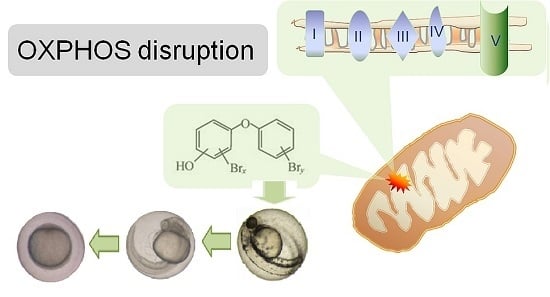
Effects of Hydroxylated Polybrominated Diphenyl Ethers in Developing Zebrafish Are Indicative of Disruption of Oxidative Phosphorylation
Abstract
:1. Introduction
2. Results
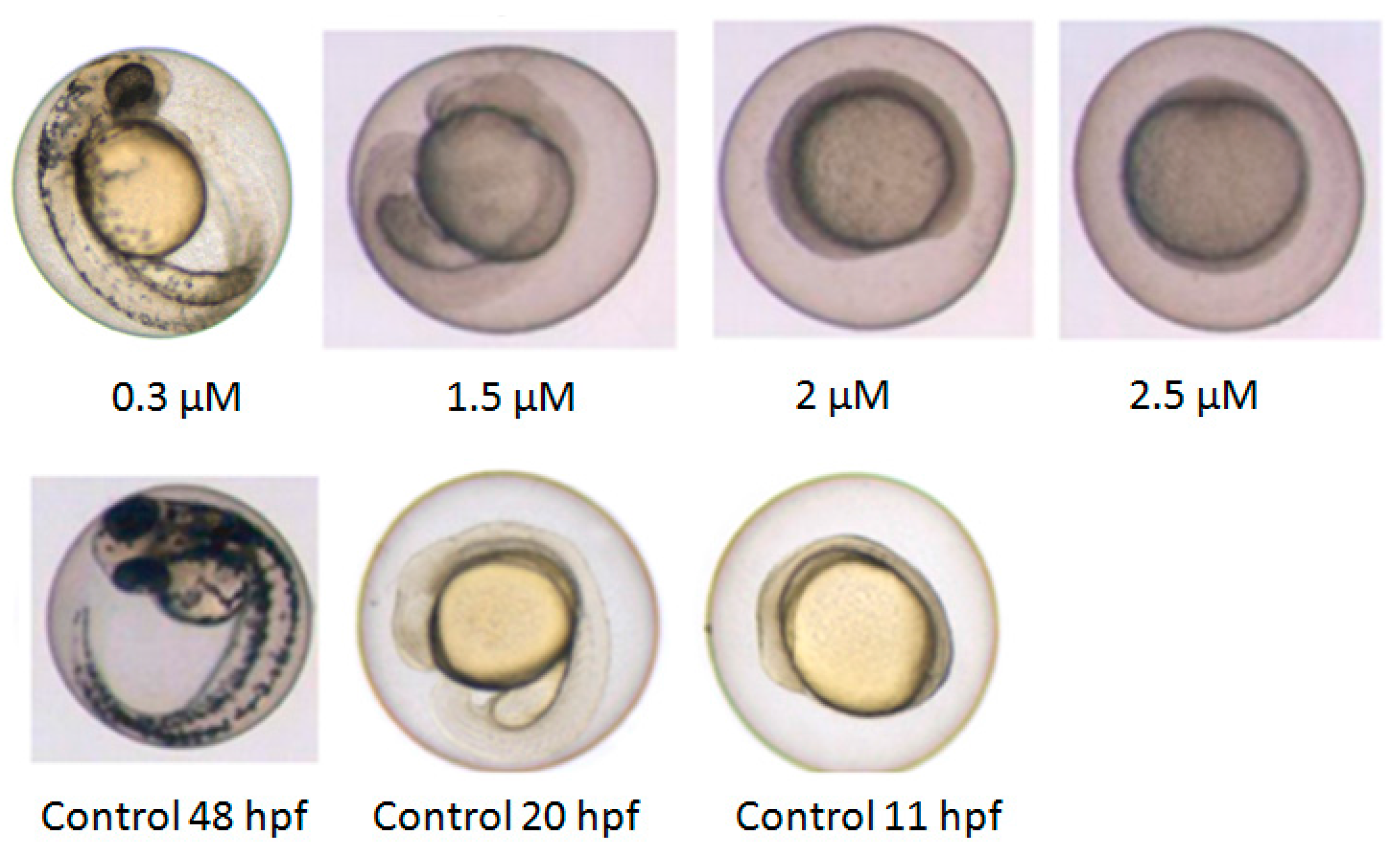
2.1. Developmental Toxicity Profile
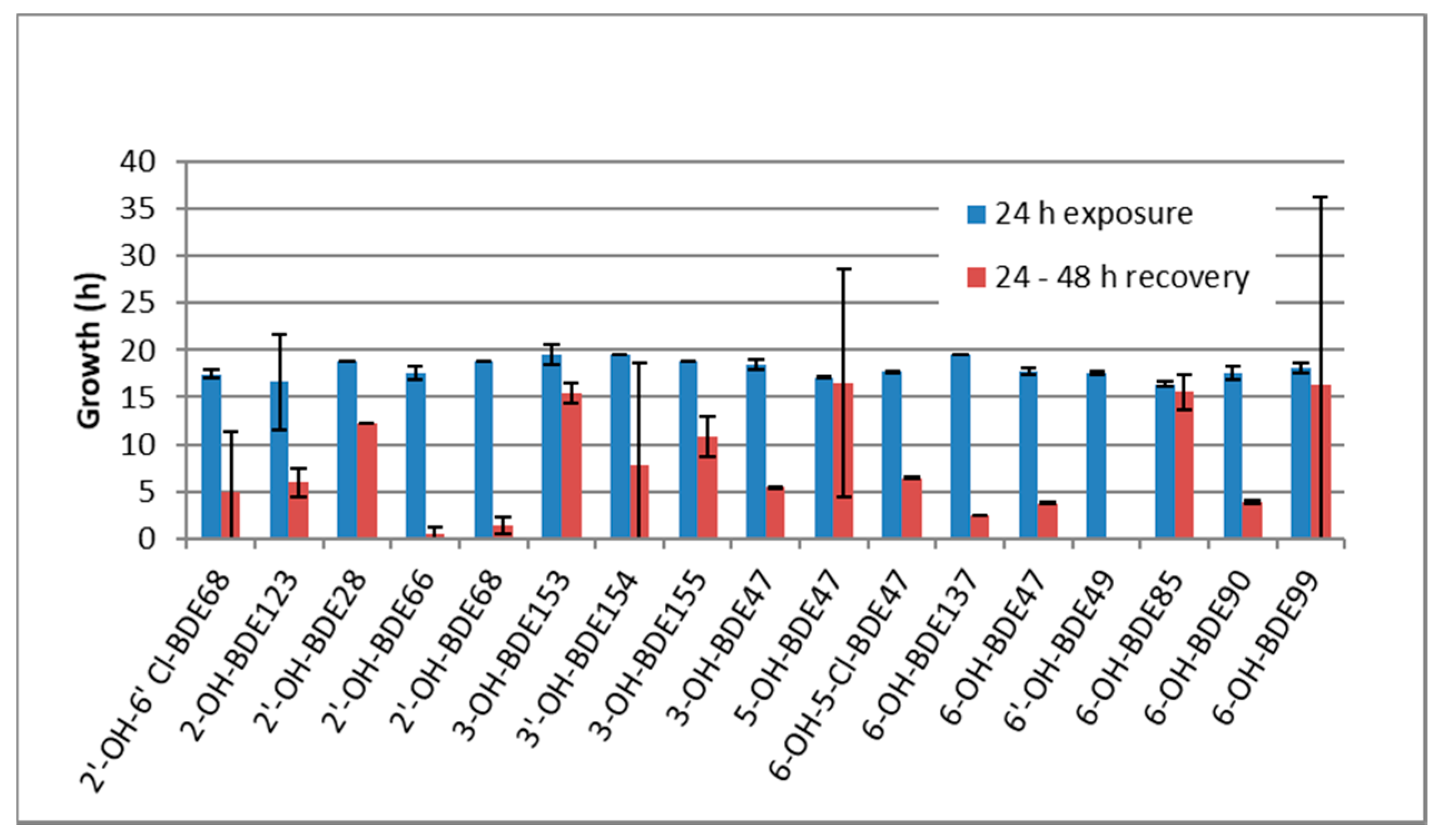
2.2. Developmental Arrest and Delay
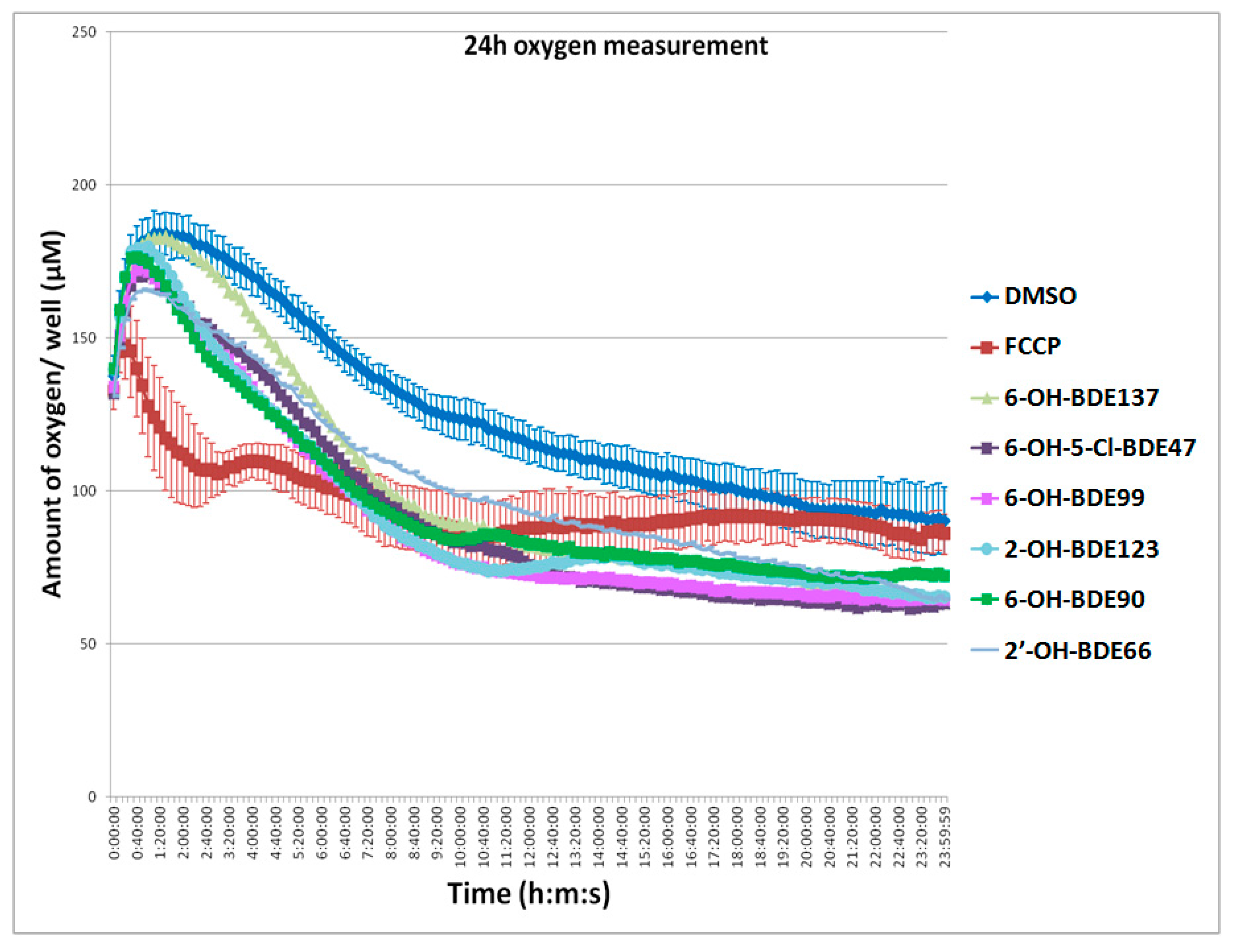
2.3. Oxygen Consumption
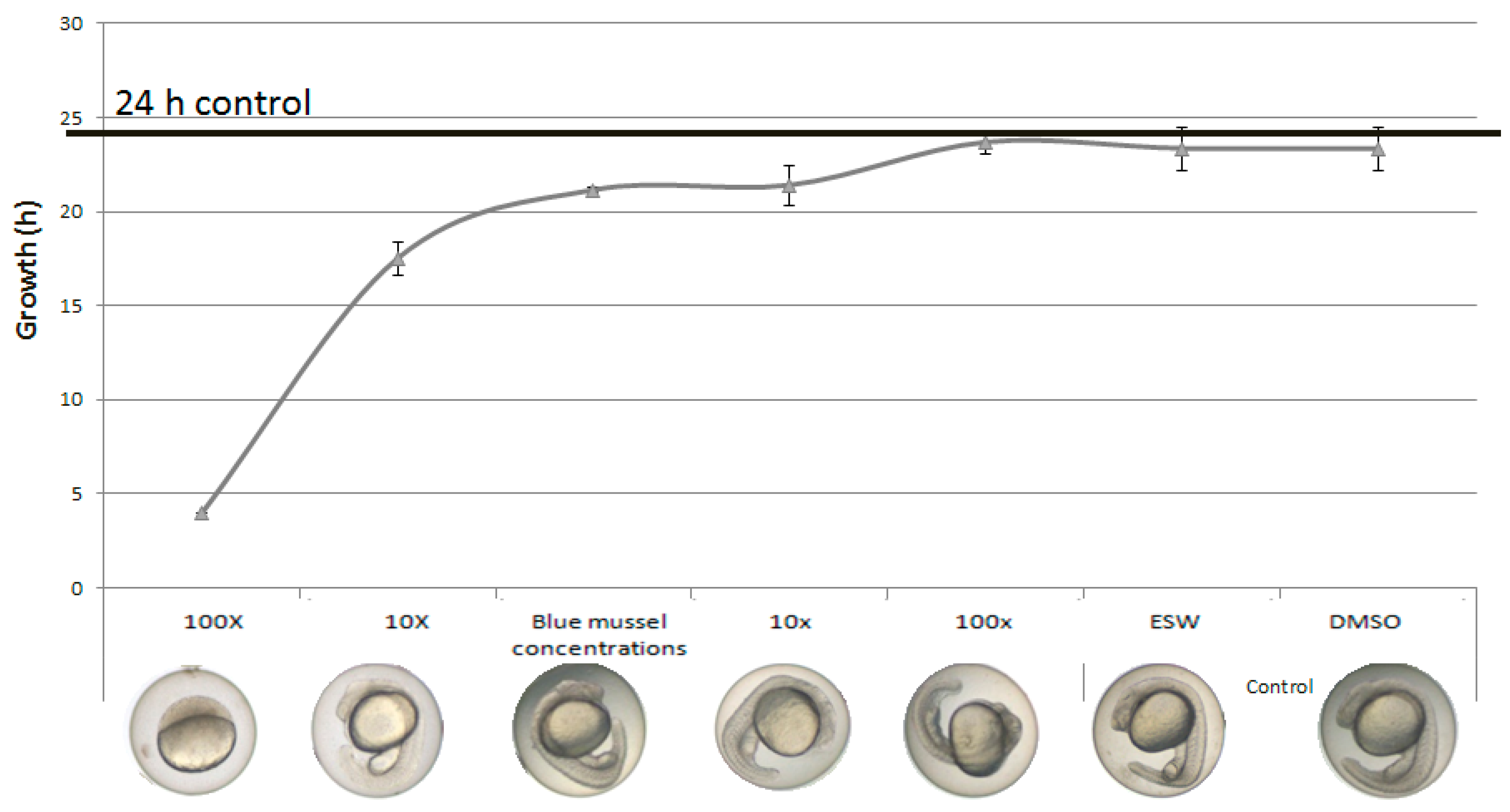
2.4. Mixtures
2.5. Actual Exposure Concentration
3. Discussion
3.1. Hydroxylated Polybrominated Diphenyl Ethers (OH-PBDEs) Developmental Toxicity
3.2. Hydroxylated Polybrominated Diphenyl Ethers (OH-PBDEs) Disrupt Oxidative Phosphorylation in Zebrafish Embryos
3.3. Mode of Action (MoA) of OH-PBDEs
3.4. Environmental Mixture Effects of OH-BDEs
4. Material and Methods
4.1. Chemicals
4.2. Zebrafish Toxicity Experiments
4.3. Oxygen Consumption Experiments
4.4. Measurement of 6-OH-BDE47 Exposure Concentration
4.4.1. Chemicals
4.4.2. Extraction and Derivatization
4.4.3. Instrumental Analysis
4.4.4. Quality Assurance/Quality Control
4.5. Data Analysis and Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Malmv, A. Brominated Natural Products at Different Trophic Levels in the Baltic Sea. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 1 January 2007. [Google Scholar]
- Malmberg, T.; Athanasiadou, M.; Marsh, G.; Brandt, I.; Bergman, A. Identification of hydroxylated polybrominated diphenyl ether metabolites in blood plasma from polybrominated diphenyl ether exposed rats. Environ. Sci. Technol. 2005, 39, 5342–5348. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Covaci, A.; Harrad, S.; Herzke, D.; Abdallahe, M.A.-E.; Fernie, K.; Toms, L.-M.L.; Takigami, H. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environ. Int. 2014, 65, 147–158. [Google Scholar] [CrossRef] [PubMed]
