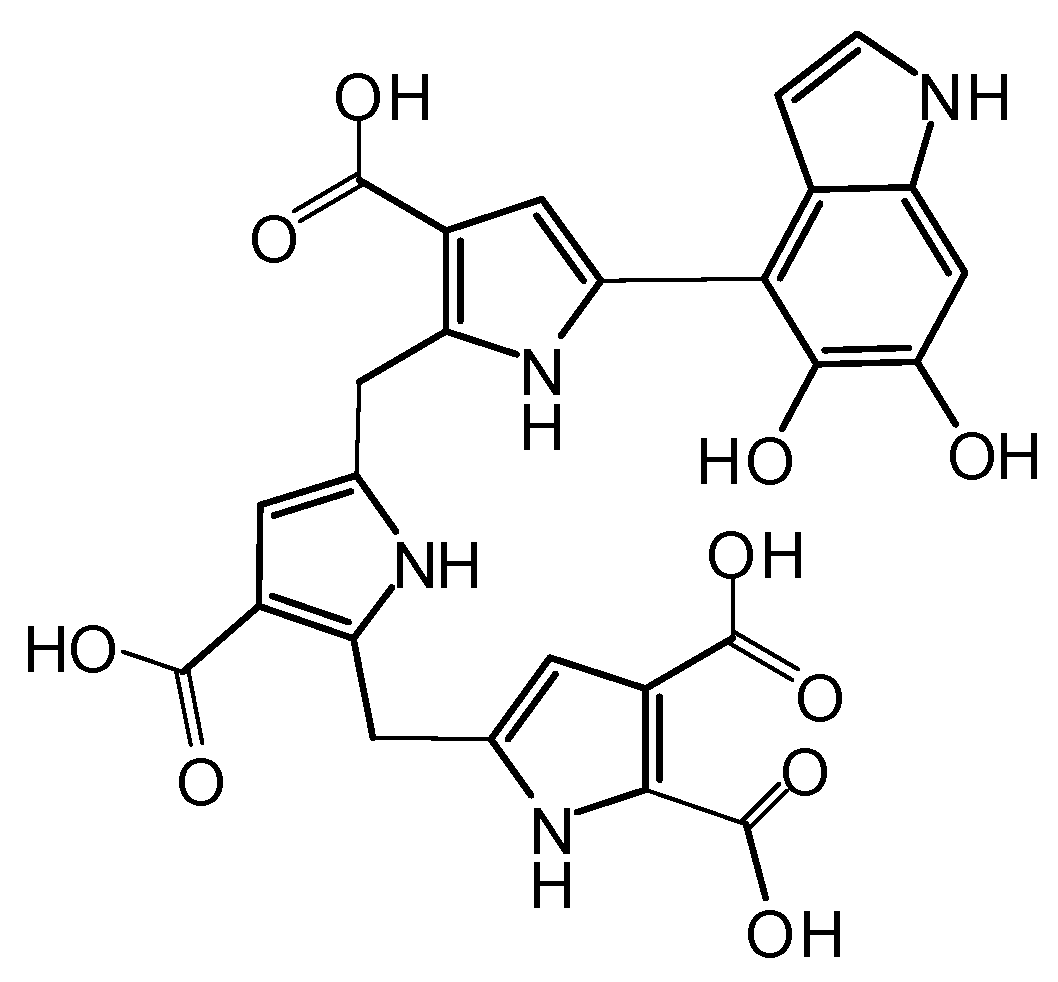
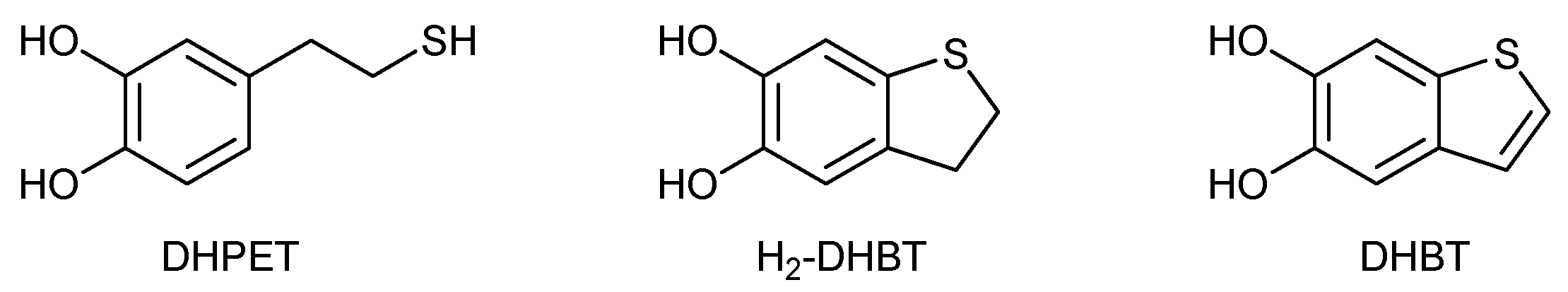
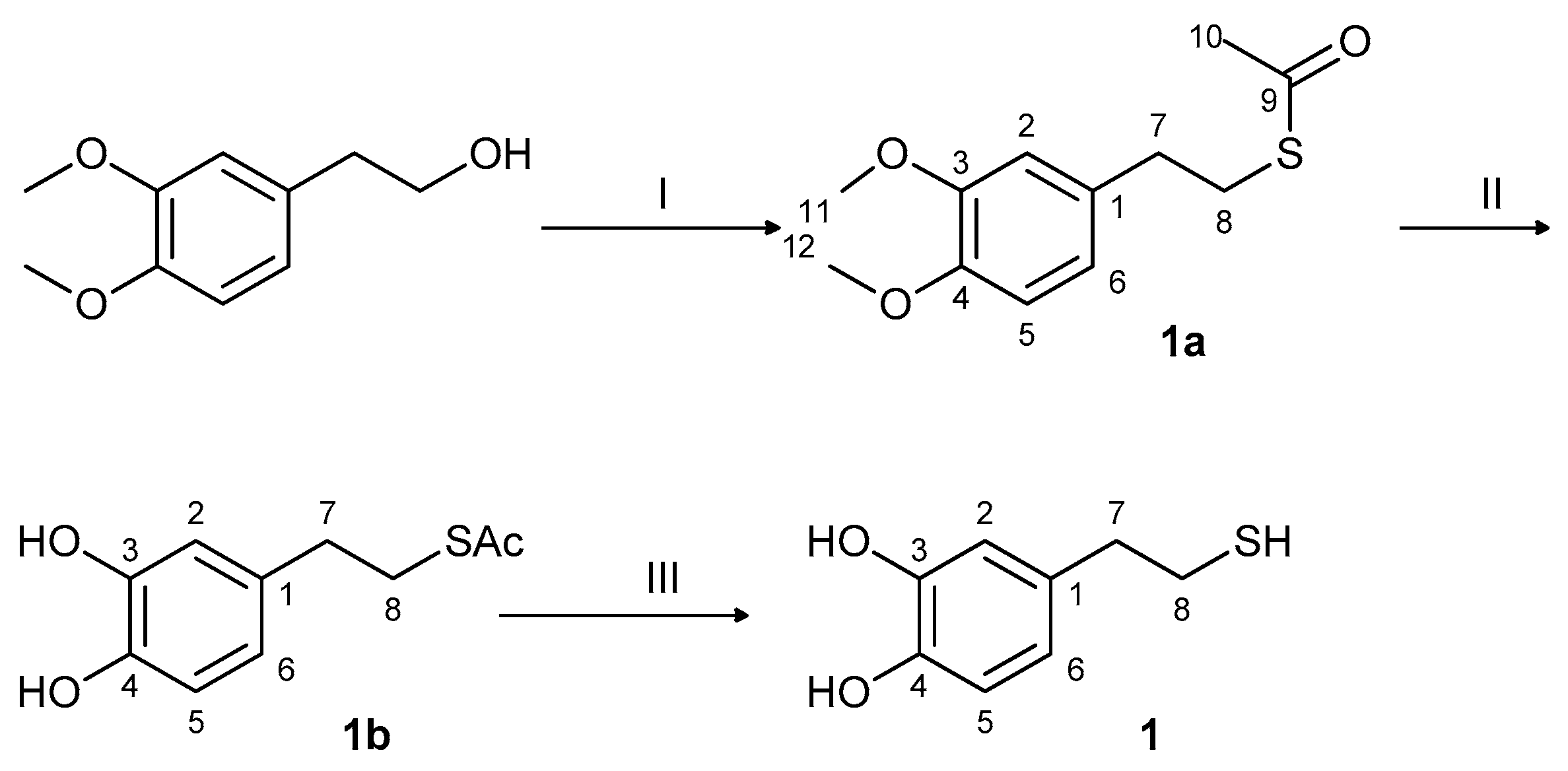
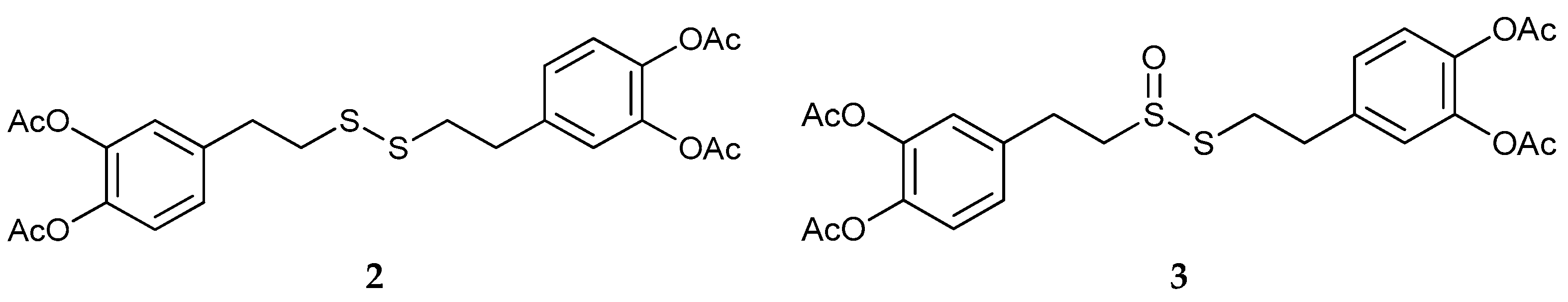
