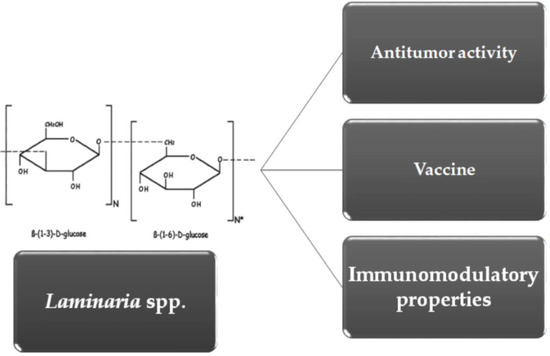
Overview of β-Glucans from Laminaria spp.: Immunomodulation Properties and Applications on Biologic Models
Abstract
:1. Introduction
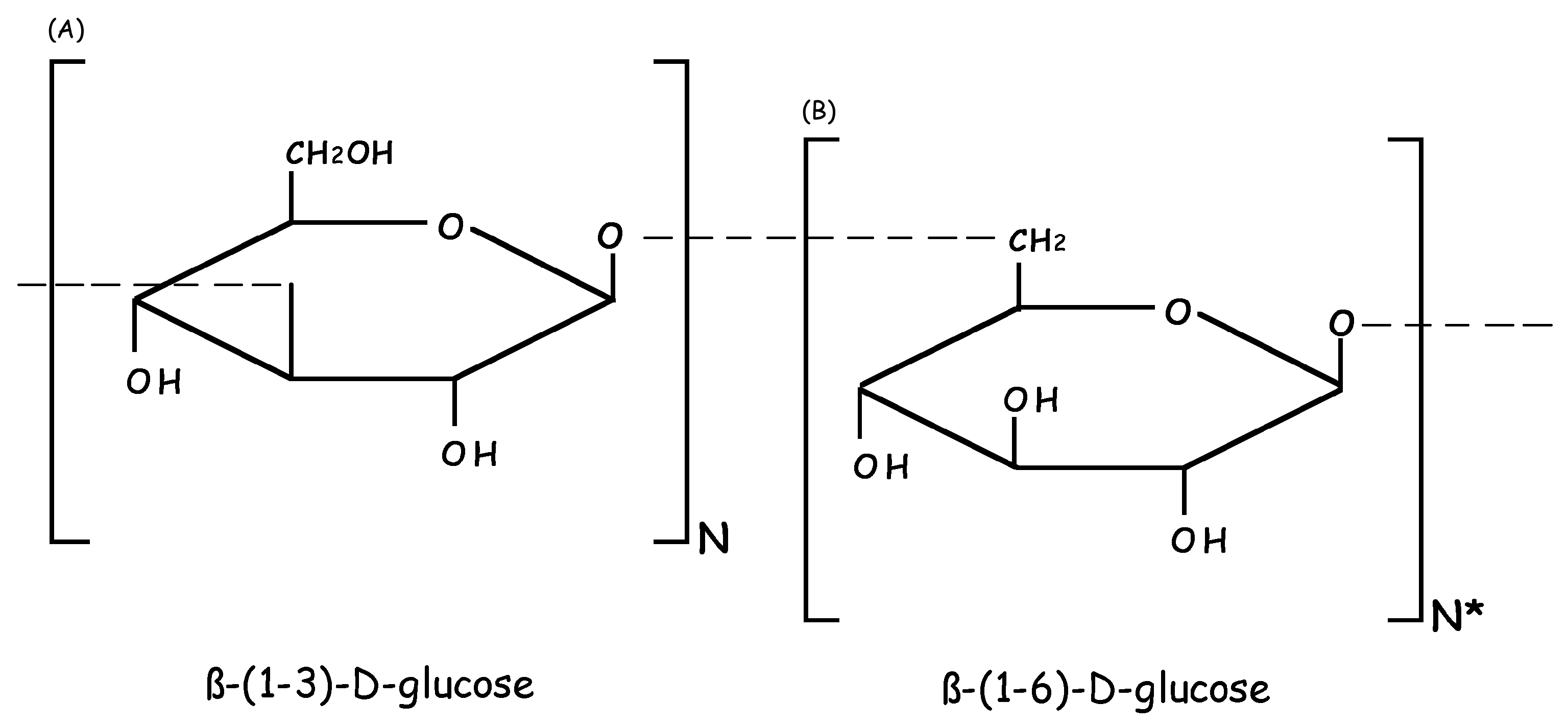
2. Molecular and Biochemical Characteristics of Laminarin (LAM)
3. Immunomodulatory Properties of LAM
3.1. Immunomodulation by LAM in Animal Cells
3.1.1. Macrophages
3.1.2. Human Neutrophils
3.1.3. Other Phagocytic Cells
3.2. Immunomodulation by LAM in Animal Nutrition
3.2.1. Fish Model
3.2.2. Rat Model
3.2.3. Pig Model
3.3. Immunomodulation by LAM in Microorganism-Host Interaction
4. Antitumor Activity of LAM
5. LAM as Vaccine
6. New Perspectives of the Application of LAM
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LAM | Laminarin or Laminaram |
| SPSs | Soluble polysaccharides |
| EE | Ethanolic extract |
| LPS | Lipopolysaccharide |
| H2O2 | Hydrogen peroxide |
| NO | Nitric oxide |
| MCP-1 | Monocyte chemotactic protein-1 |
| VEGF | Vascular endothelial growth factor |
| G-CSF | Granulocyte-colony stimulating factor |
| MIP-1α | Macrophage inflammatory proteins |
| COX-2 | Cyclooxygenase-2 |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| HClO | Hypochlorous acid |
| IgM | Immunoglobulin M |
| IGF-IR | Insulin-like growth factor |
| MAPK | Protein kinases |
| drLN | Draining lymph nodes |
| DCs | Dendritic cells |
| Ag | Antigen |
| Ab | Antibodies |
| OVA | Ovalbumin |
| Th1 | T helper 1 |
References
- Read, S.M.; Currie, G.; Bacic, A. Analysis of the structural heterogeneity of laminarin by electrospray-ionisation-mass spectrometry. Carbohydr. Res. 1996, 281, 187–201. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Immune recognition of fungal β-glucans. Cell Microbial. 2005, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Passarelli, V.; Evangelista, V.; Frassanito, A.M.; Gualtieri, P. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat. Prod. Rep. 2011, 28, 457–466. [Google Scholar] [CrossRef] [PubMed]
- De Souza Bonfim-Mendonça, P.; Ratti, B.A.; Godoy, J.D.S.R.; Negri, M.; de Lima, N.C.A.; Fiorini, A.; Hatanaka, E.; Consolaro, M.E.L.; de Oliveira Silva, S.; Svidzinski, T.I.E. β-Glucan induces reactive oxygen species production in human neutrophils to improve the killingof Candida albicans and Candida glabrata isolates from vulvovaginal candidiasis. PLoS ONE 2014, 9, e107805. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Li, M.; Jin, M. Influence of Laminarin polysaccahrides on oxidative damage. Int. J. Biol. Macromol. 2011, 48, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Pagano, M.; Dottore, A.; Genovese, G.; Morabito, M. Evaluation of anticoagulant activity of two algal polysaccharides. Nat. Prod. Res. 2016, 30, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Guselle, N.J.; Markham, R.J.F.; Speare, D.J. Intraperitoneal administration of β-1,3/1,6-glucan to rainbow trout, Oncorhynchus mykiss (Walbaum), protects against Loma salmonae. J. Fish. Dis. 2006, 29, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Roudi, R.; Mohammadi, S.R.; Roudbary, M.; Mohsenzadegan, M. Lung cancer and β-glucans: Review of potential therapeutic applications. Investig. New Drugs 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Akramiene, D.; Kondrotas, A.; Didziapetriene, J.; Kevelaitis, E. Effects of β-glucans on the immune system. Medicina 2007, 43, 597–606. [Google Scholar] [PubMed]