- De Wit, C.A.; Herzke, D.; Vorkamp, K. Brominated flame retardants in the arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885–2918. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Naturally Occuring Organohalogen Compounds—A Comprehensive Update; Springer: New York, NY, USA, 2010. [Google Scholar]
- Ekdahl, A.; Pedersen, M.; Collen, J.; Abrahamsson, K. Production of halocarbons from seaweeds: An oxidative stress reaction? Sci. Mar. 1996, 60, 257–263. [Google Scholar]
- Dring, M.J. Stress resistance and disease resistance in seaweeds: The role of reactive oxygen metabolism. Adv. Bot. Res. 2005, 43, 175–207. [Google Scholar]
- Dahlgren, E.; Lindqvist, D.; Dahlgren, H.; Asplund, L.; Lehtil, K. Trophic transfer of naturally produced brominated aromatic compounds in a Baltic Sea food chain. Chemosphere 2016, 144, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Asplund, L.; Kierkegaard, A.; Burreau, S.; Marsh, G.; Klasson-Wehler, E.; de Wit, C. P164 metabolism and distribution of 2,2′,4,4′-tetrabromo [14C] diphenyl ether in pike (Esox lucius) after dietary exposure. Organohalogen Compd. 2001, 52, 58–61. [Google Scholar]
- Van Boxtel, A.L.; Kamstra, J.H.; Cenijn, P.H.; Pieterse, B.; Wagner, M.J.; Antink, M.; Krab, K.; van der Burg, B.; Marsh, B.; Brouwer, A.; et al. Microarray analysis reveals a mechanism of phenolic polybrominated diphenylether toxicity in zebrafish. Environ. Sci. Technol. 