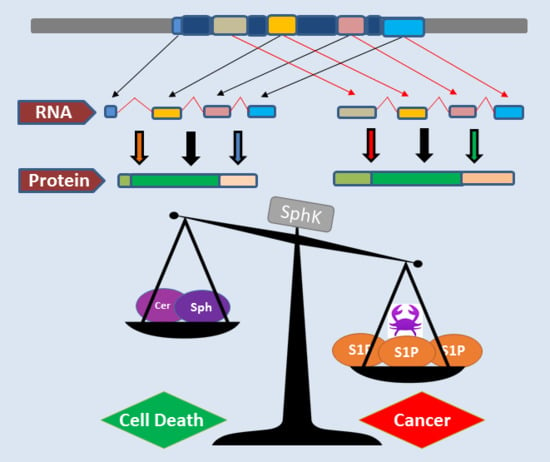
“Dicing and Splicing” Sphingosine Kinase and Relevance to Cancer
Abstract
1. Introduction
1.1. Importance of Isoenzymes (Isozymes) and Variant Isoforms in the Future of Cancer Treatment
2. SphK Isozymes and Isoforms
2.1 Clarification of SphK Nomenclature
2.2. SphK1 and SphK2 Isozymes Are Transcribed from Different Genes and Evolutionary Conserved
2.3. Lessons from the SphK “Isozyme” Knockout Mouse Models—From Mouse to Human
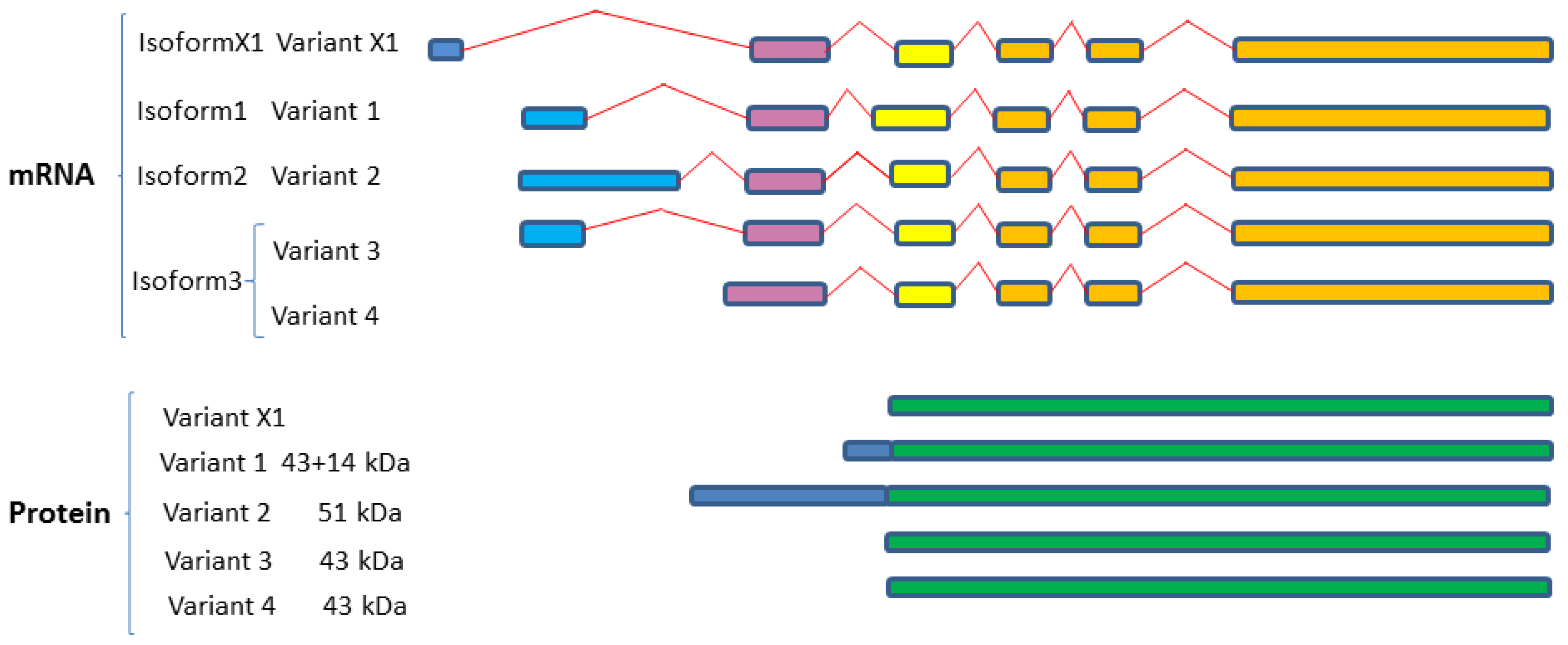
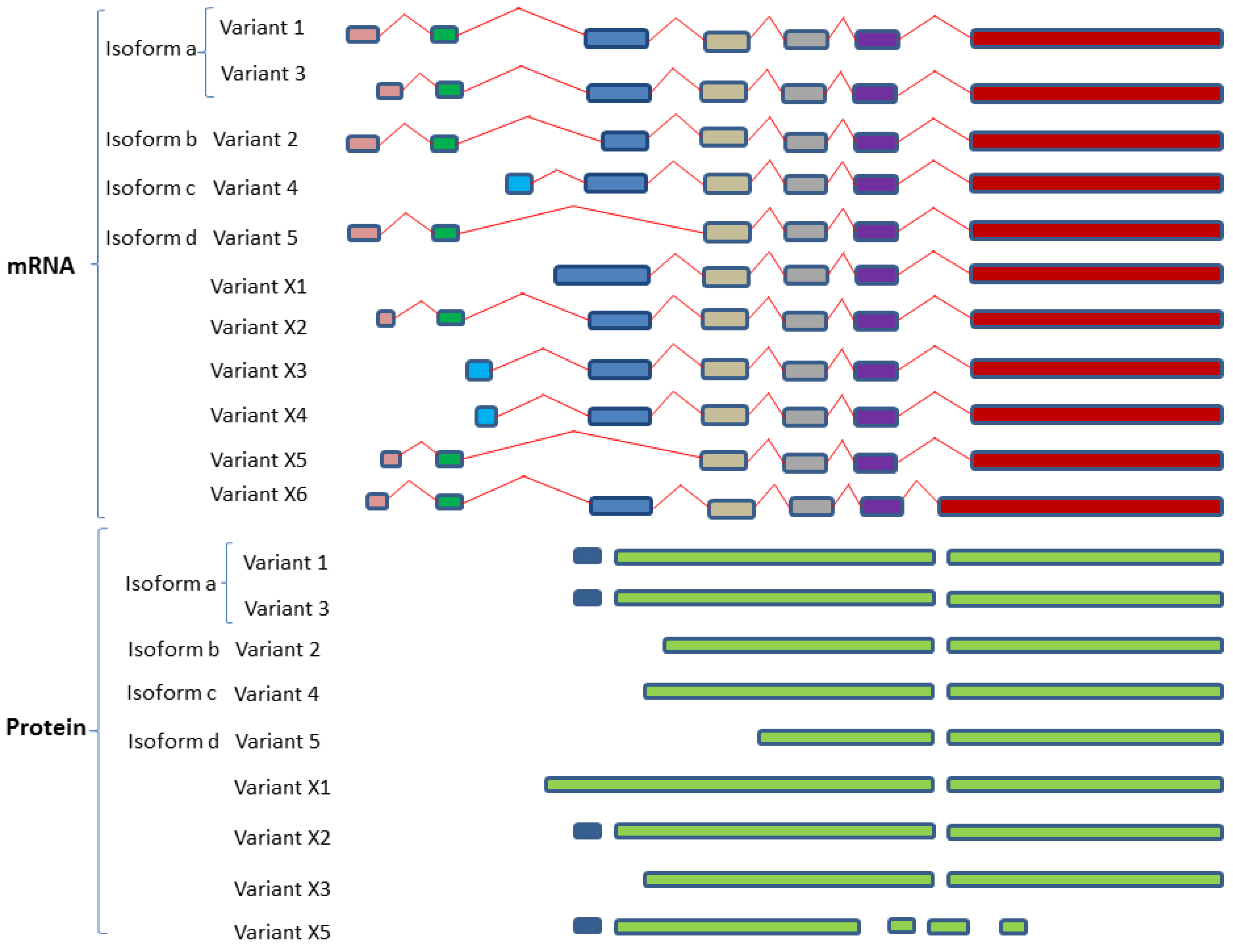
2.4. SphK1 and SphK2 Isozymes Transcribe Multiple Variant Isoforms
2.4.1. SphK1 Variant Isoforms—Differences in Dicing, Splicing and Localization
2.4.2. SphK2 Variant Isoforms—Differences in Dicing, Splicing and Localization
2.5. SphK “Isoform” Specificity—Lessons from the Mouse Model
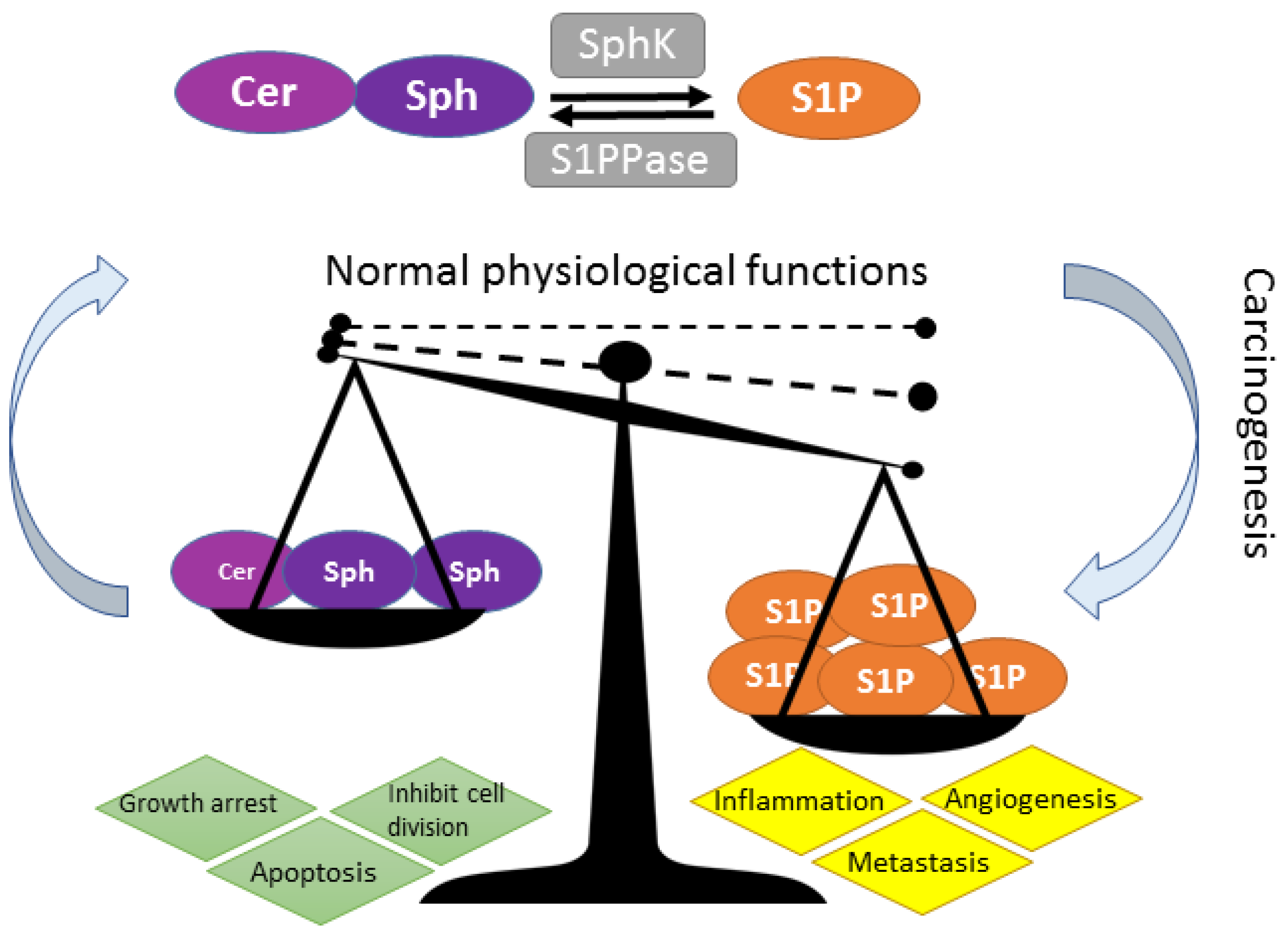
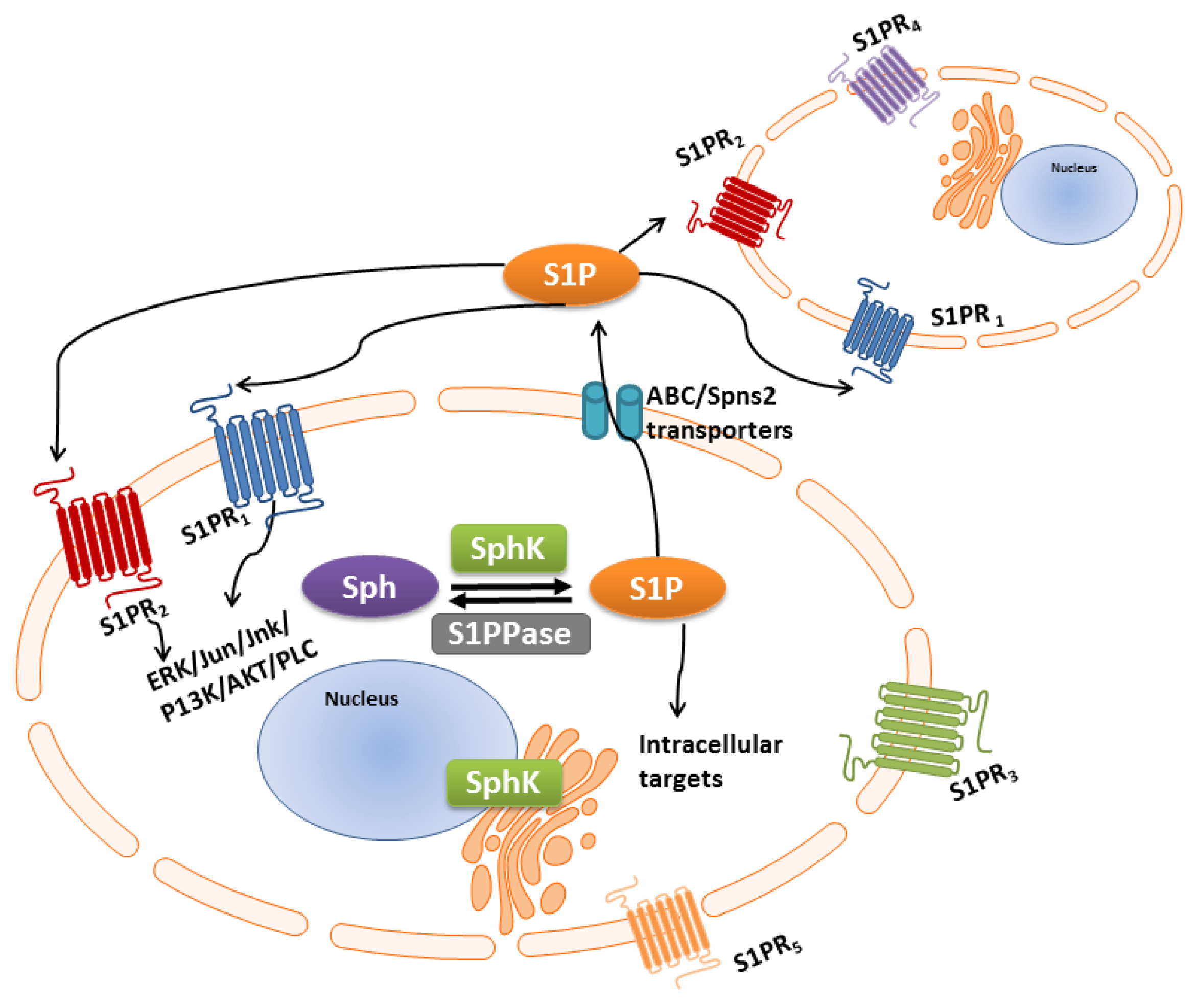
3. S1P-S1PR1-5 Signaling Rheostat—Multiple Functions, Multiple Signaling
S1P/S1P Receptor “Inside-Outside” Signaling
4. Over-Active SPHK-S1P Signaling and Relevance to Cancer
4.1. SphK1 Isozyme Is Overexpressed in Multiple Cancer Types
4.2. SphK2 Isozyme—A Promising Cancer Therapeutic Target
4.3. Targeting S1PRs in Cancer Therapy
5. “Dicing and Splicing” Sphingosine Kinase Variant Isoforms and Relevance to Cancer
5.1. Homing into SphK1 Isoform Expression in Anti-Cancer Targets
5.2. SphK2 Isoforms as Anti-Cancer Targets
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, H.; Yang, J.; Xiang, H.; Peng, H. Sphingosine 1-phosphate in metabolic syndrome. Int. J. Mol. Med. 2016, 38, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cheng, C.; Tan, Z.; Li, N.; Tang, M.; Yang, L.; Cao, Y. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 2017, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Tonelli, F.; Lim, K.G.; Long, J.S.; Edwards, J.; Pyne, S. Sphingosine 1-phosphate signalling in cancer. Biochem. Soc. Trans. 2012, 40, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Tonelli, F.; Lim, K.G.; Long, J.; Edwards, J.; Pyne, S. Targeting sphingosine kinase 1 in cancer. Adv. Biol Regul. 2012, 52, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, G.T.; Maceyka, M.; Milstien, S.; Spiegel, S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: Therapeutic targets. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Haass, N.K.; Nassif, N.; McGowan, E.M. Switching the sphingolipid rheostat in the treatment of diabetes and cancer comorbidity from a problem to an advantage. Biomed. Res. Int. 2015, 2015, 165105. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, D.; Haddadi, N.; Lin, Y.; Nassif, N.T.; McGowan, E.M. Mammalian sphingosine kinase (SphK) isoenzymes and isoform expression: Challenges for sphk as an oncotarget. Oncotarget 2017, 8, 36898–36929. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Im, D.S. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol. Ther. 2017, 25, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Han, M.H. Sphingosine-1-phosphate (S1P) and S1P signaling pathway: Therapeutic targets in autoimmunity and inflammation. Drugs 2016, 76, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Pyne, S.; Adams, D.R.; Pyne, N.J. Sphingosine 1-phosphate and sphingosine kinases in health and disease: Recent advances. Prog. Lipid Res. 2016, 62, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; McNaughton, M.; Boomkamp, S.; MacRitchie, N.; Evangelisti, C.; Martelli, A.M.; Jiang, H.R.; Ubhi, S.; Pyne, S. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv. Biol. Regul. 2016, 60, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.R.; Costabile, M.; Pitson, S.M. Recent advances in the development of sphingosine kinase inhibitors. Cell. Signal. 2016, 28, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Adams, D.R.; Pyne, S. Sphingosine kinase 2 in autoimmune/inflammatory disease and the development of sphingosine kinase 2 inhibitors. Trends Pharmacol. Sci. 2017, 38, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Weiss, W.A. Alternative splicing in cancer: Implications for biology and therapy. Oncogene 2015, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Venables, J.P.; Klinck, R.; Koh, C.; Gervais-Bird, J.; Bramard, A.; Inkel, L.; Durand, M.; Couture, S.; Froehlich, U.; Lapointe, E.; et al. Cancer-associated regulation of alternative splicing. Nat. Struct. Mol. Biol. 2009, 16, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lee, C. Discovery of novel splice forms and functional analysis of cancer-specific alternative splicing in human expressed sequences. Nucleic Acids Res. 2003, 31, 5635–5643. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Zhang, X.; Wu, X.; Lin, Z.; Wang, Q.; Li, Y.; Hu, G. Identification of alternatively spliced mRNA variants related to cancers by genome-wide ests alignment. Oncogene 2004, 23, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, B.W.; Pitson, S.M.; Raben, D.M. The sphingosine and diacylglycerol kinase superfamily of signaling kinases: Localization as a key to signaling function. J. Lipid Res. 2006, 47, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; Lin, M.Z.; McGowan, E.M.; Baxter, R.C. Potentiation of growth factor signaling by insulin-like growth factor-binding protein-3 in breast epithelial cells requires sphingosine kinase activity. J. Biol. Chem. 2009, 284, 25542–25552. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.L.; de Silva, H.C.; Lin, M.Z.; Scott, C.D.; Baxter, R.C. Inhibition of insulin-like growth factor-binding protein-3 signaling through sphingosine kinase-1 sensitizes triple-negative breast cancer cells to egf receptor blockade. Mol. Cancer Ther. 2014, 13, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.A.; Wang, L.; Albanese, N.; Pitson, S.M.; Vadas, M.A.; Xia, P. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol. Endocrinol. 2003, 17, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wadham, C.; Holmes, A.; Albanese, N.; Verrier, E.; Feng, F.; Bernal, A.; Derian, C.K.; Ullrich, A.; Vadas, M.A.; et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: The role of sphingosine kinase-1. J. Cell. Biol. 2006, 173, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Long, J.S.; Orange, C.; Tannahill, C.L.; Mallon, E.; McGlynn, L.M.; Pyne, S.; Pyne, N.J.; Edwards, J. High expression of sphingosine 1-phosphate receptors, S1P 1 and S1P 3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am. J. Pathol. 2010, 177, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.E.; Hobson, J.P.; Murthy, S.; Milstien, S.; Spiegel, S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp. Cell. Res. 2002, 281, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Edwards, J.; Watson, C.; Tovey, S.; Mair, K.M.; Schiff, R.; Natarajan, V.; Pyne, N.J.; Pyne, S. Sphingosine kinase 1 induces tolerance to human epidermal growth factor receptor 2 and prevents formation of a migratory phenotype in response to sphingosine 1-phosphate in estrogen receptor-positive breast cancer cells. Mol. Cell. Biol. 2010, 30, 3827–3841. [Google Scholar] [CrossRef] [PubMed]
