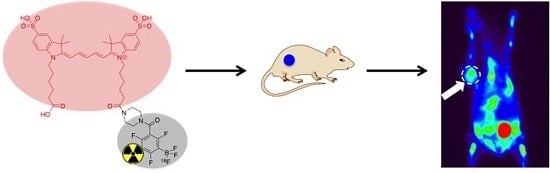
A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging
Abstract
:1. Introduction
2. Results and Discussions
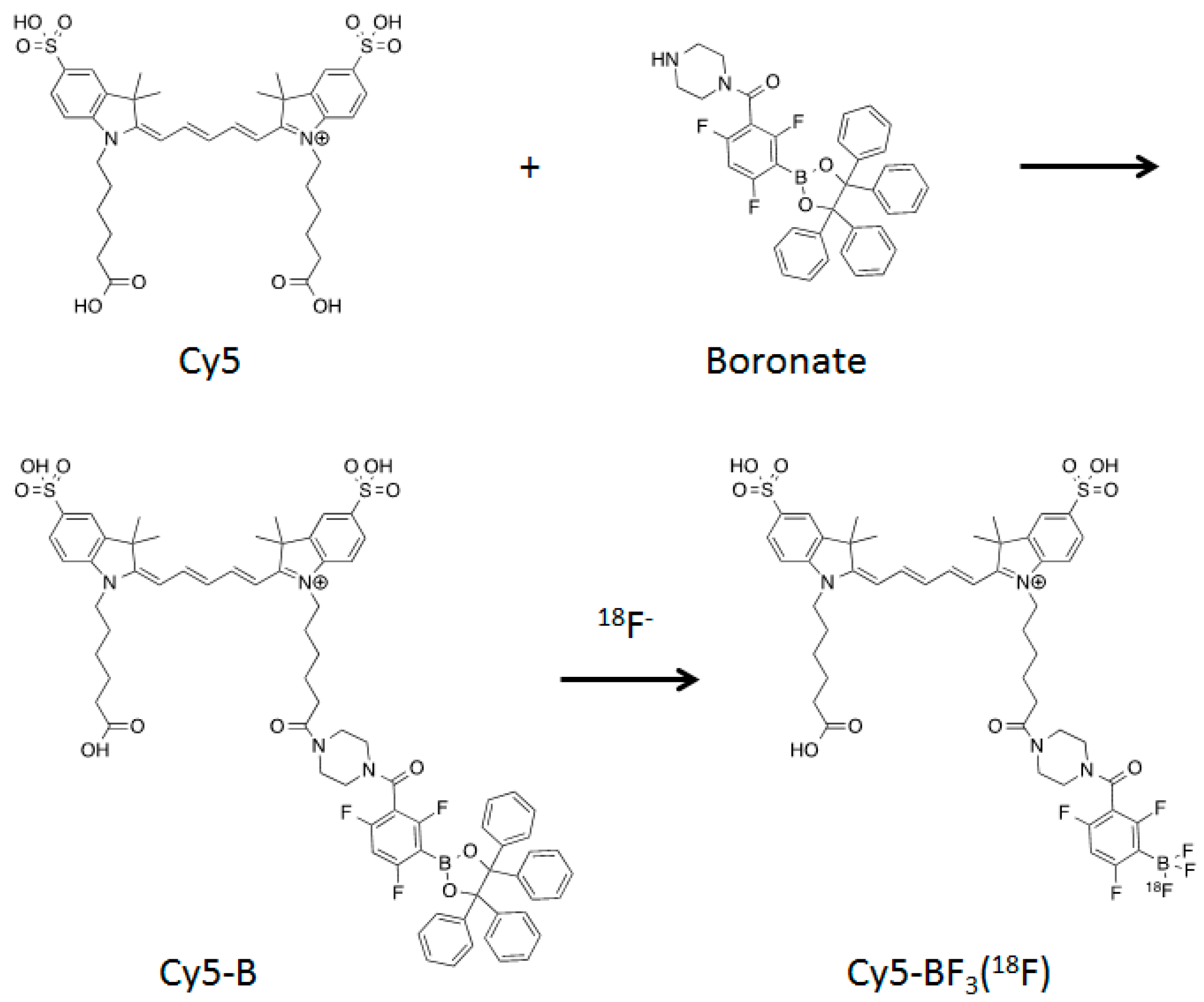
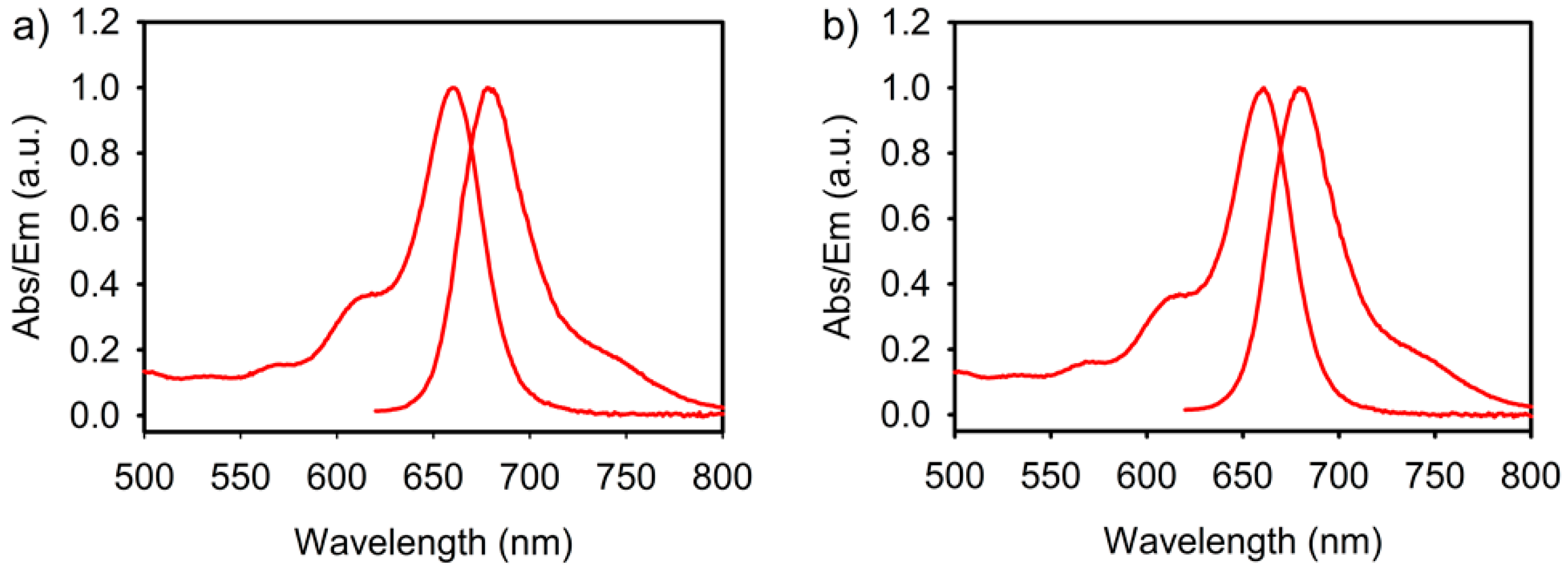
2.1. Synthesis and Fluoride Reaction
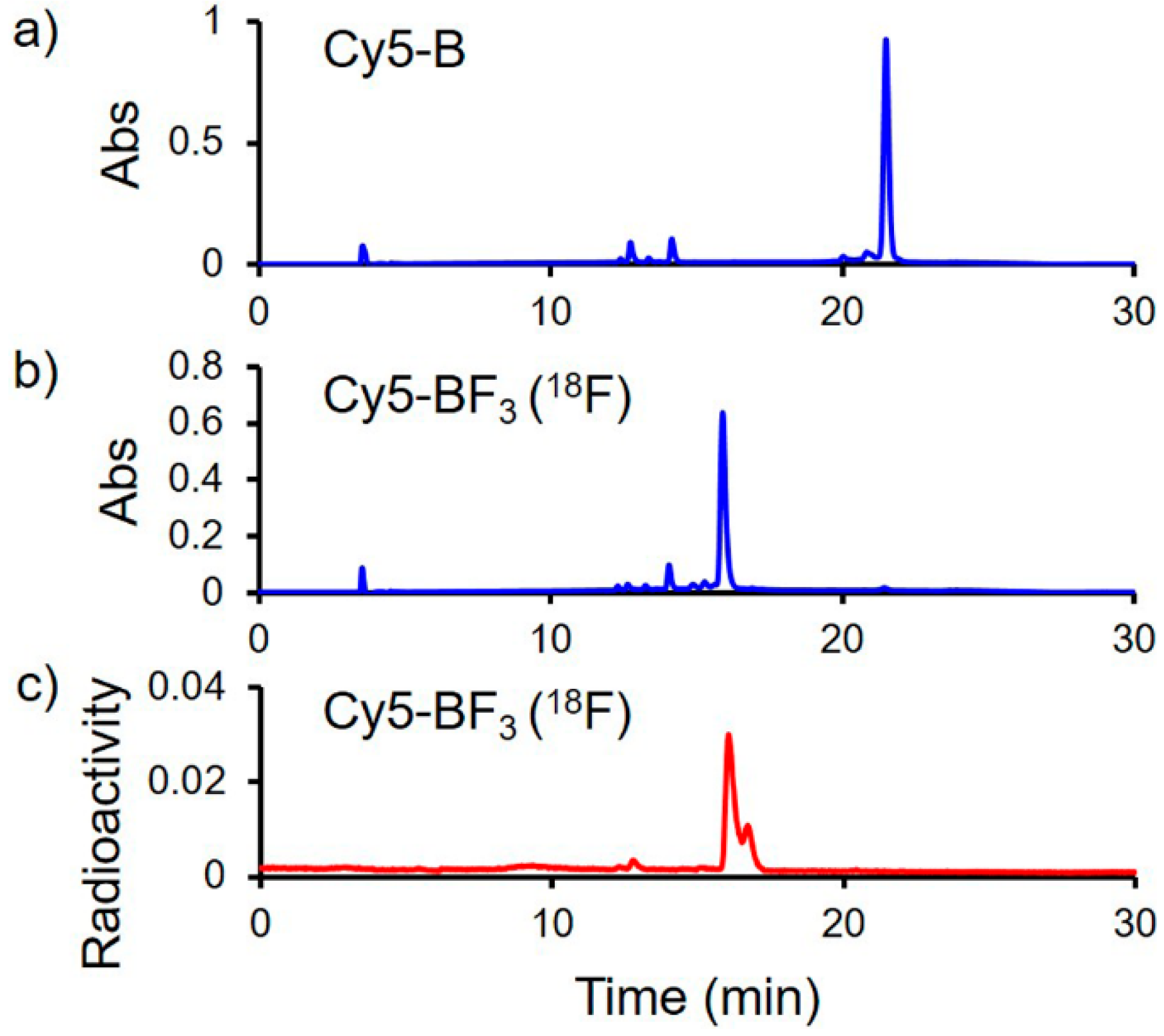
2.2. Radiolabeling
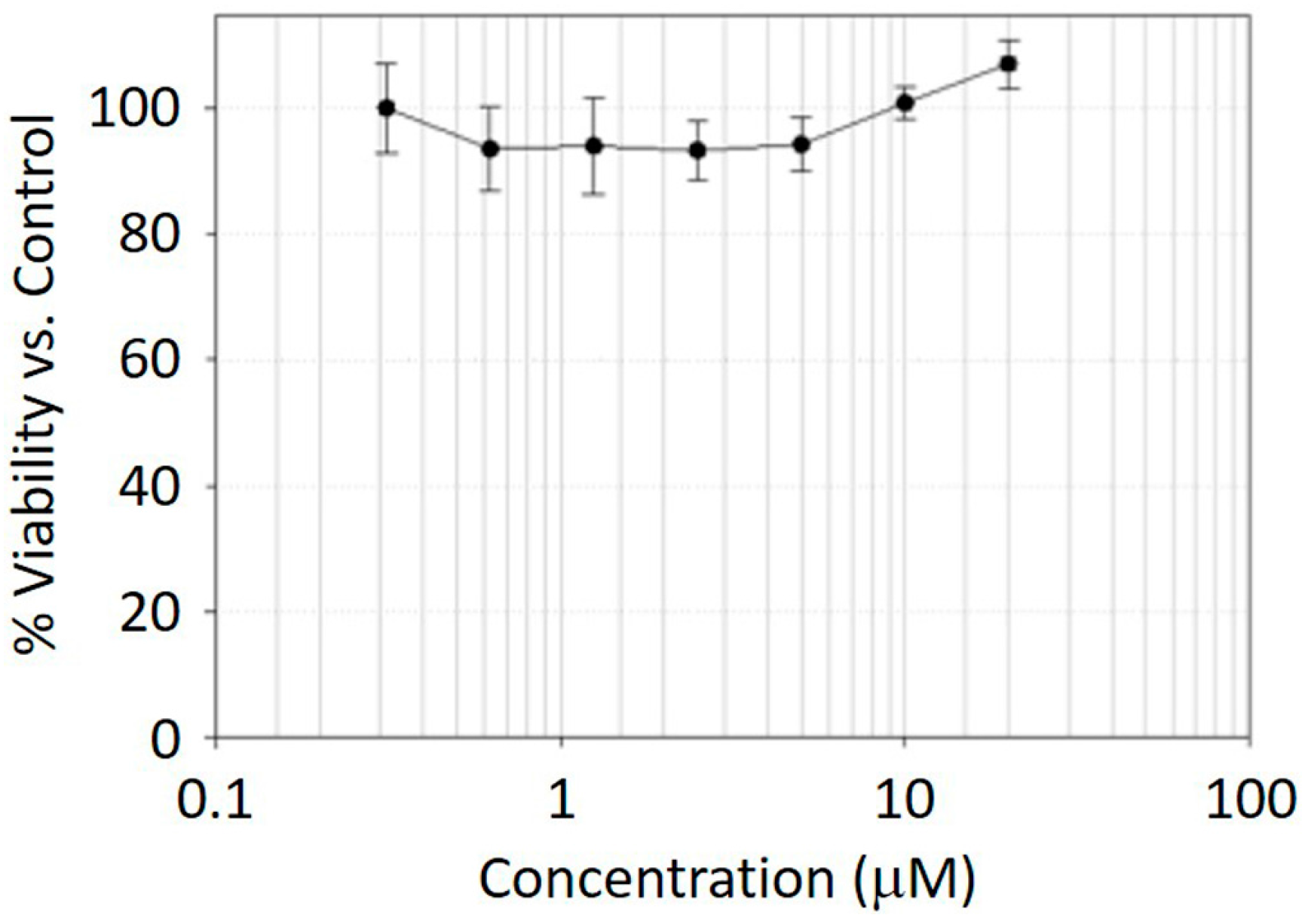
2.3. MTT Assay
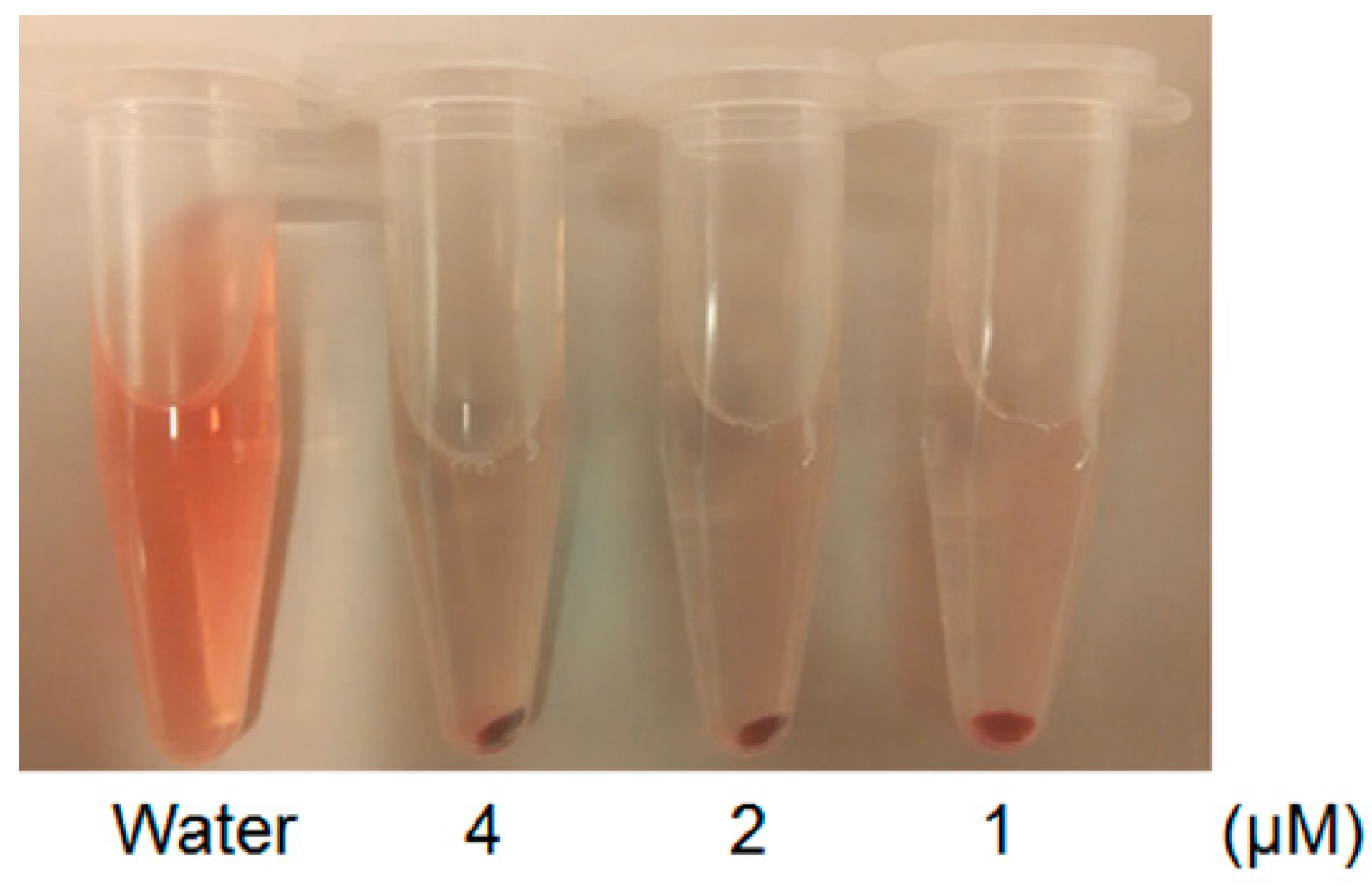
2.4. Hemolysis Assay
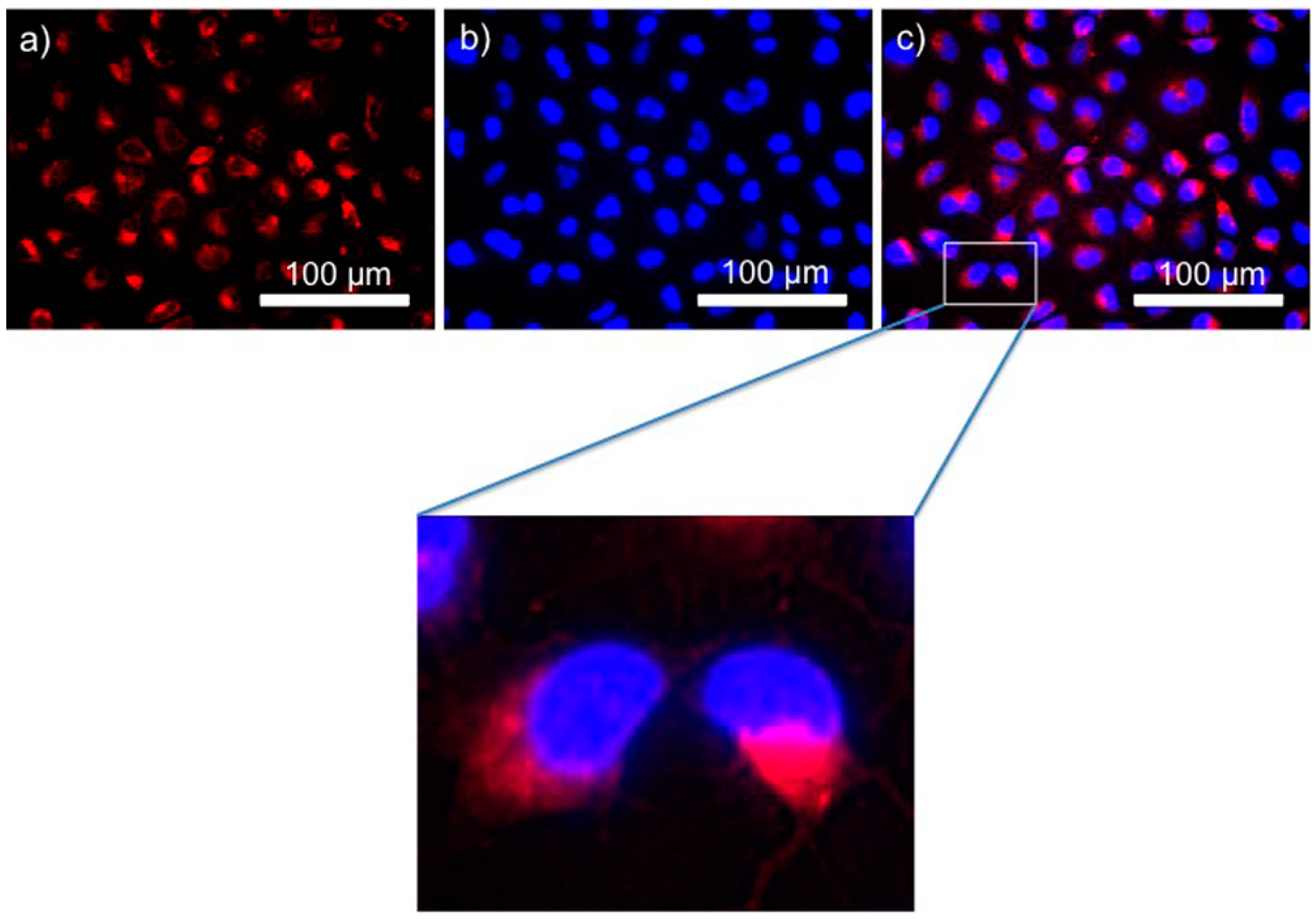
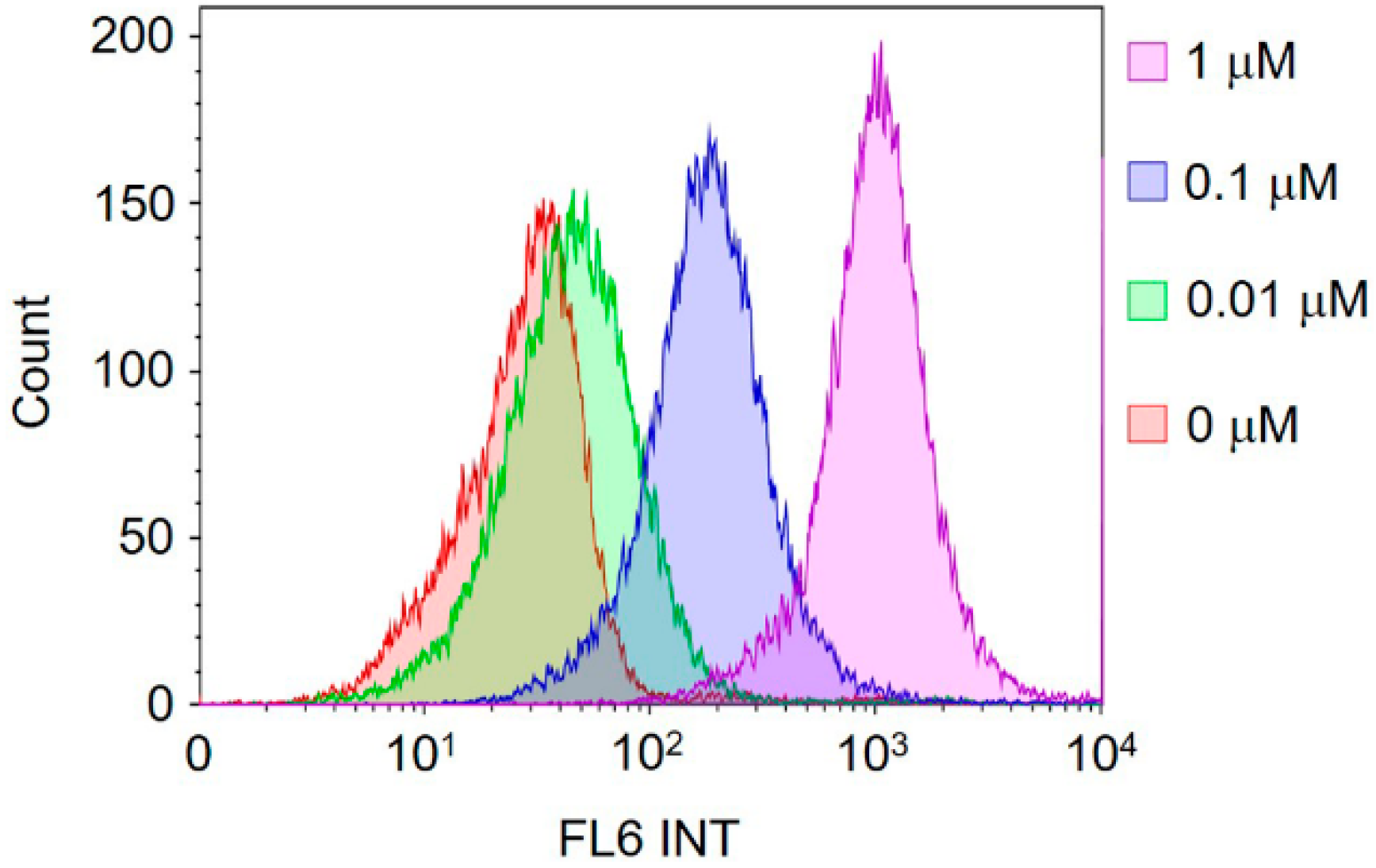
2.5. In Vitro Cell Imaging and Fluorescence Activated Cell Sorting (FACS) Quantification
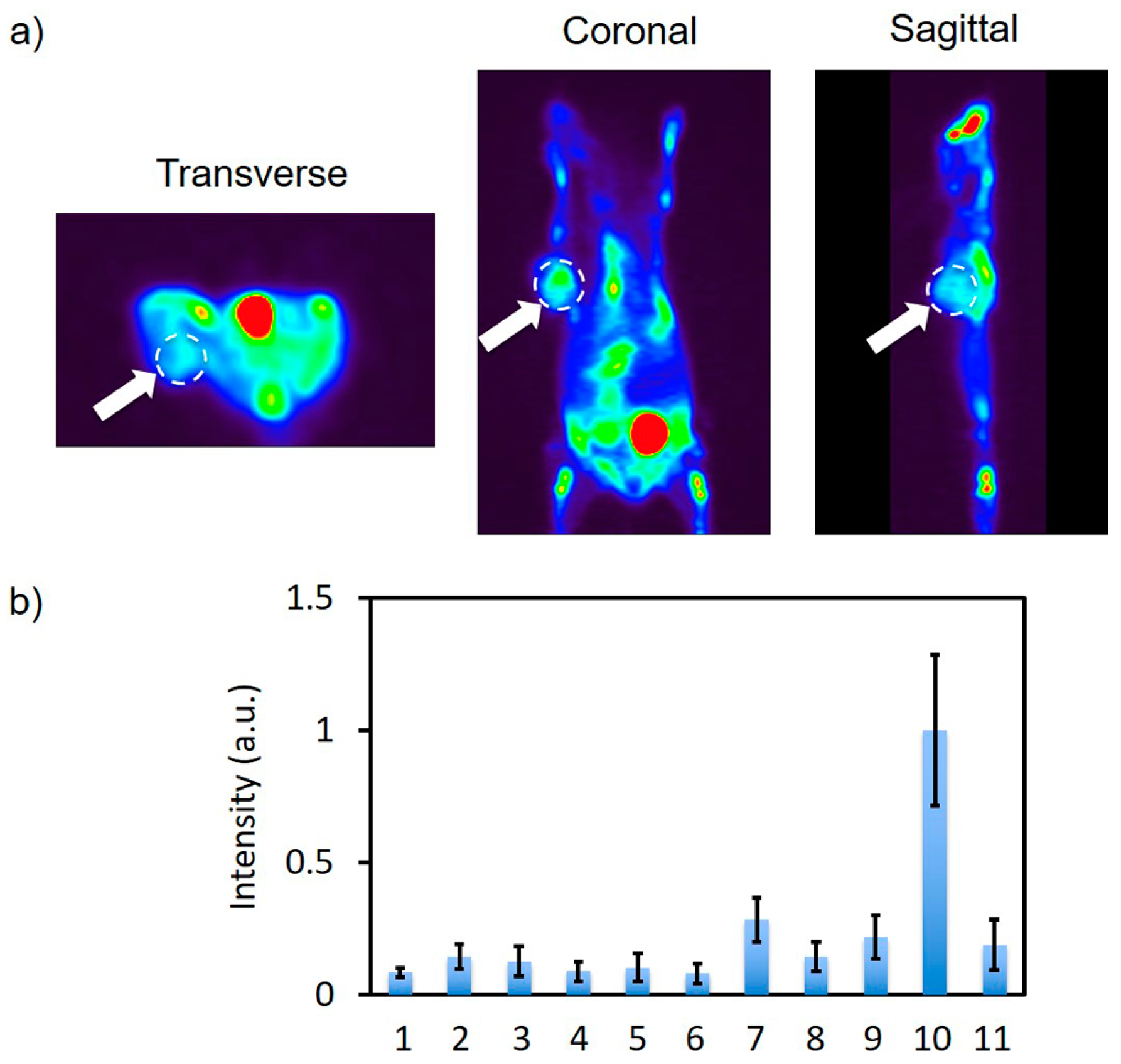
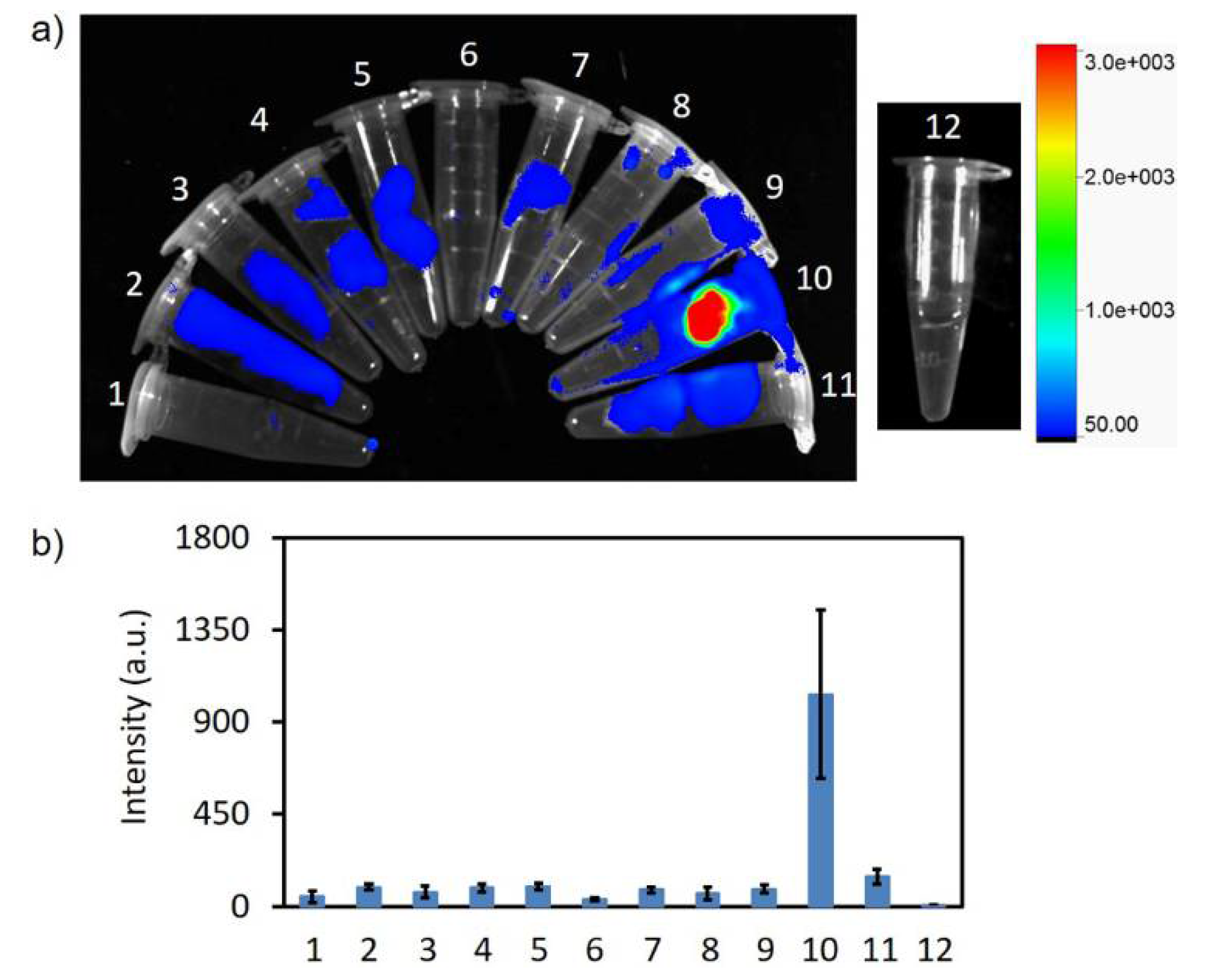
2.6. In Vivo PET Imaging and In Vitro Fluorescence Imaging
3. Materials and Methods
3.1. Cy5-B Synthesis
3.2. 18F Radiolabeling
3.3. MTT Assay
3.4. Hemolysis Assay
3.5. In Vitro Cell Imaging and FACS Quantification
3.6. In Vivo PET Imaging and In Vitro Fluorescence Imaging
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- James, N.S.; Chen, Y.; Joshi, P.; Ohulchanskyy, T.Y.; Ethirajan, M.; Henary, M.; Strekowsk, L.; Pandey, R.K. Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: Part-1. Theranostics 2013, 3, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; Van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Inomoto, T.; Miwa, M.; Shikayama, T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 2005, 12, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, I.; Dissemond, J.; Pöppel, T.; Schadendorf, D.; Klode, J. Intraoperative fluorescence imaging for sentinel lymph node detection: Prospective clinical trial to compare the usefulness of indocyanine green vs technetium Tc 99m for identification of sentinel lymph nodes. JAMA Surg. 2015, 150, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Verjans, J.W.; Osborn, E.A.; Ughi, G.J.; Press, M.A.C.; Hamidi, E.; Antoniadis, A.P.; Papafaklis, M.I.; Conrad, M.F.; Libby, P.; Stone, P.H. Targeted near-infrared fluorescence imaging of atherosclerosis: Clinical and intracoronary evaluation of indocyanine green. JACC Cardiovasc. Imaging 2016, 9, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, T.; Su, Y.; Luo, S.; Zhu, Y.; Tan, X.; Fan, S.; Zhang, L.; Zhou, Y.; Cheng, T. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials 2010, 31, 6612–6617. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, C.; Tong, R.; Qian, W.; Zhau, H.E.; Wang, R.; Zhu, G.; Cheng, J.; Yang, V.W.; Cheng, T.; et al. Near IR heptamethine cyanine dye-mediated cancer imaging. Clin. Cancer Res. 2010, 16, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Tan, X.; Qi, Q.; Guo, Q.; Ran, X.; Zhang, L.; Zhang, E.; Liang, Y.; Weng, L.; Zheng, H.; et