2008, 42, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Liu, H.L.; Zhu, Y.T.; Zheng, X.M.; Su, G.Y.; Zhang, X.W.; Yu, H.X.; Giesy, J.P.; Lam, M.H. Maternal transfer, distribution, and metabolism of BDE-47 and its related hydroxylated, methoxylated analogs in zebrafish (Danio rerio). Chemosphere 2015, 120, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, J.R.; Norman, A.; Norrgren, L.; Haglund, P.; Andersson, P.L. Maternal transfer of brominated flame retardants in zebrafish (Danio rerio). Chemosphere 2008, 73, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Meerts, I.A.; Letcher, R.J.; Hoving, S.; Marsh, G.; Bergman, A.; Lemmen, J.G.; van der Burg, B.; Brouwer, A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001, 109, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, S.B.; Wan, Y.; Chang, H.; Zhang, X.; Hecker, M.; Jones, P.D.; Giesy, J.P. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Mar. Pollut. Bull. 2011, 63, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Legler, J.; Brouwer, A. Are brominated flame retardants endocrine disruptors? Environ. Int. 2003, 29, 879–885. [Google Scholar] [CrossRef]
- Legradi, J.; Dahlberg, A.K.; Cenijn, P.; Marsh, G.; Asplund, L.; Bergman, Å.; Legler, J. Disruption of oxidative phosphorylation (OXPHOS) by hydroxylated polybrominated diphenyl ethers (OH-PBDEs) present in the marine environment. Environ. Sci. Technol. 2014, 48, 14703–14711. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.H. Fish mitochondrial genomics: Sequence, inheritance and functional variation. J. Fish Biol. 2008, 72, 355–374. [Google Scholar] [CrossRef]