- Fuentes, A.-L.; Millis, L.; Sigola, L.B. Laminarin, a soluble β-glucan, inhibits macrophage phagocytosis of zymosan but has no effect on lipopolysaccharide mediated augmentation of phagocytosis. Int. Immunopharmacol. 2011, 11, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, T.; Fitieh, A.; Pierre, J.S.; Ostergaard, H.L.; Bundle, D.R.; Touret, N. Enhanced immunogenicity of a tricomponent mannan tetanus toxoid conjugate vaccine targeted to dendritic cells via Dectin-1 by incorporating β-glucan. J. Immunol. 2013, 190, 4116–4128. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Sonck, E.; Stuyven, E.; Goddeeris, B.; Cox, E. The effect of β-glucans on porcine leukocytes. Vet. Immunol. Immunopathol. 2010, 135, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; O’Doherty, J.V.; Reilly, P.; Ryan, M.T.; Bahar, B.; Sweeney, T. The effects of laminarin derived from Laminaria digitata on measurements of gut health: Selected bacterial populations, intestinal fermentation, mucin gene expression and cytokine gene expression in the pig. Br. J. Nutr. 2011, 105, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.; Collins, C.B.; Reilly, P.; Pierce, K.M.; Ryan, M.; O’Doherty, J.V. Effect of purified β-glucans derived from Laminaria digitata, Laminaria hyperborea and Saccharomyces cerevisiae on piglet performance, selected bacterial populations, volatile fatty acids and pro-inflammatory cytokines in the gastrointestinal tract of pigs. Br. J. Nutr. 2012, 108, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.J.; Wouters, I.M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kim, I.H.; Kim, J.; Nam, T.J. Induction of apoptosis and the regulation of ErbB signaling by laminarin in HT-29 human colon cancer cells. Int. J. Mol. Med. 2013, 32, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, M.; Fang, Z.; Zhang, Q. In vitro antioxidant effects and cytotoxicity of polysaccharides extracted from Laminaria japonica. Int. J. Biol. Macromol. 2012, 50, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Bromuro, C.; Romano, M.; Chiani, P.; Berti, F.; Tontini, M.; Proietti, D.; Mori, E.; Torosantucci, A.; Costantino, P.; Rappuoli, R. β-Glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine 2010, 28, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Torosantucci, A.; Bromuro, C.; Chiani, P.; De Bernardis, F.; Berti, F.; Galli, C.; Norelli, F.; Bellucci, C.; Polonelli, L.; Costantino, P. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005, 202, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Torosantucci, A.; Chiani, P.; Bromuro, C.; De Bernardis, F.; Palma, A.S.; Liu, Y.; Mignogna, G.; Maras, B.; Colone, M.; Stringaro, A. Protection by anti-β-glucan antibodies is associated with restricted β-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE 2009, 4, e5392. [Google Scholar] [CrossRef] [PubMed]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Reason, A.J.; Haslam, S.M.; McDowell, R.A.; Chizhov, O.S.; Usov, A.I. Structural analysis of laminarans by MALDI and FAB mass spectrometry. Carbohydr. Res. 1998, 310, 203–210. [Google Scholar] [CrossRef]