- Maczis, M.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate and estrogen signaling in breast cancer. Adv. Biol. Regul. 2016, 60, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Van Brocklyn, J.R.; Edsall, L.; Nava, V.E.; Spiegel, S. Sphingosine-1-phosphate inhibits motility of human breast cancer cells independently of cell surface receptors. Cancer Res. 1999, 59, 6185–6191. [Google Scholar] [PubMed]
- Takabe, K.; Kim, R.H.; Allegood, J.C.; Mitra, P.; Ramachandran, S.; Nagahashi, M.; Harikumar, K.B.; Hait, N.C.; Milstien, S.; Spiegel, S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 2010, 285, 10477–10486. [Google Scholar] [CrossRef] [PubMed]
- Yagoub, D.; Wilkins, M.R.; Lay, A.J.; Kaczorowski, D.C.; Hatoum, D.; Bajan, S.; Hutvagner, G.; Lai, J.H.; Wu, W.; Martiniello-Wilks, R.; et al. Sphingosine kinase 1 isoform-specific interactions in breast cancer. Mol. Endocrinol. 2014, 28, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 1 promotes breast cancer progression by stimulating angiogenesis and lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Takabe, K.; Hait, N.C. Metastatic triple-negative breast cancer is dependent on sphks/s1p signaling for growth and survival. Cell. Signal. 2017, 32, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, J.; Nagahashi, M.; Takabe, K.; Wakai, T. Clinical impact of sphingosine-1-phosphate in breast cancer. Mediat. Inflamm. 2017. [Google Scholar] [CrossRef]
- Katsuta, E.; Yan, L.; Nagahashi, M.; Raza, A.; Sturgill, J.; Lyon, D.; Rashid, O.; Hait, N.; Takabe, K. Doxorubicin effect is enhanced by sphingosine-1- phosphate signaling antagonist in breast cancer. JRS 2017, 219, 202–213. [Google Scholar] [CrossRef]
- Spiegel, S. Sphingosine-1-phosphate: A bridge from bench to clinic. FASEB J. 2016, 30, 243-1. [Google Scholar]
- Mukhopadhyay, P.; Ramanathan, R.; Takabe, K. S1P promotes breast cancer progression by angiogenesis and lymphangiogenesis. Breast Cancer Manag. 2015, 4, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Pyne, S.; Edwards, J.; Ohotski, J.; Pyne, N.J. Sphingosine 1-phosphate receptors and sphingosine kinase 1: Novel biomarkers for clinical prognosis in breast, prostate, and hematological cancers. Front. Oncol. 2012, 2, 168. [Google Scholar] [CrossRef] [PubMed]
- Pchejetski, D.; Golzio, M.; Bonhoure, E.; Calvet, C.; Doumerc, N.; Garcia, V.; Mazerolles, C.; Rischmann, P.; Teissie, J.; Malavaud, B.; et al. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005, 65, 11667–11675. [Google Scholar] [CrossRef] [PubMed]
- Pchejetski, D.; Doumerc, N.; Golzio, M.; Naymark, M.; Teissie, J.; Kohama, T.; Waxman, J.; Malavaud, B.; Cuvillier, O. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol. Cancer Ther. 2008, 7, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Pchejetski, D.; Bohler, T.; Stebbing, J.; Waxman, J. Therapeutic potential of targeting sphingosine kinase 1 in prostate cancer. Nat. Rev. Urol. 2011, 8, 569–678. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, M.; Pitman, M.; Pitson, S.M.; Pyne, N.J.; Pyne, S. Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SKi or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells. Oncotarget 2016, 7, 16663–16675. [Google Scholar] [CrossRef] [PubMed]
- Malavaud, B.; Pchejetski, D.; Mazerolles, C.; de Paiva, G.R.; Calvet, C.; Doumerc, N.; Pitson, S.; Rischmann, P.; Cuvillier, O. Sphingosine kinase-1 activity and expression in human prostate cancer resection specimens. Eur. J. Cancer 2010, 46, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.G.; Tonelli, F.; Berdyshev, E.; Gorshkova, I.; Leclercq, T.; Pitson, S.M.; Bittman, R.; Pyne, S.; Pyne, N.J. Inhibition kinetics and regulation of sphingosine kinase 1 expression in prostate cancer cells: Functional differences between sphingosine kinase 1a and 1b. Int. J. Biochem. Cell. Biol. 2012, 44, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Gestaut, M.M.; Antoon, J.W.; Burow, M.E.; Beckman, B.S. Inhibition of sphingosine kinase-2 ablates androgen resistant prostate cancer proliferation and survival. Pharmacol. Rep. 2014, 66, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Dayon, A.; Brizuela, L.; Martin, C.; Mazerolles, C.; Pirot, N.; Doumerc, N.; Nogueira, L.; Golzio, M.; Teissie, J.; Serre, G.; et al. Sphingosine kinase-1 is central to androgen-regulated prostate cancer growth and survival. PLoS ONE 2009, 4, e8048. [Google Scholar] [CrossRef] [PubMed]
- Akao, Y.; Banno, Y.; Nakagawa, Y.; Hasegawa, N.; Kim, T.J.; Murate, T.; Igarashi, Y.; Nozawa, Y. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochem. Biophys. Res. Commun. 2006, 342, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Wallington-Beddoe, C.T.; Powell, J.A.; Tong, D.; Pitson, S.M.; Bradstock, K.F.; Bendall, L.J. Sphingosine kinase 2 promotes acute lymphoblastic leukemia by enhancing MYC expression. Cancer Res. 2014, 74, 2803–2815. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Gao, M.; Wang, G.; Fu, Y. Concurrent targeting Akt and sphingosine kinase 1 by A-674563 in acute myeloid leukemia cells. Biochem. Biophys. Res. Commun. 2016, 472, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Sobue, S.; Nemoto, S.; Murakami, M.; Ito, H.; Kimura, A.; Gao, S.; Furuhata, A.; Takagi, A.; Kojima, T.; Nakamura, M.; et al. Implications of sphingosine kinase 1 expression level for the cellular sphingolipid rheostat: Relevance as a marker for daunorubicin sensitivity of leukemia cells. Int. J. Hematol. 2008, 87, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Marfe, G.; di Stefano, C.; Gambacurta, A.; Ottone, T.; Martini, V.; Abruzzese, E.; Mologni, L.; Sinibaldi-Salimei, P.; de Fabritis, P.; Gambacorti-Passerini, C.; et al. Sphingosine kinase 1 overexpression is regulated by signaling through PI3K, AKT2, and mTOR in imatinib-resistant chronic myeloid leukemia cells. Exp. Hematol. 2011, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Mathews, T.P.; Kharel, Y.; Field, S.D.; Moyer, M.L.; East, J.E.; Houck, J.D.; Lynch, K.R.; Macdonald, T.L. Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J. Med. Chem. 2011, 54, 3524–3548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, Z.; Lin, Y.; Chen, Z.; Liu, H.; Chen, Y.; Wang, N.; Song, X. Sphingosine kinase 1 enhances the invasion and migration of non-small cell lung cancer cells via the AKT pathway. Oncol. Rep. 2015, 33, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kalari, S.K.; Usatyuk, P.V.; Gorshkova, I.; He, D.; Watkins, T.; Brindley, D.N.; Sun, C.; Bittman, R.; Garcia, J.G.; et al. Intracellular generation of sphingosine 1-phosphate in human lung endothelial cells: Role of lipid phosphate phosphatase-1 and sphingosine kinase 1. J. Biol. Chem. 2007, 282, 14165–14177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Li, G.; Li, Y.; Xu, C.; Li, M.; Xu, G.; Fu, S. Prognostic significance of sphingosine kinase 2 expression in non-small cell lung cancer. Tumour Biol. 2014, 35, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Xiong, H.; Li, J.; Liao, W.; Wang, L.; Wu, J.; Li, M. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin. Cancer Res. 2011, 17, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.R.; Johnson, K.Y.; Crellin, H.G.; Ogretmen, B.; Boylan, A.M.; Harley, R.A.; Obeid, L.M. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J. Histochem. Cytochem. 2005, 53, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Aoki, M.; Katsuta, E.; Ramanathan, R.; Idowu, M.O.; Spiegel, S.; Takabe, K. Host sphingosine kinase 1 worsens pancreatic cancer peritoneal carcinomatosis. J. Surg. Res. 2016, 205, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Ma, Y.J.; Han, L.; Wang, Y.J.; Han, J.A.; Zhu, Y. Role of sphingosine 1-phosphate in human pancreatic cancer cells proliferation and migration. Int. J. Clin. Exp. Med. 2015, 8, 20349–20354. [Google Scholar] [PubMed]
- Japtok, L.; Schmitz, E.I.; Fayyaz, S.; Kramer, S.; Hsu, L.J.; Kleuser, B. Sphingosine 1-phosphate counteracts insulin signaling in pancreatic β-cells via the sphingosine 1-phosphate receptor subtype 2. FASEB J. 2015, 29, 3357–3369. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.; Allende, M.L.; Mizukami, H.; Cook, E.K.; Gavrilova, O.; Tuymetova, G.; Clarke, B.A.; Chen, W.; Olivera, A.; Proia, R.L. Sphingosine-1-phosphate phosphatase 2 regulates pancreatic islet β-cell endoplasmic reticulum stress and proliferation. J. Biol. Chem. 2016, 291, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.F.; Carroll, B.; Adada, M.; Pulkoski-Gross, M.; Hannun, Y.A.; Obeid, L.M. A novel role of sphingosine kinase-1 in the invasion and angiogenesis of VHL mutant clear cell renal cell carcinoma. FASEB J. 2015, 29, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate is a missing link between chronic inflammation and colon cancer. Cancer Cell. 2013, 23, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Huang, J.A.; Qin, M.B.; Su, Y.J.; Lai, M.Y.; Jiang, H.X.; Tang, G.D. Sphingosine kinase 1 enhances colon cancer cell proliferation and invasion by upregulating the production of MMP-2/9 and uPA via MAPK pathways. Int. J. Colorectal Dis. 2012, 27, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, T.; Kaneshiro, T.; Okumura, M.; Maalouf, S.; Uflacker, A.; Bielawski, J.; Hannun, Y.A.; Obeid, L.M. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009, 23, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Baudhuin, L.M.; Xu, Y. Sphingosine-1-phosphate modulates growth and adhesion of ovarian cancer cells. FEBS Lett. 1999, 460, 513–518. [Google Scholar] [CrossRef]
- Lee, J.W.; Ryu, J.Y.; Yoon, G.; Jeon, H.K.; Cho, Y.J.; Choi, J.J.; Song, S.Y.; Do, I.G.; Lee, Y.Y.; Kim, T.J.; et al. Sphingosine kinase 1 as a potential therapeutic target in epithelial ovarian cancer. Int. J. Cancer 2015, 137, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Beach, J.A.; Aspuria, P.J.; Cheon, D.J.; Lawrenson, K.; Agadjanian, H.; Walsh, C.S.; Karlan, B.Y.; Orsulic, S. Sphingosine kinase 1 is required for TGF-β mediated fibroblastto- myofibroblast differentiation in ovarian cancer. Oncotarget 2016, 7, 4167–4182. [Google Scholar] [CrossRef] [PubMed]
- White, M.D.; Chan, L.; Antoon, J.W.; Beckman, B.S. Targeting ovarian cancer and chemoresistance through selective inhibition of sphingosine kinase-2 with ABC294640. Anticancer Res. 2013, 33, 3573–3579. [Google Scholar] [PubMed]
- Wang, D.; Zhao, Z.; Caperell-Grant, A.; Yang, G.; Mok, S.C.; Liu, J.; Bigsby, R.M.; Xu, Y. S1P differentially regulates migration of human ovarian cancer and human ovarian surface epithelial cells. Mol. Cancer Ther. 2008, 7, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jarman, K.E.; Lokman, N.A.; Neubauer, H.A.; Davies, L.T.; Gliddon, B.L.; Taing, H.; Moretti, P.A.B.; Oehler, M.K.; Pitman, M.R.; et al. CIB2 negatively regulates oncogenic signaling in ovarian cancer via sphingosine kinase 1. Cancer Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Quint, K.; Stiel, N.; Neureiter, D.; Schlicker, H.U.; Nimsky, C.; Ocker, M.; Strik, H.; Kolodziej, M.A. The role of sphingosine kinase isoforms and receptors S1P1, S1P2, S1P3, and S1P5 in primary, secondary, and recurrent glioblastomas. Tumour Biol. 2014, 35, 8979–8989. [Google Scholar] [CrossRef] [PubMed]
- Bien-Moller, S.; Lange, S.; Holm, T.; Bohm, A.; Paland, H.; Kupper, J.; Herzog, S.; Weitmann, K.; Havemann, C.; Vogelgesang, S.; et al. Expression of S1P metabolizing enzymes and receptors correlate with survival time and regulate cell migration in glioblastoma multiforme. Oncotarget 2016, 7, 13031–13046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Sun, S.; Yu, S.; Zhang, M.; Zou, F. Inhibition of sphingosine kinase 1 suppresses proliferation of glioma cells under hypoxia by attenuating activity of extracellular signal-regulated kinase. Cell Prolif. 2012, 45, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklyn, J.R.; Jackson, C.A.; Pearl, D.K.; Kotur, M.S.; Snyder, P.J.; Prior, T.W. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: Roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J. Neuropathol. Exp. Neurol. 2005, 64, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Yoon, G.; Ryu, J.Y.; Cho, Y.J.; Choi, J.J.; Lee, Y.Y.; Kim, T.J.; Choi, C.H.; Song, S.Y.; Kim, B.G.; et al. Sphingosine kinase 1 is a reliable prognostic factor and a novel therapeutic target for uterine cervical cancer. Oncotarget 2015, 6, 26746–26756. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xie, X.; Ji, L.; Ruan, X.; Zheng, Z. Sphingosine kinase 1: A novel independent prognosis biomarker in hepatocellular carcinoma. Oncol. Lett. 2017, 13, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, C.X.; Ma, Y.; He, H.W.; Wang, J.P.; Shao, R.G. SphK1 inhibitor SKI II inhibits the proliferation of human hepatoma HepG2 cells via the Wnt5A/β-catenin signaling pathway. Life Sci. 2016, 151, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xiao, Z.; Liu, F.; Cui, M.; Li, W.; Yang, Z.; Li, J.; Ye, L.; Zhang, X. Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1). Oncotarget 2016, 7, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Uranbileg, B.; Ikeda, H.; Kurano, M.; Enooku, K.; Sato, M.; Saigusa, D.; Aoki, J.; Ishizawa, T.; Hasegawa, K.; Kokudo, N.; et al. Increased mRNA levels of sphingosine kinases and S1P lyase and reduced levels of S1P were observed in hepatocellular carcinoma in association with poorer differentiation and earlier recurrence. PLoS ONE 2016, 11, e0149462. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zheng, H.; Chan, M.T.; Wu, W.K. HULC: An oncogenic long non-coding RNA in human cancer. J. Cell. Mol. Med. 2017, 21, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Vadas, M.; Xia, P.; McCaughan, G.; Gamble, J. The role of sphingosine kinase 1 in cancer: Oncogene or non-oncogene addiction? Biochim. Biophys. Acta 2008, 1781, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Pitson, S.M. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 2011, 36, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.E.; Lacana, E.; Poulton, S.; Liu, H.; Sugiura, M.; Kono, K.; Milstien, S.; Kohama, T.; Spiegel, S. Functional characterization of human sphingosine kinase-1. FEBS Lett. 2000, 473, 81–84. [Google Scholar] [CrossRef]