- Holcomb, M.; Cloud, J.G.; Woolsey, J.; Ingermann, R.L. Oxygen consumption in unfertilized salmonid eggs: An indicator of egg quality? Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, B.A.; Gitlin, J.D. Coordination of development and metabolism in the pre-midblastula transition zebrafish embryo. Dev. Dyn. 2008, 237, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.; Selinger, D.W.; Solomon, J.M.; Wu, H.; Schmitt, E.; Serluca, F.C.; Curtis, D.; Benson, J.D. Integrated compound pro fi ling screens identify the mitochondrial electron transport chain as the molecular target of the natural products manassantin, sesquicillin, and arctigenin. ACS Chem. Biol. 2013, 8, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Usenko, C.Y.; Hopkins, D.C.; Trumble, S.J.; Bruce, E.D. Hydroxylated PBDEs induce developmental arrest in zebrafish. Toxicol. Appl. Pharmacol. 2012, 262, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Löfstrand, K.; Liu, X.; Lindqvist, D.; Jensen, S.; Asplund, L. Seasonal variations of hydroxylated and methoxylated brominated diphenyl ethers in blue mussels from the Baltic Sea. Chemosphere 2011, 84, 527–532. [Google Scholar]
- Ren, X.-M.; Guo, L.-H.; Gao, Y.; Zhang, B.-T.; Wan, B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol. Appl. Pharmacol. 2013, 268, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, L.J.; Chen, A.; Rock, K.D.; Dishaw, L.V.; Dong, W.; Hinton, D.E.; Stapleton, H.M. Developmental toxicity of the PBDE metabolite 6-OH-BDE-47 in zebrafish and the potential role of thyroid receptor β. Aquat. Toxicol. 