al. A multifunctional heptamethine near-infrared dye for cancer theranosis. Biomaterials 2013, 34, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Luo, S.; Tan, X.; Shi, C. Mechanistic study of IR-780 dye as a potential tumor targeting and drug delivery agent. Biomaterials 2014, 35, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Pansare, V.J.; Hejazi, S.; Faenza, W.J.; Prud’homme, R.K. Review of long-wavelength optical and NIR imaging materials: Contrast agents, fluorophores, and multifunctional nano carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, F.; Wang, W.; Liu, J.; Xu, M.; Wu, D.; Shuai, X.; Shen, J.; Cao, Z. Chitosan coated gold nanorod chelating gadolinium for MRI-visible photothermal therapy of cancer. RSC Adv. 2016, 6, 111337–111344. [Google Scholar] [CrossRef]
- Harrison, V.S.R.; Carney, C.E.; MacRenaris, K.W.; Waters, E.A.; Meade, T.J. Multimeric near IR-MR contrast agent for multimodal in vivo imaging. J. Am. Chem. Soc. 2015, 137, 9108–9116. [Google Scholar] [CrossRef] [PubMed]
- An, F.-F.; Chan, M.; Kommidi, H.; Ting, R. Dual PET and near-infrared fluorescence imaging probes as tools for imaging in oncology. Am. J. Roentgenol. 2016, 207, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Koo, H.