- Liu, H.; Sugiura, M.; Nava, V.E.; Edsall, L.C.; Kono, K.; Poulton, S.; Milstien, S.; Kohama, T.; Spiegel, S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 2000, 275, 19513–19520. [Google Scholar] [CrossRef] [PubMed]
- Melendez, A.J.; Carlos-Dias, E.; Gosink, M.; Allen, J.M.; Takacs, L. Human sphingosine kinase: Molecular cloning, functional characterization and tissue distribution. Gene 2000, 251, 19–26. [Google Scholar] [CrossRef]
- Liu, H.; Chakravarty, D.; Maceyka, M.; Milstien, S.; Spiegel, S. Sphingosine kinases: A novel family of lipid kinases. Prog. Nucleic Acid Res. Mol. Biol. 2002, 71, 493–511. [Google Scholar] [PubMed]
- Wang, Z.; Min, X.; Xiao, S.H.; Johnstone, S.; Romanow, W.; Meininger, D.; Xu, H.; Liu, J.; Dai, J.; An, S.; et al. Molecular basis of sphingosine kinase 1 substrate recognition and catalysis. Structure 2013, 21, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Pitson, S.M.; D’Andrea R, J.; Vandeleur, L.; Moretti, P.A.; Xia, P.; Gamble, J.R.; Vadas, M.A.; Wattenberg, B.W. Human sphingosine kinase: Purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem. J. 2000, 350, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Pulkoski-Gross, M.J.; Donaldson, J.C.; Obeid, L.M. Sphingosine-1-phosphate metabolism: A structural perspective. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.A.; Hannun, Y.A.; Obeid, L.M. Sphingosine kinase: Biochemical and cellular regulation and role in disease. J. Biochem. Mol. Biol. 2006, 39, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Taniguchi, Y.; Kihara, A.; Mitsutake, S.; Igarashi, Y. Asp177 in C4 domain of mouse sphingosine kinase 1a is important for the sphingosine recognition. FEBS Lett. 2004, 578, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Ding, G.; Sonoda, H.; Kajimoto, T.; Haga, Y.; Khosrowbeygi, A.; Gao, S.; Miwa, N.; Jahangeer, S.; Nakamura, S. Involvement of N-terminal-extended form of sphingosine kinase 2 in serum-dependent regulation of cell proliferation and apoptosis. J. Biol. Chem. 2005, 280, 36318–36325. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Toman, R.E.; Goparaju, S.K.; Maceyka, M.; Nava, V.E.; Sankala, H.; Payne, S.G.; Bektas, M.; Ishii, I.; Chun, J.; et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 2003, 278, 40330–40336. [Google Scholar] [CrossRef] [PubMed]
- Billich, A.; Bornancin, F.; Devay, P.; Mechtcheriakova, D.; Urtz, N.; Baumruker, T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 2003, 278, 47408–47415. [Google Scholar] [CrossRef] [PubMed]
- Maceyka, M.; Sankala, H.; Hait, N.C.; Le Stunff, H.; Liu, H.; Toman, R.; Collier, C.; Zhang, M.; Satin, L.S.; Merrill, A.H., Jr.; et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005, 280, 37118–37129. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Gamble, J.R.; Wang, L.; Pitson, S.M.; Moretti, P.A.; Wattenberg, B.W.; D’Andrea, R.J.; Vadas, M.A. An oncogenic role of sphingosine kinase. Curr. Biol. 2000, 10, 1527–1530. [Google Scholar] [CrossRef]
- Sukocheva, O.; Wadham, C.; Xia, P. Role of sphingolipids in the cytoplasmic signaling of estrogens. Steroids 2009, 74, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Pitson, S.M.; Moretti, P.A.; Zebol, J.R.; Lynn, H.E.; Xia, P.; Vadas, M.A.; Wattenberg, B.W. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003, 22, 5491–5500. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; van Koppen, C.J.; Danneberg, K.; Ter Braak, M.; Meyer Zu Heringdorf, D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 374, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Delon, C.; Manifava, M.; Wood, E.; Thompson, D.; Krugmann, S.; Pyne, S.; Ktistakis, N.T. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J. Biol. Chem. 2004, 279, 44763–44774. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Nishi, T.; Hirata, T.; Kihara, A.; Sano, T.; Igarashi, Y.; Yamaguchi, A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J. Lipid Res. 2006, 47, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, S.; Simmons, S.; Kawamura, S.; Inoue, A.; Orba, Y.; Tokudome, T.; Sunden, Y.; Arai, Y.; Moriwaki, K.; Ishida, J.; et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 2012, 122, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Takabe, K.; Terracina, K.P.; Soma, D.; Hirose, Y.; Kobayashi, T.; Matsuda, Y.; Wakai, T. Sphingosine-1-phosphate transporters as targets for cancer therapy. Biomed. Res. Int. 2014, 2014, 651727. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, N.; Okada, T.; Hayashi, S.; Fujita, T.; Jahangeer, S.; Nakamura, S. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 2003, 278, 46832–46839. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Panneer Selvam, S.; De Palma, R.M.; Oaks, J.J.; Oleinik, N.; Peterson, Y.K.; Stahelin, R.V.; Skordalakes, E.; Ponnusamy, S.; Garrett-Mayer, E.; Smith, C.D.; et al. Binding of the sphingolipid s1p to htert stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci. Signal. 2015, 8, ra58. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.R.; Pyne, S.; Pyne, N.J. Sphingosine kinases: Emerging structure-function insights. Trends Biochem. Sci. 2016, 41, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Kihara, A.; Igarashi, Y. Distribution of sphingosine kinase activity in mouse tissues: Contribution of sphk1. Biochem. Biophys. Res. Commun. 2003, 309, 155–160. [Google Scholar] [CrossRef]
- Michaud, J.; Kohno, M.; Proia, R.L.; Hla, T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 2006, 580, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Sasaki, T.; Kawai, H.; Olivera, A.; Mi, Y.; van Echten-Deckert, G.; Hajdu, R.; Rosenbach, M.; Keohane, C.A.; Mandala, S.; et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 2004, 279, 52487–52492. [Google Scholar] [CrossRef] [PubMed]
- Kharel, Y.; Lee, S.; Snyder, A.H.; Sheasley-O’neill S, L.; Morris, M.A.; Setiady, Y.; Zhu, R.; Zigler, M.A.; Burcin, T.L.; Ley, K.; et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem. 2005, 280, 36865–36872. [Google Scholar] [CrossRef] [PubMed]