- Gahan, D.A.; Lynch, M.B.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. Performance of weanling piglets offered low-, medium-or high-lactose diets supplemented with a seaweed extract from Laminaria spp. Animal 2009, 3, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Reilly, P.; O’doherty, J.V.; Pierce, K.M.; Callan, J.J.; O’sullivan, J.T.; Sweeney, T. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal 2008, 2, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effects of supplementing dietary laminarin and fucoidan on intestinal morphology and the immune gene expression in the weaned pig. J. Anim. Sci. 2012, 90, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.M.; Sweeney, T.; O'Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of dietary laminarin and fucoidan on selected microbiota, intestinal morphology and immune status of the newly weaned pig. Br. J. Nutr. 2013, 110, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Pagano, M.; Morabito, M.; Armeli Minicante, S.; Arfuso, F.; Genovese, G. In vitro assessment of the effect of Undaria pinnatifida extracts on erythrocytes membrane integrity and blood coagulation parameters of Equus caballus. J. Coastal Life Med. 2014, 2, 614–616. [Google Scholar] [CrossRef]
- Faggio, C.; Morabito, M.; Minicante, S.A.; Lo Piano, G.; Pagano, M.; Genovese, G. Potential use of polysaccharides from the brown alga Undaria pinnatifida as anticoagulants. Braz. Arch. Biol. Technol. 2015, 58, 798–804. [Google Scholar] [CrossRef]
- Bold, H.C.; Wynne, M.J. Introduction to the Algae; Prentice-Hall: Englewood Cliffs, NJ, USA, 1978. [Google Scholar]
- Schaal, G.; Leclerc, J.C.; Droual, G.; Leroux, C.; Riera, P. Biodiversity and trophic structure of invertebrate assemblages associated with understorey red algae in a Laminaria digitata bed. Mar. Biol. Res. 2016, 12, 513–523. [Google Scholar] [CrossRef]
- Schultze, K.; Janke, K.; Krüß, A.; Weidemann, W. The macrofauna and macroflora associated with Laminaria digitata and L. hyperborea at the island of Helgoland (German Bight, North Sea). Helgol. Meeresunters. 1990, 44, 39–51. [Google Scholar] [CrossRef]
- Nitschke, U.; Dixneuf, S.; Ruth, A.A.; Schmid, M.; Stengel, D.B. Molecular iodine (I2) emission from two Laminaria species (Phaeophyceae) and impact of irradiance and temperature on I2 emission into air and iodide release into seawater from Laminaria digitata. Mar. Environ. Res. 2013, 92, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Kraan, S. Review of the Potential Mechanisation of Kelp Harvesting in Ireland; National University of Ireland: Galway, Ireland, 2004. [Google Scholar]
- Peng, Z.; Liu, M.; Fang, Z.; Wu, J.; Zhang, Q. Composition and cytotoxicity of a novel polysaccharide from brown alga (Laminaria japonica). Carbohydr. Polym. 2012, 89, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kuda, T.; Nakamura, S.; An, C.; Takahashi, H.; Kimura, B.; Nishizawa, M. Effects of holdfast of Laminaria japonica on Listeria invasion on enterocyte-like Caco-2 cells and NO production of macrophage RAW 264.7 cells. Appl. Biochem. Biotechnol. 2012, 168, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Park, W. Immunostimulatory effect of laminarin on RAW 264.7 mouse macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef] [PubMed]
- Wakshull, E.; Brunke-Reese, D.; Lindermuth, J.; Fisette, L.; Nathans, R.S. PGG-glucan, a soluble β-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-κB-like factor in human PMN: Evidence for a glycosphingolipid β-(1,3)-glucan receptor. Immunopharmacology 1999, 41, 89–107. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish. Shellfish Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, J.O.; Kim, J.H.; Jung, J.H.; You, G.Y.; Park, S.H.; Kim, H.S. C-peptide stimulates nitrites generation via the calcium-JAK2/STAT1 pathway in murine macrophage RAW264. 7 cells. Life Sci. 2010, 86, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Webel, D.M.; Finck, B.N.; Baker, D.H.; Johnson, R.W. Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide. J. Anim. Sci. 1997, 75, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 1997, 75, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Davies, M.J.; Hawkins, C.L. Reactions and reactivity of myeloperoxidase-derived oxidants: Differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 2012, 46, 975–995. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Ji, I.H.; Chang, H.I.; Kim, C.W. High-level TNF-α secretion and macrophage activity with soluble β-glucans from Saccharomyces cerevisiae. Biosci. Biotechnol. Biochem. 2002, 66, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal β-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Mueller, A.; Raptis, J.; Rice, P.J.; Kalbfleisch, J.H.; Stout, R.D.; Ensley, H.E.; Browder, W.; Williams, D.L. The influence of glucan polymer structure and solution conformation on binding to (1-3)-β-d-glucan receptors in a human monocyte-like cell line. Glycobiology 2000, 10, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.L.; Rice, P.J.; Graves, B.; Ensley, H.E.; Yu, H.; Brown, G.D.; Gordon, S.; Monteiro, M.A.; Papp-Szabo, E.; Lowman, D.W.; et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J. Pharmacol. Exp. Ther. 2008, 325, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Kew, S. The immune system: A target for functional foods? Br. J. Nutr. 2002, 88, S165–S176. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Fazio, F.; Marafioti, S.; Arfuso, F.; Piccione, G. Oral administration of Gum Arabic: Effects on haematological parameters and oxidative stress markers in Mugil cephalus. Iran. J. Fish. Sci. 2015, 14, 60–72. [Google Scholar]
- Carbone, D.; Faggio, C. Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish. Shellfish Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M.A. Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish. Shellfish Immunol. 2016, 52, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Lockhart, B.E.; Barker, L.A.; Adams, E.L.; Ensley, H.E.; Williams, D.L. Pharmacokinetics of fungal (1–3)-β-d-glucans following intravenous administration in rats. Int. Immunopharmacol. 2004, 4, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Rice, P.J.; Adams, E.L.; Ozment-Skelton, T.; Gonzalez, A.J.; Goldman, M.P.; Lockhart, B.E. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 2005, 314, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Li, W.; Lin, Q.; Lin, X.; Lin, J.; Zhu, Q.; Jiang, H.; Huang, Z. Dietary administration of laminarin improves the growth performance and immune responses in Epinephelus coioides. Fish. Shellfish Immunol. 2014, 41, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Villanueva, L.T.; Tovar-Ramírez, D.; Gisbert, E.; Cordero, H.; Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Ascencio-Valle, F.; Esteban, M.A. Dietary administration of β-1,3/1,6-glucan and probiotic strain Shewanella putrefaciens, single or combined, on gilthead seabream growth, immune responses and gene expression. Fish. Shellfish Immunol. 2014, 39, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Aceves, M.; Cohen, A.; Smith, Y.; Faggio, C. Estrogen regulation of gene expression in the teleost fish immune system. Fish. Shellfish Immunol. 2016, 58, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Gao, Y.H.; Wang, R.X.; Sun, Y.N.; Xu, T.J. Characterization, genomic organization, and expression profiles of MyD88, a key adaptor molecule in the TLR signaling pathways in miiuy croaker (Miichthys miiuy). Fish. Physiol. Biochem. 