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multi-functional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Tsuchimochi, M.; Hayama, K.; Kawase, T.; Tsubokawa, N. Dual-labeled near-infrared/99mTc imaging probes using PAMAM-coated silica nanoparticles for the imaging of HER2-expressing cancer cells. Int. J. Mol. Sci. 2016, 17, 1086. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, Y.-H.; Kim, J.H.; Kang, K.W.; Tae, E.L.; Youn, H.; Kim, D.; Kim, S.-K.; Kwon, J.-T.; Cho, M.-H. Development and in vivo imaging of a PET/MRI nanoprobe with enhanced NIR fluorescence by dye encapsulation. Nanomedicine 2012, 7, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhao, J.; Conti, P.S.; Chen, K. Radiolabeled nanoparticles for multimodality tumor imaging. Theranostics 2014, 4, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Serwotka-Suszczak, A.M.; Sochaj-Gregorczyk, A.M.; Pieczykolan, J.; Krowarsch, D.; Jelen, F.; Otlewski, J. A conjugate based on anti-HER2 diaffibody and auristatin E targets HER2-positive cancer cells. Int. J. Mol. Sci. 2017, 18, 401. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, Y.; Yue, W.; Xie, X.; Wang, J.-P.; Chordia, M.D.; Chung, L.W.K.; Pan, D. Heptamethine cyanine based 64Cu-PET probe PC-1001 for cancer imaging: Synthesis and in vivo evaluation. Nucl. Med. Biol. 2013, 40, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Huang, P.; Weiss, O.J.; Yan, X.; Yue, X.; Zhang, M.G.; Tang, Y.; Nie, L.; Ma, Y.; Niu, G. PET and NIR optical imaging using self-illuminating 64Cu-doped chelator-free gold nanoclusters. Biomaterials 2014, 35, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ellison, P.A.; Lewis, C.M.; Hong, H.; Zhang, Y.; Shi, S.; Hernandez, R.; Meyerand, M.E.; Barnhart, T.E.; Cai, W. Chelator-free synthesis of a dual-modality PET/MRI agent. Angew. Chem. Int. Ed. 2013, 52, 13319–13323. [Google Scholar] [CrossRef] [PubMed]
- Ting, R.; Adam, M.J.; Ruth, T.J.; Perrin, D.M. Arylfluoroborates and alkylfluorosilicates as potential PET imaging agents: High-yielding aqueous biomolecular 18F-labeling. J. Am. Chem. Soc. 2005, 127, 13094–13095. [Google Scholar] [CrossRef] [PubMed]
- Ting, R.; Harwig, C.; auf dem Keller, U.; McCormick, S.; Austin, P.; Overall, C.M.; Adam, M.J.; Ruth, T.J.; Perrin, D.M. Toward [18F]-labeled aryltrifluoroborate radiotracers: In vivo positron emission tomography imaging of stable aryltrifluoroborate clearance in mice. J. Am. Chem. Soc. 2008, 130, 12045–12055. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Gauthier, V.; Bailey, J.J.; Liu, Z.; Wängler, B.; Wängler, C.; Jurkschat, K.; Perrin, D.M.; Schirrmacher, R. From unorthodox to established: The