Subcutaneous and Visceral Adipose Tissue Secretions from Extremely Obese Men and Women both Acutely Suppress Muscle Insulin Signaling
Abstract
:1. Introduction
2. Results
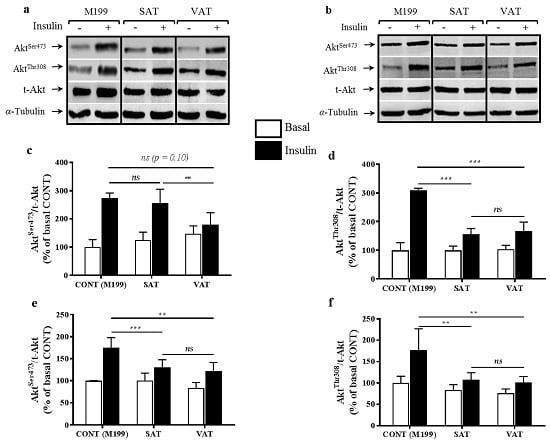
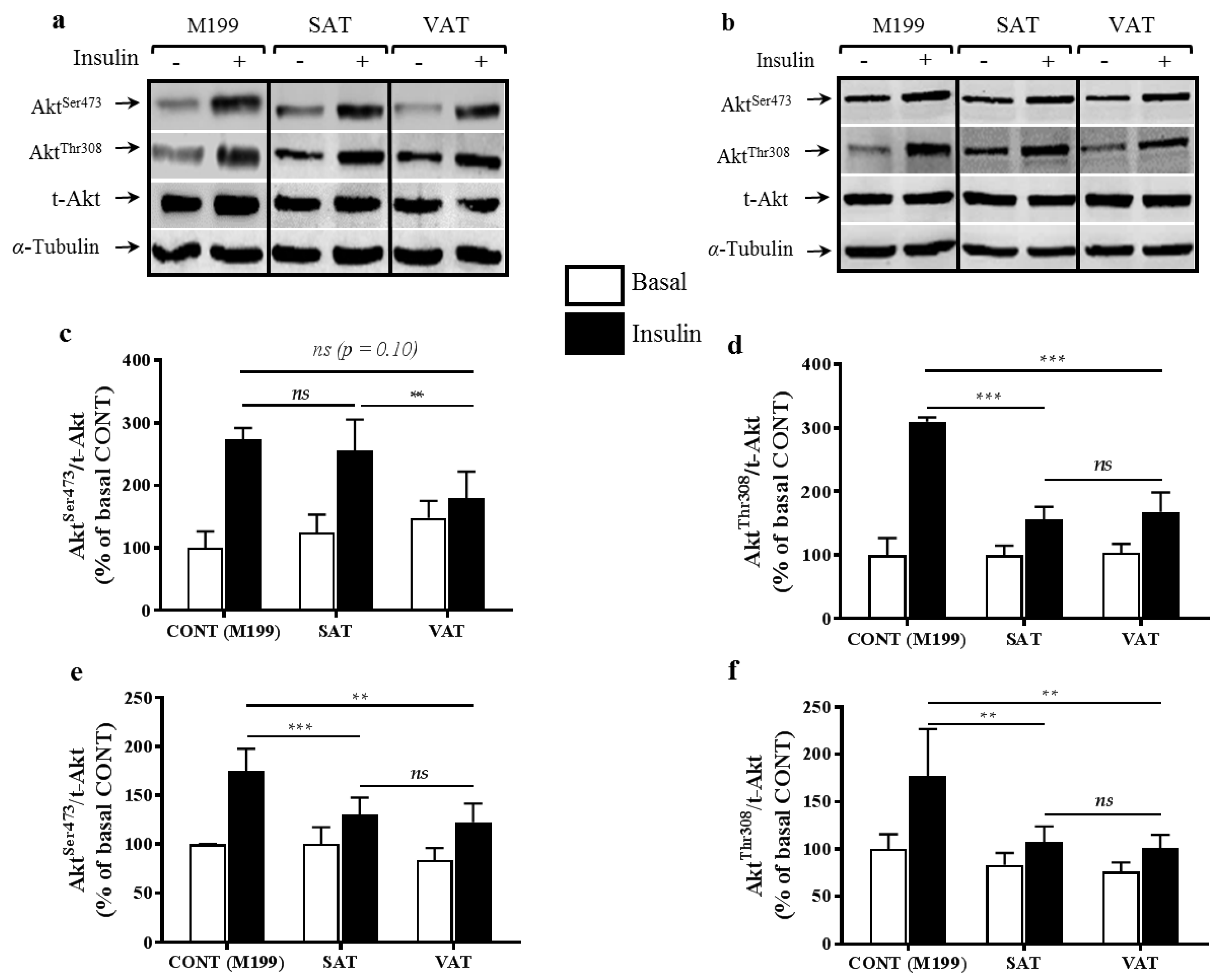
2.1. Effect of Secretion Media on Protein Kinase B (Akt) Proteins in Human Myotubes
2.2. Adipokine Content of Secretion Media
3. Discussion
4. Materials and Methods
4.1. Characteristics of Muscle Cells and Donors of Adipose Tissues
4.2. Adipose Tissue Culture
4.3. Determining Adipokine Content in Secretion Media
4.4. Culture of Human Skeletal Myoblasts
4.5. Immunoblotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| MCP-1 | Monocyte chemotactic protein-1 |
| Ser | Serine |
| Thr | Threonine |
| TNF-α | Tumor necrosis-α |
References
- World Health Organization. Global Report on Diabetes. Available online: http://www.who.int/diabetes/publications/grd-2016/en/ (accessed on 8 December 2016).