2012, 38, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Munoz, V.; Gallardo-Escarate, C. TLR and IMD signaling pathwaysfrom Caligus rogercresseyi (Crustacea: Copepoda): In silico gene expressionand SNPs discovery. Fish. Shellfish Immunol. 2014, 36, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Amar, E.C.; Kiron, V.; Satoh, S.; Watanabe, T. Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish. Shellfish Immunol. 2004, 16, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Gatlin Iii, D.M.; Li, P. Dietary supplementation of prebiotic for health management of hybrid striped bass Morone chrysops × M. saxatilis. Aqua Feeds Formul. Beyond 2004, 1, 19–21. [Google Scholar] [CrossRef]
- Herre, J.; Gordon, S.; Brown, G.D. Dectin-1 and its role in the recognition of β-glucans by macrophages. Mol. Immunol. 2004, 40, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Mouson, A.; Delzenne, N.M. Dietary supplementation with laminarin, a fermentable marine β (1–3) glucan, protects against hepatotoxicity induced by LPS in rat by modulating immune response in the hepatic tissue. Int. Immunopharmacol. 2007, 7, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Salvemini, D.; Cuzzocrea, S. Oxidative stress in septic shock and disseminated intravascular coagulation. Free Radic. Biol. Med. 2002, 33, 1173–1185. [Google Scholar] [CrossRef]
- Barton, B.E. The biological effects of interleukin 6. Med. Res. Rev. 1996, 16, 87–109. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [PubMed]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [PubMed]
- Trinchieri, G.; Sher, A. Cooperation of Toll-like receptor signals in innate immune defence. Nat. Rev. Immunol. 2007, 7, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. β-glucan recognition by the innate immune system. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Tsoni, S.V.; Brown, G.D. β-Glucans and Dectin-1. Ann. N. Y. Acad. Sci. 2008, 1143, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.T.; O’Shea, C.J.; Collins, C.B.; O’Doherty, J.V.; Sweeney, T. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon. J. Anim. Sci. 2012, 90, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Korn, T.; Oukka, M.; Kuchroo, V.K. Induction and effector functions of Th17 cells. Nature 2008, 453, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effect of maternal fish oil and seaweed extract supplementation on colostum and milk composition, humoral immune response, and performance of suckled piglets. J. Anim. Sci. 2010, 88, 2988–2997. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; O’Doherty, J.V. Effect of dietary seaweed extracts and fish oil supplementation in sows on performance, intestinal microflora, intestinal morphology, volatile fatty acid concentrations and immune status of weaned pigs. Br. J. Nutr. 2011, 105, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Lynch-Devaney, K.; Kindon, H. Trefoil peptides promote epithelial migration through a transforming growth factor β-independant pathway. J. Clin. Investig. 1994, 94, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; O’Doherty, J.V. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J. Anim. Sci. 2012, 90, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Strangfeld, A.; Listing, L. Bacterial and opportunistic infections during anti-TNF therapy. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Nathan, C.F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 1989, 169, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Bouwhuis, M.A.; Sweeney, T.; Mukhopadhya, A.; Thornton, K.; McAlpine, P.O.; O'Doherty, J.V. Zinc methionine and laminarin have growth-enhancing properties in newly weaned pigs influencing both intestinal health and diarrhoea occurrence. J. Anim. Physiol. Anim. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bouwhuis, M.A.; McDonnell, M.J.; Sweeney, T.; Mukhopadhya, A.; O’Shea, C.J.; O’Doherty, J.V. Seaweed extracts and galacto-oligosaccharides improve intestinal health in pigs following Salmonella Typhimurium challenge. Animal 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ratti, B.A.; Godoy, J.S.; Bonfim Mendonça, P.S.; Bidóia, D.L.; Nakamura, T.U.; Nakamura, C.V.; Consolaro, M.E.L.; Svidzinski, T.I.E.; Silva, S.O. Microbicidal activity of neutrophils is inhibited by isolates from recurrent vaginal candidiasis (RVVC) caused by Candida albicans through fungal thioredoxin reductase. Cell. Immunol. 2015, 293, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Kim, I.H.; Kim, J.; Nam, T.J. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells. Inter. J. Mol. Med. 2012, 30, 734–738. [Google Scholar] [CrossRef]
- Dërmaku-Sopjani, M.; Abazi, S.; Faggio, C.; Kolgeci, J.; Sopjani, M. AMPK-sensitive cellular transport. J. Biochem. Mar. 2014, 155, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Xu, L.; Zhang, W.; Cai, Y.; Jang, B.; Oh, J.; Jin, J.O. Laminarin promotes anti-cancer immunity by the maturation of dendritic cells. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Li, X.; Yang, Y.; Yu, L.; Yao, Y. Antitumor activity of a polysaccharide fraction from Laminaria japonica on U14 cervical carcinoma-bearing mice. Tumor Biol. 2014, 35, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, W.S.; Lu, J.N.; Kim, G.; Jung, J.M.; Ryu, C.H.; Kim, G.Y.; Hwang, H.J.; Kwon, T.K.; Choi, Y.H. Citrus aurantium L. exhibits apoptotic effects on U937 human leukemia cells partly through inhibition of Akt. Int. J. Oncol. 2012, 40, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Liu, M.; Fang, Z.; Chen, L.; Wu, J.; Zhang, Q. In vitro antiproliferative effect of a water-soluble Laminaria japonica polysaccharide on human melanoma cell line A375. Food Chem. Toxicol. 2013, 58, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, A. Regulation of apoptosis by Bcl-2 family proteins. J. Cell. Mol. Med. 2003, 7, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, Y.W.; Kim, H.B.; Lee, B.J.; Lee, D.S. Anti-apoptotic activity of laminarin polysaccharides and their enzymatically hydrolyzed oligosaccharides from Laminaria japonica. Biotechnol. Lett. 2006, 28, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Tontini, M.; Romano, M.R.; Proietti, D.; Balducci, E.; Micoli, F.; Balocchi, C.; Santini, L.; Masignani, V.; Berti, F.; Costantino, P. Preclinical studies on new proteins as carrier for glycoconjugate vaccines. Vaccine 2016, 34, 4235–4242. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.H.; Jin, H.J.; Lee, S.Y. Antimicrobial activity of ethanol extracts of Laminaria japonica against oral microorganisms. Anaerobe 2013, 21, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, L.M.; Albina, J.E.; Reichner, J.S. β-Glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J. Immunol. 2006, 177, 8667–8675. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, J. Surface glycans of Candida albicans and other pathogenic fungi: Physiological roles, clinical uses, and experimental challenges. Clin. Microbiol. Rev. 2004, 17, 281–310. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Long, K.; Dong, H.L.; Gao, X.M. Adjuvanticity of a recombinant calreticulin fragment in assisting anti-β-glucan IgG responses in T cell-deficient mice. Clin. Vaccine Immunol. 2013, 20, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Cassone, A.; Torosantucci, A. Opportunistic fungi and fungal infections: The challenge of a single, general antifungal vaccine. Expert Rev. Vaccines 2006, 5, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, S.G.; Klein, B.S. Vaccine immunity against fungal infections. Curr. Opin. Immunol. 2014, 28, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Miró, M.S.; Rodríguez, E.; Vigezzi, C.; Icely, P.A.; Gonzaga de Freitas Araújo, M.; Riera, F.O.; Vargas, L.; Abiega, C.; Caeiro, J.P.; Sotomayor, C.E. Vulvovaginal candidiasis: An old disease with new challenges. Rev. Iberoam. Micol. 2017, 34, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Akimoto-Gunther, L.; Bonfim-Mendonça, P.S.; Takahashi, G.; Irie, M.M.; Miyamoto, S.; Consolaro, M.E.; Svidzinski, T.I.E. Highlights Regarding Host Predisposing Factors to Recurrent Vulvovaginal Candidiasis. PLoS ONE 2016, 11, e0158870. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Edwards, L. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004, 351, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, Z.F.; Silva, D.D.; Lazera, M.; Petri, V.; Oliveira, R.M.; Sabroza, P.C.; Wanke, B. Paracoccidioidomycosis mortality in Brazil (1980–1995). Cad. Saude. Publica 2002, 18, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Benard, G. An overview of the immunopathology of human paracoccidioidomycosis. Mycopathologia 2008, 165, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; de Gregori, W.; Velez, D.; Restrepo, A.; Cano, L.E. Nitric Oxide Participation in the Fungicidal Mechanism of γ Interferon-Activated Murine Macrophages against Paracoccidioides brasiliensis Conidia. Infect. Immun. 2000, 68, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, H.C.; Assato, P.A.; Marcos, C.M.; Scorzoni, L.; de Paula E Silva, A.C.; Da Silva Jde, F.; Singulani Jde, L.; Alarcon, K.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. Paracoccidioides-host Interaction: An Overview on Recent Advances in the Paracoccidioidomycosis. Front. Microbiol. 2015, 25, 1319. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Silva Jde, F.; Silva, J.L.; Andreotti, P.F.; Soares, C.P.; Benard, G.; Giannini, M.J. Induction of apoptosis in A549 pulmonary cells by two Paracoccidioides brasiliensis samples. Mem. Inst. Oswaldo Cruz 2009, 104, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.F.D.; Vicentim, J.; Oliveira, H.C.; Marcos, C.M.; Assato, P.A.; Andreotti, P.F.; Silva, J.L.; Soares, C.P.; Benard, G.; Almeida, A.M.; et al. Influence of the Paracoccidioides brasiliensis 14-3-3 and gp43 proteins on the induction of apoptosis in A549 epithelial cells. Mem. Inst. Oswaldo Cruz 2015, 110, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Verícimo, M.A.; França, K.M.; Arnholdt, A.C.; Kipnis, T.L. Increased apoptosis during the early phase of experimental paracoccidioidomycosis as a phenotypic marker of resistance. Microbes Infect. 2006, 8, 2811–2820. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hernandez, O. New insights into a complex fungal pathogen: The case of Paracoccidioides spp. Yeast 2016, 33, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Underhill, D.M. β-Glucan signaling connects phagocytosis to autophagy. Glycobiology 2013, 23, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