current status of 18F-trifluoroborate- and 18F-SiFA-based radiopharmaceuticals in PET nuclear imaging. Bioconjug. Chem. 2016, 27, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Perrin, D.M. [18F]-organotrifluoroborates as radioprosthetic groups for PET imaging: From design principles to preclinical applications. Acc. Chem. Res. 2016, 49, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Chin, J.W. Fluorescent imaging: Shining a light into live cells. Nat. Chem. 2013, 5, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Kaloyanova, S.; Zagranyarski, Y.; Ritz, S.; Hanulová, M.; Koynov, K.; Vonderheit, A.; Müllen, K.; Peneva, K. Water-soluble NIR-absorbing rylene chromophores for selective staining of cellular organelles. J. Am. Chem. Soc. 2016, 138, 2881–2884. [Google Scholar] [CrossRef] [PubMed]
- Khandelia, R.; Bhandari, S.; Pan, U.N.; Ghosh, S.S.; Chattopadhyay, A. Gold nanocluster embedded albumin nanoparticles for two-photon imaging of cancer cells accompanying drug delivery. Small 2015, 11, 4075–4081. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Mujumdar, R.B.; Ernst, L.A.; Mujumdar, S.R.; Lewis, C.J.; Waggoner, A.S. Cyanine dye labeling reagents: Sulfoindocyanine succinimidyl esters. Bioconjug. Chem. 1993, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ting, R.; Aguilera, T.A.; Crisp, J.L.; Hall, D.J.; Eckelman, W.C.; Vera, D.R.; Tsien, R.Y. Fast 