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose: Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Felber, J.P.; Golay, A. Pathways from obesity to diabetes. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dietze, D.; Koenen, M.; Röhrig, K.; Horikoshi, H.; Hauner, H.; Eckel, J. Impairment of insulin signaling in human skeletal muscle cells by co-culture with human adipocytes. Diabetes 2002, 51, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Coppack, S.W. Adipose tissue changes in obesity. Biochem. Soc. Trans. 2005, 33, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Van Harmelen, V.; Reynisdottir, S.; Eriksson, P.; Thörne, A.; Hoffstedt, J.; Lönnqvist, F.; Arner, P. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1988, 47, 913–917. [Google Scholar] [CrossRef]
- Lihn, A.S.; Bruun, J.M.; He, G.; Pedersen, S.B.; Jensen, P.F.; Richelsen, B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol. Cell. Endocrinol. 2004, 219, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Monocyte chemoattractant protein 1 release is higher in the visceral than subcutaneous human adipose tissue (AT): Implication of macrophages resident in the AT. J. Clin. Endocrinol. Metab. 2005, 90, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Lihn, A.S.; Madan, A.K.; Pedersen, S.B.; Schiøtt, K.M.; Fain, J.N.; Richelsen, B. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue: Implication of nonadipose cells in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2004, 286, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clément, K. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different adipose depots: Their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J. Obes. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Carswell, K.A.; Lee, M.J.; Fried, S.K. Culture of isolated human adipocytes and isolated adipose tissue. Methods Mol. Biol. 2012, 806, 203–214. [Google Scholar] [PubMed]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Randeva, H.S. Differential effects of leptin and adiponectin in endothelial angiogenesis. J. Diabetes Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, K.; Kantartzis, K.; Machann, J.; Schick, F.; Thamer, C.; Machicao, F.; Fritsche, A.; Häring, H.U.; Stefan, N. Impact of different fat depots on insulin sensitivity: Predominant role of liver fat. J. Diabetes Sci. Technol. 2007, 1, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Roytblat, L.; Rachinsky, M.; Fisher, A.; Greemberg, L.; Shapira, Y.; Douvdevani, A. Raised interleukin-6 levels in obese patients. Obes. Res. 2000, 8, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stêpieñ, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-α system. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef] [PubMed]
- Svensson, H.; Odén, B.; Edén, S.; Lönn, M. Adiponectin, chemerin, cytokines, and dipeptidyl peptidase 4 are released from human adipose tissue in a depot-dependent manner: An in vitro system including human serum albumin. BMC Endocr. Disord. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Madani, R.; Karastergiou, K.; Ogston, N.C.; Miheisi, N.; Bhome, R.; Haloob, N.; Tan, G.D.; Karpe, F.; Malone-Lee, J.; Hashemi, M.; et al. RANTES release by human adipose tissue in vivo and evidence for depot-specific differences. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Chacón, M.R.; Fernández-Real, J.M.; Richart, C.; Megía, A.; Gómez, J.M.; Miranda, M.; Caubet, E.; Pastor, R.; Masdevall, C.; Vilarrasa, N.; et al. Monocyte chemoattractant protein-1 in obesity and type 2 diabetes. Insulin sensitivity study. Obesity 2007, 15, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Dietze-Schroeder, D.; Kaiser, U.; Eckel, J. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology 2006, 147, 2458–2467. [Google Scholar] [CrossRef] [PubMed]