- Zemann, B.; Kinzel, B.; Muller, M.; Reuschel, R.; Mechtcheriakova, D.; Urtz, N.; Bornancin, F.; Baumruker, T.; Billich, A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 2006, 107, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, M.; Kim, M.; D’Agati, V.D.; Lee, H.T. Sphingosine kinase 1 protects against renal ischemia-reperfusion injury in mice by sphingosine-1-phosphate1 receptor activation. Kidney Int. 2011, 80, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, I.A.; Wang, H.; Orbelyan, G.A.; Goya, J.; Natarajan, V.; Beiser, D.G.; Vanden Hoek, T.L.; Berdyshev, E.V. Inhibition of sphingosine-1-phosphate lyase rescues sphingosine kinase-1-knockout phenotype following murine cardiac arrest. Life Sci. 2013, 93, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Shafique, A.; Shang, K.; Couttas, T.A.; Zhao, H.; Don, A.S.; Karl, T. Contextual fear conditioning is enhanced in mice lacking functional sphingosine kinase 2. Behav. Brain Res. 2017, 333, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, X.; Qiang, X.; Luo, L.; Hylemon, P.B.; Jiang, Z.; Zhang, L.; Zhou, H. Taurocholate induces cyclooxygenase-2 expression via the sphingosine 1-phosphate receptor 2 in a human cholangiocarcinoma cell line. J. Biol. Chem. 2015, 290, 30988–31002. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, R.; Zhou, X.; Liang, X.; Campbell, D.J.; Zhang, X.; Zhang, L.; Shi, R.; Wang, G.; Pandak, W.M. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology 2014, 60, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aoki, H.; Yang, J.; Peng, K.; Liu, R.; Li, X.; Qiang, X.; Sun, L.; Gurley, E.C.; Lai, G. The role of sphingosine 1-phosphate receptor 2 in bile-acid–induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology 2017, 65, 2005–2018. [Google Scholar] [CrossRef] [PubMed]
- Kwong, E.K.; Li, X.; Hylemon, P.B.; Zhou, H. Sphingosine kinases/sphingosine 1-phosphate signaling in hepatic lipid metabolism. Curr. Pharmacol. Rep. 2017, 3, 176–183. [Google Scholar] [CrossRef]
- Nagahashi, M.; Takabe, K.; Liu, R.; Peng, K.; Wang, X.; Wang, Y.; Hait, N.C.; Wang, X.; Allegood, J.C.; Yamada, A.; et al. Conjugated bile acid-activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology 2015, 61, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Nagahashi, M.; Kim, E.Y.; Harikumar, K.B.; Yamada, A.; Huang, W.C.; Hait, N.C.; Allegood, J.C.; Price, M.M.; Avni, D.; et al. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell 2013, 23, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.A.; Pitson, S.M. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS J. 2013, 280, 5317–5336. [Google Scholar] [CrossRef] [PubMed]
- Loveridge, C.; Tonelli, F.; Leclercq, T.; Lim, K.G.; Long, J.S.; Berdyshev, E.; Tate, R.J.; Natarajan, V.; Pitson, S.M.; Pyne, N.J.; et al. The sphingosine kinase 1 inhibitor 2-(p-Hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J. Biol Chem 2010, 285, 38841–38852. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, F.; Lim, K.G.; Loveridge, C.; Long, J.; Pitson, S.M.; Tigyi, G.; Bittman, R.; Pyne, S.; Pyne, N.J. FTY720 and (S)-FTY720 vinylphosphonate inhibit sphingosine kinase 1 and promote its proteasomal degradation in human pulmonary artery smooth muscle, breast cancer and androgen-independent prostate cancer cells. Cell. Signal. 2010, 22, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Momoi, M.; Oo, M.L.; Paik, J.H.; Lee, Y.M.; Venkataraman, K.; Ai, Y.; Ristimaki, A.P.; Fyrst, H.; Sano, H.; et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 2006, 26, 7211–7223. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Thangada, S.; Michaud, J.; Oo, M.L.; Ai, Y.; Lee, Y.M.; Wu, M.; Parikh, N.S.; Khan, F.; Proia, R.L.; et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem. J. 2006, 397, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A.; Anada, Y.; Igarashi, Y. Mouse sphingosine kinase isoforms SPHK1a and SPHK1b differ in enzymatic traits including stability, localization, modification, and oligomerization. J. Biol. Chem. 2006, 281, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell. Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.G.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate: Dual messenger functions. FEBS Lett. 2002, 531, 54–57. [Google Scholar] [CrossRef]
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstien, S.; Spiegel, S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155. [Google Scholar] [PubMed]
- Patmanathan, S.N.; Wang, W.; Yap, L.F.; Herr, D.R.; Paterson, I.C. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cell. Signal. 2017, 34, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Hla, T.; Maciag, T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to g-protein-coupled receptors. J. Biol. Chem. 1990, 265, 9308–9313. [Google Scholar] [PubMed]
- Liu, Y.; Wada, R.; Yamashita, T.; Mi, Y.; Deng, C.X.; Hobson, J.P.; Rosenfeldt, H.M.; Nava, V.E.; Chae, S.S.; Lee, M.J.; et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Investig. 2000, 106, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Wu, L.; Acharya, S.; Arac, A.; Blaho, V.A.; Huang, Y.; Moon, B.S.; Axtell, R.C.; Ho, P.P.; Steinberg, G.K.; et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 2013, 14, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Oo, M.L.; Chang, S.H.; Thangada, S.; Wu, M.T.; Rezaul, K.; Blaho, V.; Hwang, S.I.; Han, D.K.; Hla, T. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Investig. 2011, 121, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Mi, Y.; Liu, Y.; Sasaki, T.; Allende, M.L.; Wu, Y.P.; Yamashita, T.; Proia, R.L. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 2004, 279, 29367–29373. [Google Scholar] [CrossRef] [PubMed]
- Kluk, M.J.; Hla, T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta 2002, 1582, 72–80. [Google Scholar] [CrossRef]
- Wang, W.; Graeler, M.H.; Goetzl, E.J. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005, 19, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Jaillard, C.; Harrison, S.; Stankoff, B.; Aigrot, M.S.; Calver, A.R.; Duddy, G.; Walsh, F.S.; Pangalos, M.N.; Arimura, N.; Kaibuchi, K.; et al. Edg8/S1P5: An oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. 2005, 25, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, R.; Kusmartseva, I.; Loughran, T.P. Characterization of a human sphingosine-1-phosphate receptor gene (S1P5) and its differential expression in LGL leukemia. Biochim. Biophys. Acta 2002, 1579, 117–123. [Google Scholar] [CrossRef]