2015, 168, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Macaulay, L.J.; Kwok, K.W.; Hinton, D.E.; Ferguson, P.L.; Stapleton, H.M. The PBDE metabolite 6-OH-BDE 47 affects melanin pigmentation and THRβ MRNA expression in the eye of zebrafish embryos. Endocr. Disruptors 2014, 2, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, Y.; Liu, C.; Liu, H.; Giesy, J.P.; Hecker, M.; Lam, M.H.; Yu, H. Accumulation and biotransformation of BDE-47 by zebrafish larvae and teratogenicity and expression of genes along the hypothalamus—Pituitary—Thyroid Axis. Environ. Sci. Technol. 2012, 46, 12943–12951. [Google Scholar] [CrossRef] [PubMed]
- Hamers, T.; Kamstra, J.H.; Sonneveld, E.; Murk, A.J.; Visser, T.J.; van Velzen, M.J.; Brouwer, A.; Bergman, A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47). Mol. Nutr. Food Res. 2008, 52, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, A.-K.; Bignert, A.; Legradi, J.; Legler, J.; Asplund, L. Anthropogenic and naturally produced brominated substances in Baltic herring (Clupea harengus membras) from two sites in the Baltic Sea. Chemosphere 2016, 144, 2408–2414. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G.; Hu, J.; Jakobsson, E.; Rahm, S.; Bergman, A. Synthesis and characterization of 32 polybrominated diphenyl ethers. Environ. Sci. Technol. 1999, 33, 3033–3037. [Google Scholar] [CrossRef]
- Rydén, A.; Nestor, G.; Jakobsson, K.; Marsh, G. Synthesis and tentative identification of novel polybrominated diphenyl ether metabolites in human blood. Chemosphere 2012, 88, 1227–1234. [Google Scholar]
- Marsh, G.; Stenutz, R.; Bergman, Å. Synthesis of hydroxylated and methoxylated polybrominated diphenyl ethers—Natural products and potential polybrominated diphenyl ether metabolites. Eur. J. Org. Chem. 2003, 2003, 2566–2576. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals Section 2; OECD: Paris, France, 2013; pp. 1–22. [Google Scholar]