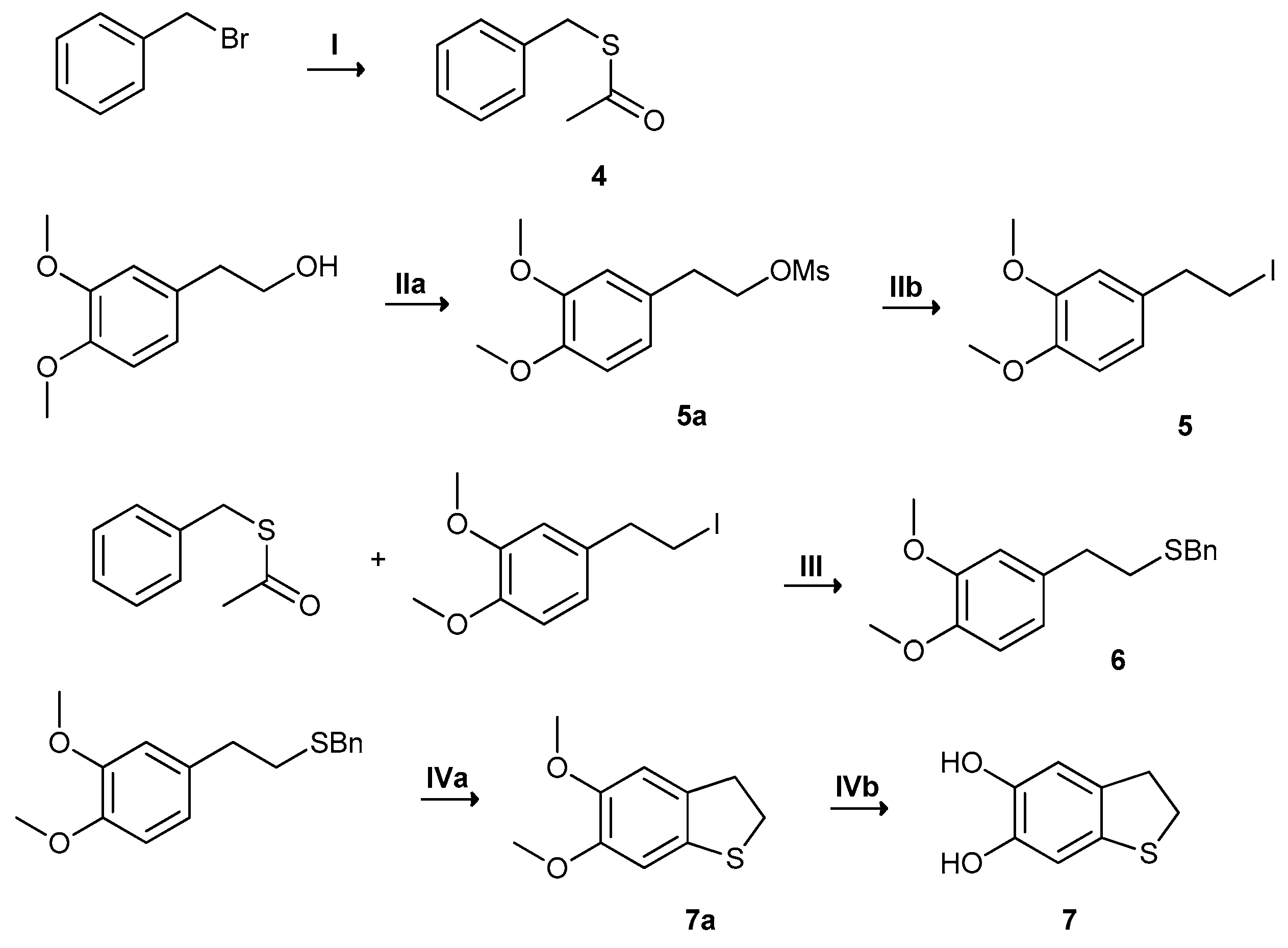
2.1. Synthesis and Oxidation of DHPET (1)
- to verify the feasibility of this approach as an expedient entry to DHBT via nucleophilic attack of the thiol (-SH) group onto the o-quinone groups;
- to assess whether a PDA-like material is formed regardless of DHBT formation, and to investigate its absorption, free radical, and adhesion properties.