- Walzer, T.; Chiossone, L.; Chaix, J.; Calver, A.; Carozzo, C.; Garrigue-Antar, L.; Jacques, Y.; Baratin, M.; Tomasello, E.; Vivier, E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 2007, 8, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Andrieu, G.; Ledoux, A.; Branka, S.; Bocquet, M.; Gilhodes, J.; Walzer, T.; Kasahara, K.; Inagaki, M.; Sabbadini, R.A.; Cuvillier, O.; et al. Sphingosine 1-phosphate signaling through its receptor S1P5 promotes chromosome segregation and mitotic progression. Sci. Signal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gillies, L.; Lee, S.C.; Long, J.S.; Ktistakis, N.; Pyne, N.J.; Pyne, S. The sphingosine 1-phosphate receptor 5 and sphingosine kinases 1 and 2 are localised in centrosomes: Possible role in regulating cell division. Cell. Signal. 2009, 21, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ohotski, J.; Rosen, H.; Bittman, R.; Pyne, S.; Pyne, N.J. Sphingosine kinase 2 prevents the nuclear translocation of sphingosine 1-phosphate receptor-2 and tyrosine 416 phosphorylated c-Src and increases estrogen receptor negative MDA-MB-231 breast cancer cell growth: The role of sphingosine 1-phosphate receptor-4. Cell. Signal. 2014, 26, 1040–1047. [Google Scholar] [PubMed]
- Yester, J.W.; Tizazu, E.; Harikumar, K.B.; Kordula, T. Extracellular and intracellular sphingosine-1-phosphate in cancer. Cancer Metastasis Rev. 2011, 30, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Sauer, L.; Nunes, J.; Salunkhe, V.; Skalska, L.; Kohama, T.; Cuvillier, O.; Waxman, J.; Pchejetski, D. Sphingosine kinase 1 inhibition sensitizes hormone-resistant prostate cancer to docetaxel. Int. J. Cancer 2009, 125, 2728–2736. [Google Scholar] [CrossRef] [PubMed]
- Bonhoure, E.; Pchejetski, D.; Aouali, N.; Morjani, H.; Levade, T.; Kohama, T.; Cuvillier, O. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting shingosine kinase-1. Leukemia 2006, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, D.; Allegood, J.C.; Mitchell, C.; Hait, N.C.; Almenara, J.A.; Adams, J.K.; Zipkin, R.E.; Dent, P.; Kordula, T.; Milstien, S. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009, 69, 6915–6923. [Google Scholar] [CrossRef] [PubMed]
- Paugh, S.W.; Paugh, B.S.; Rahmani, M.; Kapitonov, D.; Almenara, J.A.; Kordula, T.; Milstien, S.; Adams, J.K.; Zipkin, R.E.; Grant, S. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood 2008, 112, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Price, M.M.; Oskeritzian, C.A.; Falanga, Y.T.; Harikumar, K.B.; Allegood, J.C.; Alvarez, S.E.; Conrad, D.; Ryan, J.J.; Milstien, S.; Spiegel, S. A specific sphingosine kinase 1 inhibitor attenuates airway hyperresponsiveness and inflammation in a mast cell-dependent murine model of allergic asthma. J. Allergy Clin. Immunol. 2013, 131, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Anelli, V.; Gault, C.R.; Snider, A.J.; Obeid, L.M. Role of sphingosine kinase-1 in paracrine/transcellular angiogenesis and lymphangiogenesis in vitro. FASEB J. 2010, 24, 2727–2738. [Google Scholar] [CrossRef] [PubMed]
- French, K.J.; Schrecengost, R.S.; Lee, B.D.; Zhuang, Y.; Smith, S.N.; Eberly, J.L.; Yun, J.K.; Smith, C.D. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003, 63, 5962–5969. [Google Scholar] [PubMed]
- Guillermet-Guibert, J.; Davenne, L.; Pchejetski, D.; Saint-Laurent, N.; Brizuela, L.; Guilbeau-Frugier, C.; Delisle, M.-B.; Cuvillier, O.; Susini, C.; Bousquet, C. Targeting the sphingolipid metabolism to defeat pancreatic cancer cell resistance to the chemotherapeutic gemcitabine drug. Mol. Cancer Ther. 2009, 8, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Peterson, Y.K.; Smith, R.A.; Smith, C.D. Characterization of isoenzyme-selective inhibitors of human sphingosine kinases. PLoS ONE 2012, 7, e44543. [Google Scholar] [CrossRef] [PubMed]
- Antoon, J.W.; White, M.D.; Slaughter, E.M.; Driver, J.L.; Khalili, H.S.; Elliott, S.; Smith, C.D.; Burow, M.E.; Beckman, B.S. Targeting NFĸB mediated breast cancer chemoresistance through selective inhibition of sphingosine kinase-2. Cancer Biol. Ther. 2011, 11, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.A.; Carvajal, R.D.; Merrill, A.H.; Gonen, M.; Cane, L.M.; Schwartz, G.K. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin. Cancer Res. 2011, 17, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- French, K.J.; Zhuang, Y.; Maines, L.W.; Gao, P.; Wang, W.; Beljanski, V.; Upson, J.J.; Green, C.L.; Keller, S.N.; Smith, C.D. Pharmacology and antitumor activity of ABC294640, a selective inhibitor of sphingosine kinase-2. J. Pharm. Exp. Ther. 2010, 333, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Coward, J.; Ambrosini, G.; Musi, E.; Truman, J.-P.; Haimovitz-Friedman, A.; Allegood, J.C.; Wang, E.; Merrill, A.H., Jr.; Schwartz, G.K. Safingol (l-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy 2009, 5, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Sweeney, E.A.; Masamune, A.; Yatomi, Y.; Hakomori, S.-i.; Igarashi, Y. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: A possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 1995, 55, 691–697. [Google Scholar] [PubMed]
- Jarvis, W.D.; Fornari, F.A.; Auer, K.L.; Freemerman, A.J.; Szabo, E.; Birrer, M.J.; Johnson, C.R.; Barbour, S.E.; Dent, P.; Grant, S. Coordinate regulation of stress-and mitogen-activated protein kinases in the apoptotic actions of ceramide and sphingosine. Mol. Pharmacol. 1997, 52, 935–947. [Google Scholar] [PubMed]
- Sweeney, E.A.; Inokuchi, J.-i.; Igarashi, Y. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 1998, 425, 61–65. [Google Scholar] [CrossRef]
- Sakakura, C.; Sweeney, E.A.; Shirahama, T.; Hakomori, S.-I. Suppression of Bcl-2 gene expression by sphingosine in the apoptosis of human leukemic HL-60 cells during phorbol ester-induced terminal differentiation. FEBS Lett. 1996, 379, 177–180. [Google Scholar] [CrossRef]
- Klostergaard, J.; Auzenne, E.; Leroux, E. Characterization of cytotoxicity induced by sphingolipids in multidrug-resistant leukemia cells. Leukemia Res. 1998, 22, 1049–1056. [Google Scholar] [CrossRef]
- Jarvis, W.D.; Frank Jr, A.; Traylor, R.S.; Martin, H.A.; Kramer, L.B.; Erukulla, R.K.; Bittman, R.; Grant, S. Induction of apoptosis and potentiation of ceramide-mediated cytotoxicity by sphingoid bases in human myeloid leukemia cells. J. Biol. Chem. 1996, 271, 8275–8284. [Google Scholar] [CrossRef] [PubMed]
- Sawai, H.; Okazaki, T.; Domae, N. Sphingosine-induced c-jun expression: Differences between sphingosine- and C2-ceramide-mediated signaling pathways. FEBS Lett. 2002, 524, 103–106. [Google Scholar] [CrossRef]
- Jendiroba, D.B.; Klostergaard, J.; Keyhani, A.; Pagliaro, L.; Freireich, E.J. Effective cytotoxicity against human leukemias and chemotherapy-resistant leukemia cell lines by N-N-dimethylsphingosine. Leukemia Res. 2002, 26, 301–310. [Google Scholar] [CrossRef]
