CTC-mRNA (AR-V7) Analysis from Blood Samples—Impact of Blood Collection Tube and Storage Time
Abstract
:1. Introduction
2. Results
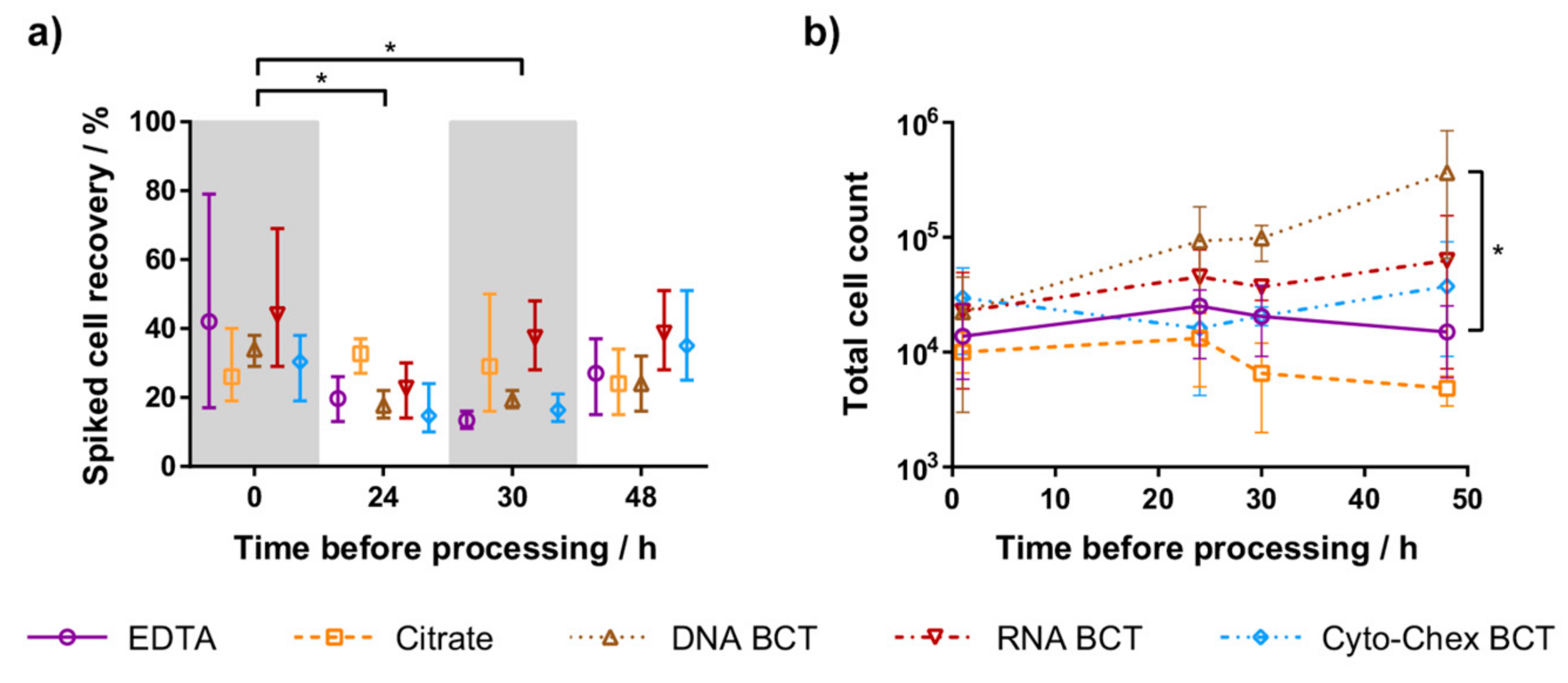
2.1. Spiked Cell Recovery
2.2. Leukocyte Contamination
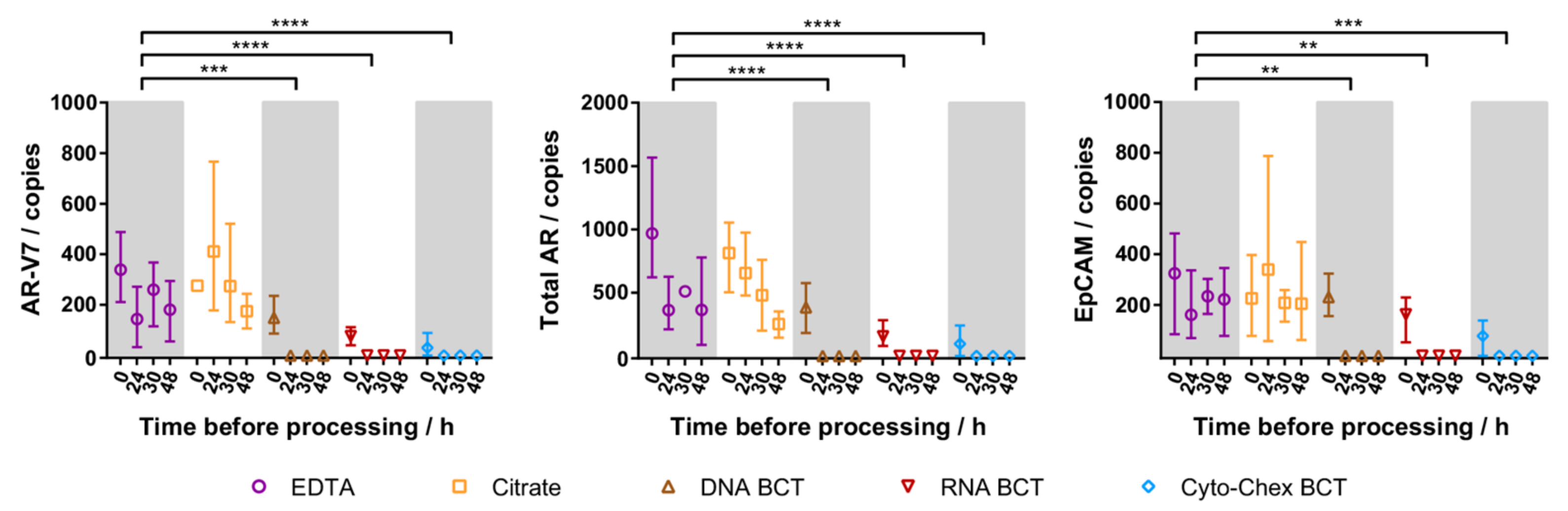
2.3. Cellular RNA Recovery