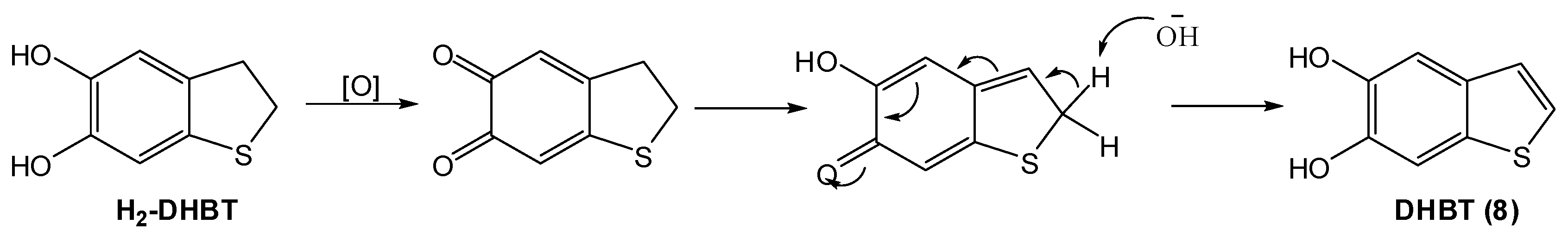
2.2.Synthesis and Oxidation of H2-DHBT (7)
2.3. Oxidative Polymerization of DHBT
2.4. Mechanistic Issues and Density Functional Theory (DFT) Calculations
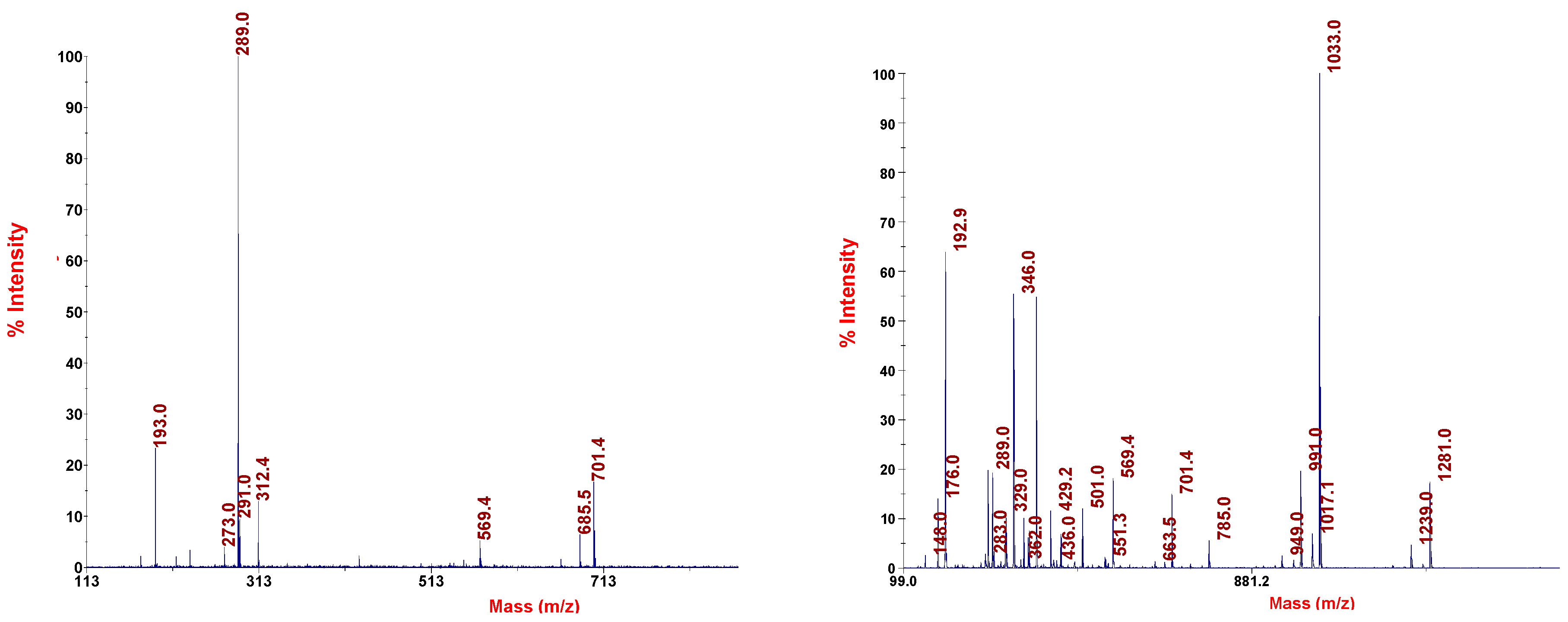
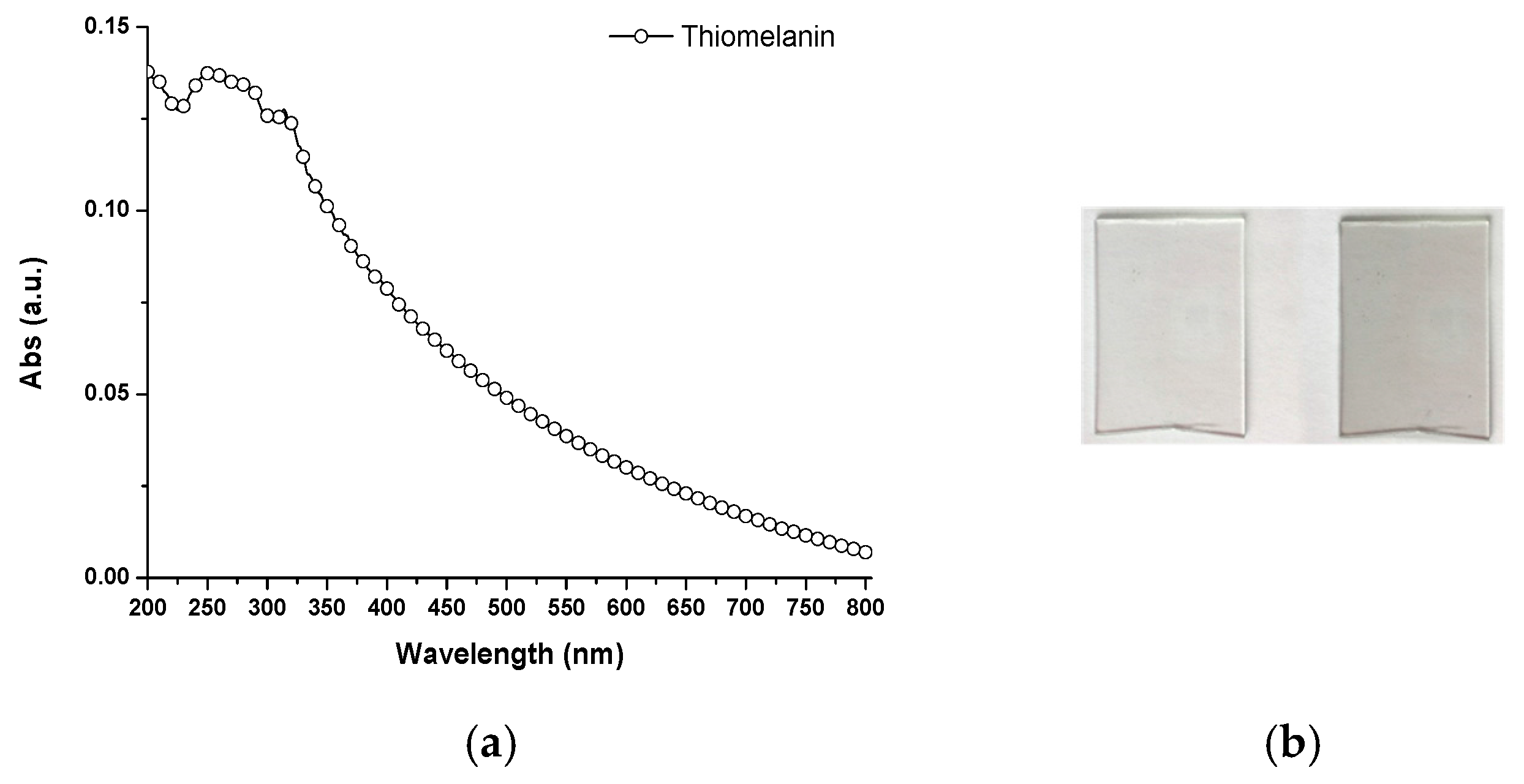
2.5. General Properties of Thiomelanin
3. Materials and Methods
4. Conclusions
Eumelanin Versus Thiomelanin: the Role of Nitrogen and Opportunities from Replacement by Sulfur
- Replacement of nitrogen by sulfur in dopamine completely precludes the formation of eumelanin-type polymers due to the inability of DHPET to undergo intramolecular cyclization. However, the observed conversion of DHPET into its disulfide and related oxygenation products may be of interest for the development of novel antioxidant systems responding to oxidizing stimuli via both the catechol and thiol/disulfide groups.
- Oxidation of H2-DHBT proceeds via a red-orange chromophoric phase that displays an absorption maximum similar to that of dopaminochrome. This chromophore was attributed to the corresponding quinone, i.e., the sulfur analog of dopaminochrome, on the basis of DFT simulations. The analogies both in the chromophore and the tendency to rearrangement would apparently rule out a specific role of nitrogen in aminochrome chemistry and properties.
- DHBT appear to be more difficult to oxidize than DHI, which makes reduced species predominate (unless subject to strong oxidizing environments), and radical species less abundant.