- PreSense. Instruction Manual OxoPlate; PreSense: Regensburg, Germany, 2003. [Google Scholar]
- Jensen, S.; Lindqvist, D.; Asplund, L. Lipid extraction and determination of halogenated phenols and alkylphenols as their pentafluorobenzoyl derivatives in marine organisms. J. Agric. Food Chem. 2009, 57, 2009. [Google Scholar] [CrossRef] [PubMed]
- Marsh, E.; Bergman, G.; Bladh, Å.; Gillner, L.-G.; Jakobsson, M. Synthesis of p-hydroxybromodiphenyl ethers and binding to the thyroid receptor. Organohalogen Compd. 1998, 37, 305–308. [Google Scholar]
| Developmental Delay | Malformations and Mortality | ||||
|---|---|---|---|---|---|
| 24 hpf | 72 hpf/144 hpf | Significant Increase in Oxygen Consumption | |||
| Compound | NOEC * | LOEC & | NOEC ** | LOEC && | LOEC † |
| 2′-OH-6′-Cl-BDE68 | 0.3 | 1.5 | 0.75 | 1 | 1.5 |
| 2-OH-BDE123 | 1.25 | 2 | 0.5 | 1 | 2.5 |
| 2′-OH-BDE28 | 5.25 | 6 | 2 | 3 | 6.5 |
| 2′-OH-BDE66 | 6 | 7 | 4 | 5 | 7.5 |
| 2′-OH-BDE68 | 0.5 | 1 | 0.4 | 0.6 | 1.75 |
| 3-OH-BDE153 | 3 | 3.5 | 0.5 | 1 | 4.75 |
| 3′-OH-BDE154 | 2 | 2.5 | 0.5 | 1 | 3.25 |
| 3-OH-BDE155 | 4 | 5 | 0.5 | 1 | 7 |
| 3-OH-BDE47 | 3.5 | 5 | 1 | 2 | 6.5 |
| 5-OH-BDE47 | 2 | 2.5 | 1 | 2 | 2.75 |
| 6-OH-5-Cl-BDE47 | 0.9 | 1.2 | 0.5 | 0.75 | 1.2 |
| 6-OH-BDE137 | 0.1 | 1.2 | 0.2 | 0.5 | 2 |
| 6-OH-BDE47 | 0.1 | 0.5 | 0.1 | 0.5 | 1 |
| 6′-OH-BDE49 | 0.05 | 0.75 | 0.1 | 0.5 | 2.5 |
| 6-OH-BDE85 | 0.5 | 1 | 0.5 | 0.75 | 1.25 |
| 6-OH-BDE90 | 0.25 | 2 | 0.5 | 1 | 2.5 |
| 6-OH-BDE99 | 0.5 | 1 | 0.5 | 1 | 2 |
| All in µM | 144 hpf | Environmental Measured Fish Concentration | Margin of Exposure | |||
|---|---|---|---|---|---|---|