| Secreted Factor a,b,c | Average in Male SAT | Average in Female SAT | Average in Male VAT | Average in Female VAT | SAT vs. VAT | Male vs. Female | Depot x Sex |
|---|---|---|---|---|---|---|---|
| IL-4 | 29.8 ± 7.2 | 40.6 ± 12.4 | 106.7 ± 13.3 † | 110.4 ± 23.3 ** | < 0.001 | 0.689 | 0.846 |
| IL-6 | 388.5 ± 72.1 | 699.6 ± 329.5 | 2183.6 ± 497.8 | 4861.1 ± 1590.1 ** | 0.004 | 0.141 | 0.242 |
| IL-21 | 287.9 ± 15.6 | 285.3 ± 19.3 | 231.9 ± 13.1 | 213.8 ± 14.0 ** | < 0.001 | 0.530 | 0.639 |
| IL-1α | 17.9 ± 0.9 | 18.4 ± 1.3 | 16.4 ± 0.7 | 15.6 ± 1.4 | 0.093 | 0.872 | 0.614 |
| IL-1RA | 65.7 ± 11.3 | 76.4 ± 18.9 | 79.2 ± 19.1 | 99.1 ± 19.9 | 0.349 | 0.427 | 0.813 |
| GRO-α | 50.2 ± 2.2 | 61.3 ± 9.0 | 125.3 ± 28.7 | 178.7 ± 54.6 * | 0.008 | 0.358 | 0.546 |
| IL-8 | 218.5 ± 61.3 | 586.9 ± 222.9 | 1427.5 ± 247.7 | 1866.5 ± 693.5 | 0.007 | 0.365 | 0.937 |
| MCP-1 | 176.2 ± 55.2 | 234.3 ± 109.9 | 844.6 ± 150.3 | 949.8 ± 295.9 * | < 0.001 | 0.682 | 0.906 |
| MIP-1α | 10.6 ± 9.0 | 18.6 ± 39.3 | 29.9 ± 14.8 | 40.2 ± 48.4 | 0.033 | 0.332 | 0.901 |
| MIP-1β | 202.4 ± 11.8 | 214.1 ± 27.8 | 304.1 ± 16.9 | 297.0 ± 53.6 | 0.014 | 0.949 | 0.798 |
| RANTES | 1.9 ± 0.5 | 2.0 ± 0.6 | 4.6 ± 0.4 † | 4.7 ± 1.0 * | < 0.001 | 0.900 | 0.933 |
| SDF-1α | 314.2 ± 65.3 | 260.6 ± 79.6 | 622.2 ± 38.2 | 578.5 ± 114.6 * | 0.001 | 0.593 | 0.956 |
| FGF-2 | 87.3 ± 4.4 | 82.6 ± 6.0 | 70.4 ± 3.2 | 63.8 ± 4.6 * | < 0.001 | 0.262 | 0.852 |
| HGF | 694.3 ± 173.5 | 484.8 ± 101.3 | 950.7 ± 136.2 | 761.4 ± 140.8 | 0.058 | 0.1538 | 0.942 |
| LIF | 32.0 ± 7.0 | 55.4 ± 16.7 | 200.5 ± 32.1 ‡ | 213.6 ± 48.9 ** | <0.001 | 0.589 | 0.879 |
| P IGF-1 | 3.5 ± 0.7 | 2.5 ± 0.8 | 9.3 ± 1.6 † | 8.4 ± 1.8 ** | <0.001 | 0.516 | 0.974 |
| VEGF-A | 117.3 ± 23.6 | 89.4 ± 14.5 | 253.6 ± 53.4 † | 169.9 ± 30.2 | 0.001 | 0.089 | 0.391 |
| Adiponectin | 3679.7 ± 648.2 | 3757.7 ± 565.4 | 3513.4 ± 426.5 | 3939.8 ± 610.0 | 0.989 | 0.670 | 0.769 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarr, O.; Strohm, R.J.; MacDonald, T.L.; Gaudio, N.; Reed, J.K.; Foute-Nelong, J.; Dyck, D.J.; Mutch, D.M. Subcutaneous and Visceral Adipose Tissue Secretions from Extremely Obese Men and Women both Acutely Suppress Muscle Insulin Signaling. Int. J. Mol. Sci. 2017, 18, 959. https://doi.org/10.3390/ijms18050959
Sarr O, Strohm RJ, MacDonald TL, Gaudio N, Reed JK, Foute-Nelong J, Dyck DJ, Mutch DM. Subcutaneous and Visceral Adipose Tissue Secretions from Extremely Obese Men and Women both Acutely Suppress Muscle Insulin Signaling. International Journal of Molecular Sciences. 2017; 18(5):959. https://doi.org/10.3390/ijms18050959
Chicago/Turabian StyleSarr, Ousseynou, Rachel Joyce Strohm, Tara Lynn MacDonald, Nicholas Gaudio, John Kenneth Reed, Jules Foute-Nelong, David James Dyck, and David Michael Mutch. 2017. "Subcutaneous and Visceral Adipose Tissue Secretions from Extremely Obese Men and Women both Acutely Suppress Muscle Insulin Signaling" International Journal of Molecular Sciences 18, no. 5: 959. https://doi.org/10.3390/ijms18050959
APA StyleSarr, O., Strohm, R. J., MacDonald, T. L., Gaudio, N., Reed, J. K., Foute-Nelong, J., Dyck, D. J., & Mutch, D. M. (2017). Subcutaneous and Visceral Adipose Tissue Secretions from Extremely Obese Men and Women both Acutely Suppress Muscle Insulin Signaling. International Journal of Molecular Sciences, 18(5), 959. https://doi.org/10.3390/ijms18050959