18F labeling of a near-infrared fluorophore enables positron emission tomography and optical imaging of sentinel lymph nodes. Bioconjug. Chem. 2010, 21, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.A.; Wang, Y.; Crisp, J.L.; Vera, D.R.; Tsien, R.Y.; Ting, R. New dioxaborolane chemistry enables [18F]-positron-emitting, fluorescent [18F]-multimodality biomolecule generation from the solid phase. Bioconjug. Chem. 2016, 27, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Auf dem Keller, U.; Bellac, C.L.; Li, Y.; Lou, Y.; Lange, P.F.; Ting, R.; Harwig, C.; Kappelhoff, R.; Dedhar, S.; Adam, M.J.; et al. Novel matrix metalloproteinase inhibitor [18F] marimastat-aryltrifluoroborate as a probe for in vivo positron emission tomography imaging in cancer. Cancer Res. 2010, 70, 7562–7569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ting, R.; Harwig, C.W.; auf dem Keller, U.; Bellac, C.L.; Lange, P.F.; Inkster, J.A.H.; Schaffer, P.; Adam, M.J.; Ruth, T.J.; et al. Towards kit-like 18F-labeling of marimastat, a noncovalent inhibitor drug for in vivo PET imaging cancer associated matrix metalloproteases. MedChemComm 2011, 2, 942–949. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, W.; Wang, W.; Zhang, C.; Xu, M.; Liu, J.; Feng, S.