- Endo, K.; Igarashi, Y.; Nisar, M.; Zhou, Q.; Hakomori, S.-i. Cell membrane signaling as target in cancer therapy: Inhibitory effect of N,N-dimethyl and N,N,N-trimethyl sphingosine derivatives on in vitro and in vivo growth of human tumor cells in nude mice. Cancer Res. 1991, 51, 1613–1618. [Google Scholar] [PubMed]
- Sweeney, E.A.; Sakakura, T.S.; Ohta, H.; Hakomori, S.-I.; Igarashi, Y. Sphingosine and its methylated derivative in a variety of human cancer cell lines. Int. J. Cancer 1996, 66, 366. [Google Scholar] [CrossRef]
- Cuvillier, O.; Edsall, L.; Spiegel, S. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J. Biol. Chem. 2000, 275, 15691–15700. [Google Scholar] [CrossRef] [PubMed]
- Cuvillier, O.; Levade, T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood 2001, 98, 2828–2836. [Google Scholar] [CrossRef] [PubMed]
- Shirahama, T.; Sweeney, E.A.; Sakakura, C.; Singhal, A.K.; Nishiyama, K.; Akiyama, S.-I.; Hakomori, S.-I.; Igarashi, Y. In vitro and in vivo induction of apoptosis by sphingosine and N,N-dimethylsphingosine in human epidermoid carcinoma KB-3-1 and its multidrug-resistant cells. Clin. Cancer Res. 1997, 3, 257–264. [Google Scholar] [PubMed]
- Edsall, L.C.; Cuvillier, O.; Twitty, S.; Spiegel, S.; Milstien, S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J. Neurochem. 2001, 76, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Edsall, L.C.; Pirianov, G.G.; Spiegel, S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J. Neurosci. 1997, 17, 6952–6960. [Google Scholar] [PubMed]
- Nava, V.E.; Cuvillier, O.; Edsall, L.C.; Kimura, K.; Milstien, S.; Gelmann, E.P.; Spiegel, S. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 2000, 60, 4468–4474. [Google Scholar] [PubMed]
- Wen-Chun, H.; CHANG, H.-C.; CHUANG, L.-Y. Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem. J. 1999, 338, 161–166. [Google Scholar]
- Shida, D.; Takabe, K.; Kapitonov, D.; Milstien, S.; Spiegel, S. Targeting SphK1 as a new strategy against cancer. Curr. Drug Targets 2008, 9, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.M.; Ergin, M.; Denning, M.F.; Quevedo, M.E.; Alkan, S. Characterization of apoptosis induced by protein kinase C inhibitors and its modulation by the caspase pathway in acute promyelocytic leukaemia. Br. J. Haematol. 2000, 110, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Chmura, S.J.; Nodzenski, E.; Beckett, M.A.; Kufe, D.W.; Quintans, J.; Weichselbaum, R.R. Loss of ceramide production confers resistance to radiation-induced apoptosis. Cancer Res. 1997, 57, 1270–1275. [Google Scholar] [PubMed]
- Sachs, C.W.; Safa, A.R.; Harrison, S.D.; Fine, R.L. Partial inhibition of multidrug resistance by safingol is independent of modulation of P-glycoprotein substrate activities and correlated with inhibition of protein kinase C. J. Biol. Chem. 1995, 270, 26639–26648. [Google Scholar] [CrossRef] [PubMed]
- Tavarini, S.; Colombaioni, L.; Garcia-Gil, M. Sphingomyelinase metabolites control survival and apoptotic death in SH-SY5Y neuroblastoma cells. Neurosci. Lett. 2000, 285, 185–188. [Google Scholar] [CrossRef]
- Pchejetski, D.; Bohler, T.; Brizuela, L.; Sauer, L.; Doumerc, N.; Golzio, M.; Salunkhe, V.; Teissié, J.; Malavaud, B.; Waxman, J. FTY720 (fingolimod) sensitizes prostate cancer cells to radiotherapy by inhibition of sphingosine kinase-1. Cancer Res. 2010, 70, 8651–8661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Qi, Y.; Wadham, C.; Wang, L.; Warren, A.; Di, W.; Xia, P. FTY720 induces necrotic cell death and autophagy in ovarian cancer cells: A protective role of autophagy. Autophagy 2010, 6, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Takahara, S.; Horie, S.; Muto, S.; Otsuki, Y.; Katsuoka, Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J. Urol. 2003, 169, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhu, J.; Ding, K.; Xu, J. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signaling pathway. Tumor Biol. 2014, 35, 10707–10714. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Man, K.; Ho, J.W.; Sun, C.K.; Ng, K.T.; Wang, X.H.; Wong, Y.C.; Ng, I.O.; Xu, R.; Fan, S.T. FTY720 induces apoptosis of human hepatoma cell lines through PI3-K-mediated Akt dephosphorylation. Carcinogenesis 2004, 25, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Guo, T.L.; Hait, N.C.; Allegood, J.; Parikh, H.I.; Xu, W.; Kellogg, G.E.; Grant, S.; Spiegel, S.; Zhang, S. Biological characterization of 3-(2-amino-ethyl)-5-[3-(4-butoxyl-phenyl)-propylidene]-thiazolidine-2, 4-dione (K145) as a selective sphingosine kinase-2 inhibitor and anticancer agent. PLoS ONE 2013, 8, e56471. [Google Scholar] [CrossRef] [PubMed]
- Sukocheva, O.; Wang, L.; Verrier, E.; Vadas, M.A.; Xia, P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology 2009, 150, 4484–4492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wan, Z.; Liu, S.; Cao, Y.; Zeng, Z. Sphingosine kinase 1 and cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e90362. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Nagahashi, M.; Terracina, K.P.; Takabe, K. Emerging role of sphingosine-1-phosphate in inflammation, cancer, and lymphangiogenesis. Biomolecules 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Aspelund, A.; Alitalo, K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014, 124, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, H.A.; Pham, D.H.; Zebol, J.R.; Moretti, P.A.; Peterson, A.L.; Leclercq, T.M.; Chan, H.; Powell, J.A.; Pitman, M.R.; Samuel, M.S.; et al. An oncogenic role for sphingosine kinase 2. Oncotarget 2016, 7, 64886–64899. [Google Scholar] [CrossRef] [PubMed]
- Britten, C.D.; Garrett-Mayer, E.; Chin, S.H.; Shirai, K.; Ogretmen, B.; Bentz, T.A.; Brisendine, A.; Anderton, K.; Cusack, S.L.; Maines, L.W.; et al. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Sanchez, T.; Yamase, H.; Hla, T.; Oo, M.L.; Pappalardo, A.; Lynch, K.R.; Lin, C.Y.; Ferrer, F. S1P/S1P1 signaling stimulates cell migration and invasion in wilms tumor. Cancer Lett. 2009, 276, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.E.; Pop, A.; Koh, W.; Anthis, N.J.; Saunders, W.B.; Davis, G.E. Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane-type matrix metalloproteinase-1-dependent signaling. Mol. Cancer 2006, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Young, N.; Pearl, D.K.; Van Brocklyn, J.R. Sphingosine-1-phosphate regulates glioblastoma cell invasiveness through the urokinase plasminogen activator system and CCN1/Cyr61. Mol. Cancer Res. 2009, 7, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lepley, D.; Paik, J.H.; Hla, T.; Ferrer, F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005, 65, 3788–3795. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Takuwa, N.; Yoshioka, K.; Okamoto, Y.; Gonda, K.; Sugihara, K.; Fukamizu, A.; Asano, M.; Takuwa, Y. S1P2, the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010, 70, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Goparaju, S.K.; Jolly, P.S.; Watterson, K.R.; Bektas, M.; Alvarez, S.; Sarkar, S.; Mel, L.; Ishii, I.; Chun, J.; Milstien, S.; et al. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol. Cell. Biol. 2005, 25, 4237–4249. [Google Scholar] [CrossRef] [PubMed]