| Compound | NOEC | LOEC | Location: Askö | Location: Ängskärsklubb | Minimum | LOEC/Minimum |
| 2-OH-BDE123 | 0.5 | 1 | 0.002 | <LOD | 0.002 | 500 |
| 2′-OH-BDE68 | 0.4 | 0.6 | 0.01 | 0.007 | 0.01 | 60 |
| 6-OH-BDE137 | 0.2 | 0.5 | <LOD | <LOD | <LOD | not applicable |
| 6-OH-BDE47 | 0.1 | 0.5 | 0.2 | 0.2 | 0.2 | 3 |
| 6-OH-BDE90 | 0.5 | 1 | 0.001 | 0.002 | 0.001 | 1000 |
| 6-OH-BDE99 | 0.5 | 1 | 0.01 | 0.2 | 0.01 | 100 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legradi, J.; Pomeren, M.V.; Dahlberg, A.-K.; Legler, J. Effects of Hydroxylated Polybrominated Diphenyl Ethers in Developing Zebrafish Are Indicative of Disruption of Oxidative Phosphorylation. Int. J. Mol. Sci. 2017, 18, 970. https://doi.org/10.3390/ijms18050970
Legradi J, Pomeren MV, Dahlberg A-K, Legler J. Effects of Hydroxylated Polybrominated Diphenyl Ethers in Developing Zebrafish Are Indicative of Disruption of Oxidative Phosphorylation. International Journal of Molecular Sciences. 2017; 18(5):970. https://doi.org/10.3390/ijms18050970
Chicago/Turabian StyleLegradi, Jessica, Marinda Van Pomeren, Anna-Karin Dahlberg, and Juliette Legler. 2017. "Effects of Hydroxylated Polybrominated Diphenyl Ethers in Developing Zebrafish Are Indicative of Disruption of Oxidative Phosphorylation" International Journal of Molecular Sciences 18, no. 5: 970. https://doi.org/10.3390/ijms18050970
APA StyleLegradi, J., Pomeren, M. V., Dahlberg, A.-K., & Legler, J. (2017). Effects of Hydroxylated Polybrominated Diphenyl Ethers in Developing Zebrafish Are Indicative of Disruption of Oxidative Phosphorylation. International Journal of Molecular Sciences, 18(5), 970. https://doi.org/10.3390/ijms18050970