-T.; Jiang, Q.; Xie, X. Stable cerasomes for simultaneous drug delivery and magnetic resonance imaging. Int. J. Nanomed. 2014, 9, 5103–5116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; An, F.-F.; Liu, J.; Jin, S.; Zhang, J.-C.; Wang, P.C.; Zhang, X.; Lee, C.-S.; Liang, X.-J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale 2015, 7, 13503–13510. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, F.-F.; Kommidi, H.; Chen, N.; Ting, R. A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging. Int. J. Mol. Sci. 2017, 18, 1214. https://doi.org/10.3390/ijms18061214
An F-F, Kommidi H, Chen N, Ting R. A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging. International Journal of Molecular Sciences. 2017; 18(6):1214. https://doi.org/10.3390/ijms18061214
Chicago/Turabian StyleAn, Fei-Fei, Harikrishna Kommidi, Nandi Chen, and Richard Ting. 2017. "A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging" International Journal of Molecular Sciences 18, no. 6: 1214. https://doi.org/10.3390/ijms18061214
APA StyleAn, F.-F., Kommidi, H., Chen, N., & Ting, R. (2017). A Conjugate of Pentamethine Cyanine and 18F as a Positron Emission Tomography/Near-Infrared Fluorescence Probe for Multimodality Tumor Imaging. International Journal of Molecular Sciences, 18(6), 1214. https://doi.org/10.3390/ijms18061214