- Thiomelanin appears to be markedly different from DHI melanin in a number of properties, including visible light absorption, redox potential, and especially, adhesion to quartz and glass surfaces by dip coating.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DHBT | 5,6-dihydroxybenzo[b]thiophene |
| DHI | 5,6-dihydroxyindole |
| DHICA | 5,6-dihydroxyindole-2-carboxylic acid |
| PDA | Polydopamine |
| DHPET | 2-(3,4-dihydroxyphenyl)ethanethiol |
| H2-DHBT | 2,3-dihydro-5,6-dihydroxybenzo[b]thiophene |
| DABT | 5,6-diacetoxybenzo[b]thiophene |
| DAI | 5,6-diacetoxyindole |
References
- Blois, M.S.; Zahalan, A.B.; Maling, J.E. Electron spin resonance studies on melanin. Biophys. J. 1964, 4, 471–490. [Google Scholar] [CrossRef]
- Pezzella, A.; Napolitano, A.; d’Ischia, M.; Prota, G. Oxidative polymerisation of 5,6-dihydroxyindole-2-carboxylic acid to melanin: A new insight. Tetrahedron 1996, 52, 7913–7920. [Google Scholar] [CrossRef]
- Pezzella, A.; Vogna, D.; Prota, G. Atropoisomeric melanin intermediates by oxidation of the melanogenic precursor 5,6-dihydroxyindole-2-carboxylic acid under biomimetic conditions. Tetrahedron 2002, 58, 3681–3687. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.B.; Powell, B.J.; Pratt, F.L.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Meredith, P. Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc. Natl. Acad. Sci. USA 2012, 109, 8943–8947. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Gentile, G.; D’Errico, G.; Della Vecchia, N.F.; Errico, M.E.; Napolitano, A.; Carfagna, C.; d’Ischia, M. Atypical structural and π-electron features of a melanin polymer that lead to superior free-radical-scavenging properties. Angew. Chem. Int. Ed. 2013, 52, 12684–12687. [Google Scholar] [CrossRef] [PubMed]
- Micillo, R.; Panzella, L.; Koike, K.; Monfrecola, G.; Napolitano, A.; d’Ischia, M. “Fifty Shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int. J. Mol. Sci. 2016, 17, 746. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Lin, X.; Zhang, W.; Chi, Z.; Lyu, R.; Cao, A.; Wan, L. Coreshell structured TiO2-polydopamine for highly active visible-light photocatalysis. Chem. Commun. 2016, 52, 7122–7125. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.R.C.; Saiz-Poseu, J.; García-Pardo, J.; García, B.; Lorenzo, J.; Ojea-Jiménez, I.; Komilis, D.; Sedó, J.; Busqué, F.; Sánchez, A.; et al. Biocompatible polydopamine-like particles for the removal of heavy metals at extremely low concentrations. RSC Adv. 2016, 6, 40058–40066. [Google Scholar] [CrossRef]
- Zhou, L.; Zong, Y.; Liu, Z.; Yu, A. A polydopamine coating ultralight graphene matrix as a highly effective polysulfide absorbent for high-energy LiS batteries. Renew. Energy 2016, 96, 333–340. [Google Scholar] [CrossRef]
- Manini, P.; Criscuolo, V.; Ricciotti, L.; Pezzella, A.; Barra, M.; Cassinese, A.; Crescenzi, O.; Maglione, M.G.; Tassini, P.; Minarini, C.; et al. Melanin-inspired organic electronics: Electroluminescence in asymmetric triazatruxenes. Chempluschem 2015, 80, 919–927. [Google Scholar] [CrossRef]
- D’ischia, M.; Napolitano, A.; Pezzella, A. 5,6-Dihydroxyindole Chemistry: Unexplored Opportunities Beyond Eumelanin. Eur. J. Org. Chem. 2011, 2011, 5501–5516. [Google Scholar] [CrossRef]