| Source | Product | Immunomodulation | Type of Study | Reference |
|---|---|---|---|---|
| Laminaria digitata | Laminarin |
| In vitro (murine RAW 264.7 macrophages) | [38] |
| Laminaria japonica | Kombu and ganiashi |
| In vitro (Caco-2 cells and RAW 264.7 macrophages) | [37] |
| Laminarina digitata | Laminarin |
| In vitro (human neutrophils and total leukocytes) | [5] |
| In vitro (murine RAW 264.7 macrophages) | [11] | ||
| In vitro (monocytes, neutrophils, and lymphocytes in pigs) | [14] | ||
| In vitro (whole blood cultures) | [17] | ||
| - | Laminarin |
| In vivo (fish, Epinephelus coioides) | [56] |
| Laminarina digitata | Laminarin |
| In vivo (fish, Sparus aurata) | [57] |
| Brown Algae | Laminarin |
| In vivo (Wistar rats) | [64] |
| - | Laminarin polysaccharides |
| In vivo (Sprague-Dawley rats) | [6] |
| Laminaria digitata | Laminarin |
| In vivo (pig) | [15] |
| Laminaria digitata and L. hyperborea | Laminarin |
| In vivo (pig) | [16] |
| In vivo(pig) | [72] | ||
| In vivo (pig) | [25] | ||
| Laminaria spp. | SWE (Laminarin + Fucoidan) |
| In vivo (pig) | [74] |
| In vivo (pig) | [76] | ||
| In vivo (pig) | [78] | ||
| Laminarin + Fucoidan |
| In vivo (pigs) | [26,27] | |
| Laminarin |
| In vivo (pigs) | [82] | |
| Laminarin + Fucoidan |
| In vivo (pigs) | [83] |
| Source | Extract | Applicability | Type of Study | Reference |
|---|---|---|---|---|
| Laminaria digitata | Laminarin |
|
| [18] |
| Laminaria japonica | Sulfated polysaccharide fraction (LJSP) |
|
| [88] |
| Novel polysaccharide WPS-2-1 |
|
| [19] | |
| Novel polysaccharide WPS-2-1 |
|
| [90] | |
| Laminarin polysaccharides (LP1) |
|
| [92] |
| Source | Extract | Applicability | Type of study | Reference |
|---|---|---|---|---|
| Laminaria digitata | Laminarin | Vaccine against C. albicans and A. fumigatus |
| [21,22] |
| Vaccine against C. albicans |
| [20] | ||
| Vaccine against C. albicans |
| [97] | ||
|
| [12] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfim-Mendonça, P.D.S.; Capoci, I.R.G.; Tobaldini-Valerio, F.K.; Negri, M.; Svidzinski, T.I.E. Overview of β-Glucans from Laminaria spp.: Immunomodulation Properties and Applications on Biologic Models. Int. J. Mol. Sci. 2017, 18, 1629. https://doi.org/10.3390/ijms18091629
Bonfim-Mendonça PDS, Capoci IRG, Tobaldini-Valerio FK, Negri M, Svidzinski TIE. Overview of β-Glucans from Laminaria spp.: Immunomodulation Properties and Applications on Biologic Models. International Journal of Molecular Sciences. 2017; 18(9):1629. https://doi.org/10.3390/ijms18091629
Chicago/Turabian StyleBonfim-Mendonça, Patrícia De Souza, Isis Regina Grenier Capoci, Flávia Kelly Tobaldini-Valerio, Melyssa Negri, and Terezinha Inez Estivalet Svidzinski. 2017. "Overview of β-Glucans from Laminaria spp.: Immunomodulation Properties and Applications on Biologic Models" International Journal of Molecular Sciences 18, no. 9: 1629. https://doi.org/10.3390/ijms18091629
APA StyleBonfim-Mendonça, P. D. S., Capoci, I. R. G., Tobaldini-Valerio, F. K., Negri, M., & Svidzinski, T. I. E. (2017). Overview of β-Glucans from Laminaria spp.: Immunomodulation Properties and Applications on Biologic Models. International Journal of Molecular Sciences, 18(9), 1629. https://doi.org/10.3390/ijms18091629