- Cattoretti, G.; Mandelbaum, J.; Lee, N.; Chaves, A.H.; Mahler, A.M.; Chadburn, A.; Dalla-Favera, R.; Pasqualucci, L.; MacLennan, A.J. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res. 2009, 69, 8686–8692. [Google Scholar] [CrossRef] [PubMed]
- Watters, R.J.; Wang, H.G.; Sung, S.S.; Loughran, T.P.; Liu, X. Targeting sphingosine-1-phosphate receptors in cancer. Anticancer Agents Med. Chem. 2011, 11, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Guerrero, M.; Urbano, M.; Rosen, H. Sphingosine 1-phosphate receptor agonists: A patent review (2010–2012). Expert. Opin. Ther. Pat. 2013, 23, 817–841. [Google Scholar] [CrossRef] [PubMed]
| Cancer Sub-Type | Reference(s) |
|---|---|
| Breast | [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] |
| Prostate | [42,43,44,45,46,47,48,49,50,51] |
| Leukaemia | [52,53,54,55,56] |
| Lung | [57,58,59,60,61] |
| Pancreas | [62,63,64,65] |
| Renal | [66] |
| Colon | [67,68,69] |
| Ovarian | [70,71,72,73,74,75] * |
| Brain | [76,77,78,79] |
| Uterine Cervical | [80] * |
| Liver | [81,82,83,84,85] * |
| Isozymes | Isoform Name | Isoform No. | Variant No. | GenBank Accession | Uniprot ID |
|---|---|---|---|---|---|
| Sphingosine kinase 1 (SphK1; SK1) | SphK1a | Isoform 3 | Variant 3 | NM_001142601 | Q9NYA1-1 |
| Variant 4 | NM_001142602 | ||||
| Variant X1 * | XM-005257766 | ||||
| SphK1b | Isoform 2 | Variant 2 | NM_182965 | Q9NYA1-2 | |
| SphK1c | Isoform 1 | Variant 1 | NM_021972 | Q9NYA1-3 | |
| SphK1a+14 | |||||
| Sphingosine kinase 2 (SphK2; SK2) | SphK2a | Isoform 1 and 3 | Variant 1 | NM_020126 | Q9NRA0-1 |
| SphK2-L | Variant 3 ** | NM_001204159 | Q9NRA0-3 | ||
| SphK2b | Isoform 2 | Variant 2 | NM_001204158 | Q9NRA0-2 | |
| SphK-S | |||||
| SphK2c | Isoform 4 | Variant 4 | NM_001204160 | Q9NRA0-4 | |
| SphK2d | Isoform 5 | Variant 5 | NM_001243876 | Q9NRA0-5 | |
| Variant X1 | XM_017027008 | Q9NRA0-5 | |||
| Variant X2 | XM_011527133 | Q9NRA0-1 | |||
| Variant X3 | XM_006723292 | Q9NRA0-2 | |||
| Variant X4 | XM_011527134 | Q9NRA0-2 | |||
| Variant X5 | XM_017027009 | ||||
| Variant X6 | XM_017027010 |
| SPHK Inhibitor | Cancer Cell Type | SphK Selectivity | References |
|---|---|---|---|
| B5354-c | Prostate (LNCaP, Du145 and PC-3) | SphK1 | [45,154] |
| Breast (MDA-MB-231) | |||
| F-12509a | Leukaemia (HL-60, LAMA-84 and HL-60 MDR) | SphK1 | [155] |
| Chronic Myeloid Leukaemia blasts | |||
| SK1-I | Glioma (U87MG, LN229 and U373) and Primary Glioma Cells (GBM6) | SphK1 | [36,129,156,157,158,159] |
| Leukaemia (U937, HL-60 and Jurkat) and Acute Myeloid Leukaemia blasts | |||
| Breast cancer (MDA-MB-231, MCF-7 and MCF-7 HER2) | |||
| Prostate (LNCaP) | |||
| SKI-II | Prostate (LNCaP, C4-2B and PC-3) | SphK1 and SphK2 | [43,160,161,162,163] |
| Pancreas (Panc-1 and BXPC-3) | |||
| Bladder (T24) | |||
| Breast (MCF7) | |||
| ABC294640 | Pancreatic (clinical trial) and (Panc-1) | SphK2 | [163,164,165] |
| Colorectal (HT-29 and Caco-2) | |||
| Breast (MCF-7, MDA-MB-231) | |||
| Ovarian (SK-OV-3) | |||
| Prostate (DU145) | |||
| Kidney (A-498) | |||
| Melanoma (1025LU) | |||
| Bladder (T24) | |||
| Liver (Hep-G2) | |||
| Safingol | Solid tumors (clinical trial) | SphK1 and SphK2 | [8,165,166] |
| Glioblastomas | |||
| Colorectal tumor | |||
| Colorectal (HCT116) | |||
| Adrenal cortical carcinoma | |||
| Sarcoma | |||
| N,N-dimethyl-d-erythro-sphingosine (DMS) | Lung (A549) | SphK1 and SphK2 | - |
| Acute myeloid leukemia (HL-60, U937, CMK7) | [167,168,169,170,171,172,173] | ||
| Chronic myeloid leukemia (JFP1, K562) | [174] | ||
| Gastric (MKN45, MKN74, Kato III) | [175,176] | ||
| Acute lymphoid leukemia (Jurkat) | [177,178] | ||
| Lung (LU65, NCI-1169) | [175] | ||
| Cervix carcinoma (KB-3-1) | [179] | ||
| Colon (Colo205, SW48, SW403, SW1116, SW1417, HT29, LS174T, LS180, HRT18) | [169,175] | ||
| Pheochromocytoma (PC-12) | [180,181] | ||
| Prostate adenocarcinoma (LNCaP) | [182] | ||
| Melanoma (M1733, F10, F1, BL6) | [175] | ||
| Hepatoma (Hep3B) | [183] | ||
| Epidermoid carcinoma (A431) | [169,175] | ||
| Breast adenocarcinoma (MCF7) | [184] | ||
| l-threo-dihydrosphingosine (DHS) | Acute myeloid leukemia (HL-60, P388, U937, NB4) Lymphoma (WEHI-231) | SphK1 and SphK2 | [171,172,185,186] |
| Breast adenocarcinoma (MCF7) | [187,188] | ||
| Hepatoma (Hep3B) | [183] | ||
| Neuroblastoma (SH-SY5Y) | |||
| Melanoma (A2058, 939, C8161) | [188] | ||
| FTY720 (fingolimod) | Prostate (PC-3, LNCaP-C4-2B, and DU145) | SphK1 | [189,190,191,192,193] |
| Ovarian cancer (OV2008, IGROV-1, A2780, SKOV-3, R182) | |||
| Bladder (T24, UMUC3 and HT1197) | |||
| Glioblastoma (U251MG and U87MG) | |||
| Hepatoma (HepG2, Huh-7 and Hep3B) | |||
| K145 | Leukemia (U937) | SphK2 | [194] |
| Drug | SphK Selectivity | Indications | ClinicalTrials.gov Identifier | Phase |
|---|---|---|---|---|
| Safingol | Sphingosine derivative, PKC inhibitor | Solid tumors, combined with fenretinide Solid tumors, combined with cisplatin | NCT01553071 NCT00084812 | I (Recruiting) I (Completed) |
| ABC294640 | SPHK2 inhibitor | Pancreatic cancer | NCT01488513 | I (Completed) |
| Diffuse Large B Cell- | NCT02229981 | I (Recruiting) | ||
| Lymphoma | ||||
| Kaposi Sarcoma | NCT02229981 | II (Recruiting) | ||
| Multiple Myeloma | NCT02757326 | Ib/II (Recruiting) | ||
| Carcinoma, Hepatocellular | NCT02939807 | II (Recruiting) | ||
| Sonepcizumab (ASONEP) | S1P-specific monoclonal antibody | Advanced Solid tumors Renal Cell Carcinoma | NCT00661414 NCT01762033 | I (Completed) II (Terminated) |
| Fingolimod | S1PR antagonist | Glioblastoma | NCT02490930 | I (Recruiting) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddadi, N.; Lin, Y.; Simpson, A.M.; Nassif, N.T.; McGowan, E.M. “Dicing and Splicing” Sphingosine Kinase and Relevance to Cancer. Int. J. Mol. Sci. 2017, 18, 1891. https://doi.org/10.3390/ijms18091891
Haddadi N, Lin Y, Simpson AM, Nassif NT, McGowan EM. “Dicing and Splicing” Sphingosine Kinase and Relevance to Cancer. International Journal of Molecular Sciences. 2017; 18(9):1891. https://doi.org/10.3390/ijms18091891
Chicago/Turabian StyleHaddadi, Nahal, Yiguang Lin, Ann M. Simpson, Najah T. Nassif, and Eileen M. McGowan. 2017. "“Dicing and Splicing” Sphingosine Kinase and Relevance to Cancer" International Journal of Molecular Sciences 18, no. 9: 1891. https://doi.org/10.3390/ijms18091891
APA StyleHaddadi, N., Lin, Y., Simpson, A. M., Nassif, N. T., & McGowan, E. M. (2017). “Dicing and Splicing” Sphingosine Kinase and Relevance to Cancer. International Journal of Molecular Sciences, 18(9), 1891. https://doi.org/10.3390/ijms18091891