- D’ISCHIA, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- D’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lee, J.S.; Kim, J.; Lee, Y.B.; Shin, H.; Um, S.H.; Kim, J.B.; Park, K.I.; Lee, H.; Cho, S.W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiu, W.-Z.; Yang, H.-C.; Qian, Y.-C.; Huang, X.-J.; Xu, Z.-K. Polydopamine-assisted deposition of heparin for selective adsorption of low-density lipoprotein. RSC Adv. 2015, 5, 12922–12930. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chen, Z.; Liu, L.; Hong, L. Determination of bisphenols in environmental water samples using polydopamine-coated extraction material coupled with high-performance liquid chromatography. Anal. Methods 2016, 8, 3391–3396. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, S.; Shi, D.; Yang, Y.; Jiang, T.; Yan, S.; Shi, H.; Luan, S.; Yin, J. Integrated antifouling and bactericidal polymer membranes through bioinspired polydopamine/poly(N-vinyl pyrrolidone) coating. Appl. Surf. Sci. 2016, 375, 9–18. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Avolio, R.; Alfè, M.; Errico, M.E.; Napolitano, A.; D’ischia, M. Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Luchini, A.; Napolitano, A.; D’Errico, G.; Vitiello, G.; Szekely, N.; D’ischia, M.; Paduano, L. Tris buffer modulates polydopamine growth, aggregation, and paramagnetic properties. Langmuir 2014, 30, 9811–9818. [Google Scholar] [CrossRef] [PubMed]
- Burzio, L.A.; Waite, J.H. Cross-linking in adhesive quinoproteins: Studies with model decapeptides. Biochemistry 2000, 39, 11147–11153. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, J.; Chen, Y.; Zhao, S.; Chen, M.; Li, X.; Maitz, M.F.; Wang, J.; Huang, N. Application of phenol/amine copolymerized film modified magnesium alloys: Anticorrosion and surface biofunctionalization. ACS Appl. Mater. Interfaces 2015, 7, 24510–24522. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Yang, Z.; Zhou, S.; Luo, R.; Maitz, M.F.; Zhao, Y.; Wang, J.; Xiong, K.; Huang, N. A simple one-step modification of various materials for introducing effective multi-functional groups. Colloids Surf. B Biointerfaces 2014, 113, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, M.; Paez, J.I.; Avolio, R.; Carpentieri, A.; Panzella, L.; Falco, G.; Pizzo, E.; Errico, M.E.; Napolitano, A.; del Campo, A.; et al. Multifunctional thin films and coatings from caffeic acid and a cross-linking diamine. Langmuir 2017. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Powell, B.J.; Meredith, P. Chemical and structural disorder in eumelanins: A possible explanation for broadband absorbance. Biophys. J. 2006, 90, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Egi, M.; Ohtsubo, M.; Saiki, T.; Takada, T.; Tohma, H. Novel and efficient synthesis of sulfur-containing heterocycles using a hypervalent iodine (III) reagent. Chem. Commun. 1996, 2225–2226. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; D’ischia, M.; Napolitano, A.; Pezzella, A. Structure of Melanins. Melanins Melanosomes Biosynth. Biogenesis Physiol. Pathol. Funct. 2011, 167–185. [Google Scholar] [CrossRef]
- Gauden, M.; Pezzella, A.; Panzella, L.; Neves-Petersen, M.T.; Skovsen, E.; Petersen, S.B.; Mullen, K.M.; Napolitano, A.; D’ischia, M.; Sundström, V.; et al. Role of solvent, pH, and molecular size in excited-state deactivation of key eumelanin building blocks: Implications for melanin pigment photostability. J. Am. Chem. Soc. 2008, 130, 17038–17043. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, A.; Crescenzi, O.; Panzella, L.; Napolitano, A.; Land, E.J.; Barone, V.; D’ischia, M. Free Radical Coupling of o-Semiquinones Uncovered. J. Am. Chem. Soc. 2013, 135, 12142–12149. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Sannino, F.; Pernice, P.; Imparato, C.; Aronne, A.; D’Errico, G.; Minieri, L.; Perfetti, M.; Pirozzi, D. Hybrid TiO2-acetylacetonate amorphous gel-derived material with stably adsorbed superoxide radical active in oxidative degradation of organic pollutants. RSC Adv. 2015, 5, 93831–93839. [Google Scholar] [CrossRef]
- Mostert, A.B.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Powell, B.J.; Meredith, P. Hydration-Controlled X-band EPR spectroscopy: A tool for unravelling the complexities of the solid-state free radical in eumelanin. J. Phys. Chem. B 2013, 117, 4965–4972. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. J. Chem. Phys. 1981, 55, 117–129. [Google Scholar]
- Cossi, M.; Scalmani, G.; Rega, N.; Barone, V. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J. Chem. Phys. 2002, 117, 43–54. [Google Scholar] [CrossRef]
- Scalmani, G.; Barone, V.; Kudin, K.N.; Pomelli, C.S.; Scuseria, G.E.; Frisch, M.J. Achieving linear-scaling computational cost for the polarizable continuum model of solvation. Theor. Chem. Acc. 2004, 111, 90–100. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Rappé, A.K.; Casewit, C.J.; Colwell, K.S.; Goddard, W.A., III; Skiff, W.M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar]
- York, D.A.; Karplus, M. A Smooth Solvation Potential Based on the Conductor-Like Screening Model. J. Phys. Chem. A 1999, 103, 11060–11079. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Adamo, C.; Scuseria, G.E.; Barone, V. Accurate excitation energies from time-dependent density functional theory: Assessing the PBE0 model. J. Chem. Phys. 1999, 111, 2889–2899. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and properties of excited states in the gas phase and in solution: Theory and application of a time-dependent density functional theory polarizable continuum model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef] [PubMed]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]



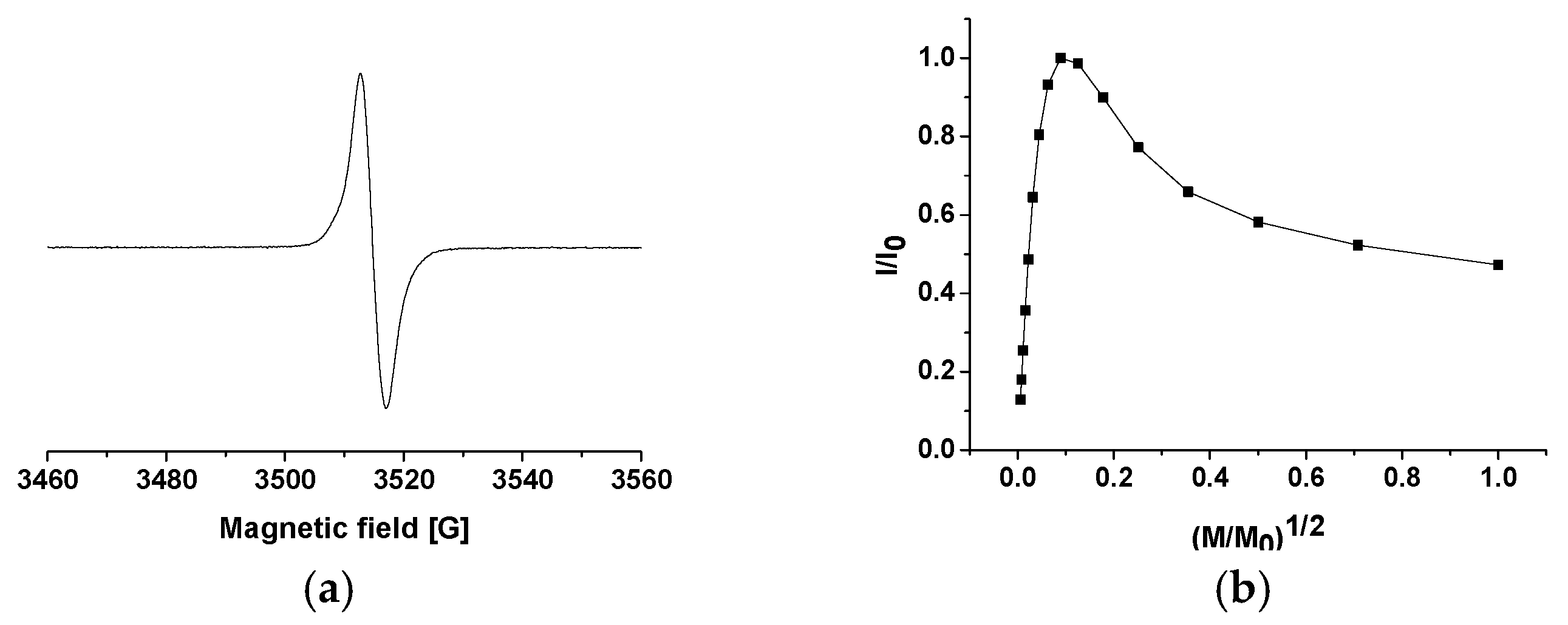
| Dimer | Coupling Mode | ΔGSMD,RRHO (kcal mol−1) |
|---|---|---|
| 2,2′ | 2 of 6-yl semiquinone on 2 of 6-yl semiquinone | 0.99 |
| 2,4′ | 2 of 6-yl semiquinone on 4 of 5-yl semiquinone | 1.08 |
| 2,7′ | 2 of 6-yl semiquinone on 7 of 6-yl semiquinone | 1.64 |
| 4,4′ | 4 of 5-yl semiquinone on 4 of 5-yl semiquinone | 0.00 |
| 4,7′ | 4 of 5-yl semiquinone on 7 of 6-yl semiquinone | 0.93 |
| 7,7′ | 7 of 6-yl semiquinone on 7 of 6-yl semiquinone | 2.53 |
| Melanin | g (±0.0003) | ΔB [G] (±0.05 G) | Spin Density (spin/g) |
|---|---|---|---|
| Thiomelanin | 2.0032 | 4.4 | 4 × 1017 |
| DHICA mel 1 | 2.0034 | 4.4 | 5 × 1017 |
| DHI mel 1 | 2.0035 | 5.6 | 4 × 1018 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacomino, M.; Mancebo-Aracil, J.; Guardingo, M.; Martín, R.; D’Errico, G.; Perfetti, M.; Manini, P.; Crescenzi, O.; Busqué, F.; Napolitano, A.; et al. Replacing Nitrogen by Sulfur: From Structurally Disordered Eumelanins to Regioregular Thiomelanin Polymers. Int. J. Mol. Sci. 2017, 18, 2169. https://doi.org/10.3390/ijms18102169
Iacomino M, Mancebo-Aracil J, Guardingo M, Martín R, D’Errico G, Perfetti M, Manini P, Crescenzi O, Busqué F, Napolitano A, et al. Replacing Nitrogen by Sulfur: From Structurally Disordered Eumelanins to Regioregular Thiomelanin Polymers. International Journal of Molecular Sciences. 2017; 18(10):2169. https://doi.org/10.3390/ijms18102169
Chicago/Turabian StyleIacomino, Mariagrazia, Juan Mancebo-Aracil, Mireia Guardingo, Raquel Martín, Gerardino D’Errico, Marco Perfetti, Paola Manini, Orlando Crescenzi, Félix Busqué, Alessandra Napolitano, and et al. 2017. "Replacing Nitrogen by Sulfur: From Structurally Disordered Eumelanins to Regioregular Thiomelanin Polymers" International Journal of Molecular Sciences 18, no. 10: 2169. https://doi.org/10.3390/ijms18102169
APA StyleIacomino, M., Mancebo-Aracil, J., Guardingo, M., Martín, R., D’Errico, G., Perfetti, M., Manini, P., Crescenzi, O., Busqué, F., Napolitano, A., D’Ischia, M., Sedó, J., & Ruiz-Molina, D. (2017). Replacing Nitrogen by Sulfur: From Structurally Disordered Eumelanins to Regioregular Thiomelanin Polymers. International Journal of Molecular Sciences, 18(10), 2169. https://doi.org/10.3390/ijms18102169