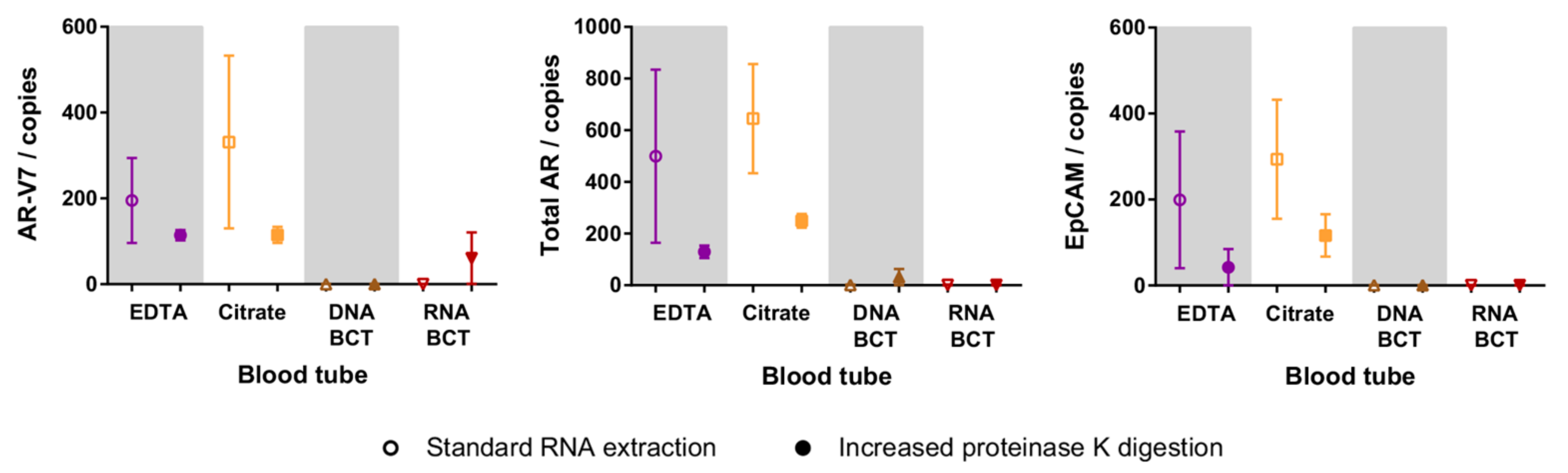
2.4. Increased Proteinase K Treatment
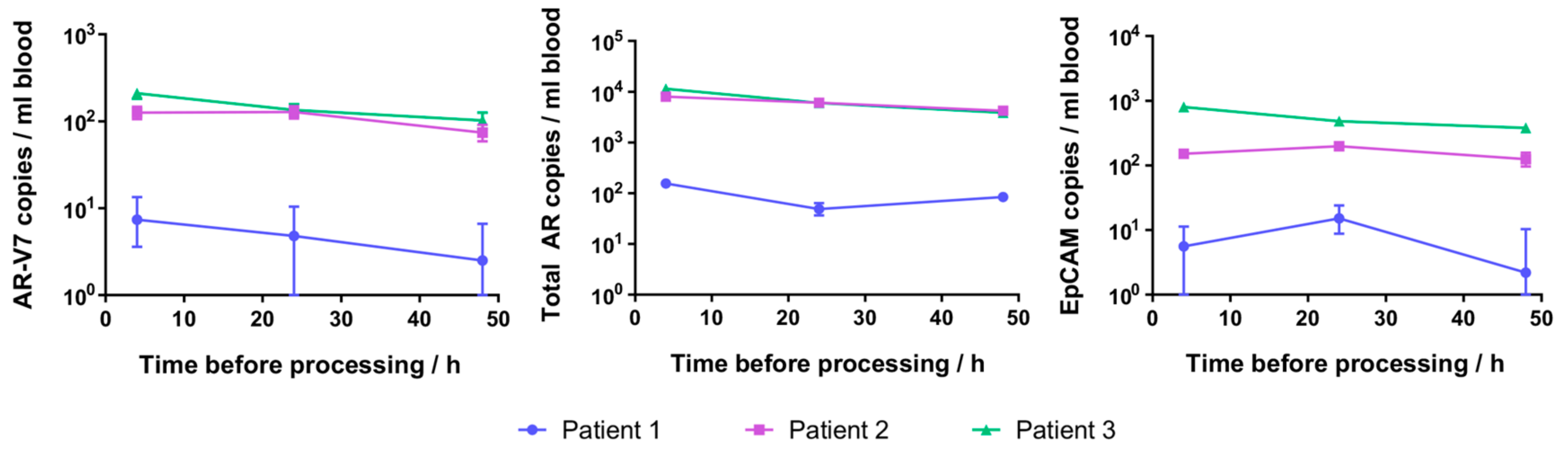
2.5. Patient CTC Cellular RNA Detection
3. Discussion
3.1. Tumour Cell Preservation
3.2. Cellular RNA Preservation
4. Materials and Methods
4.1. Blood Collection
4.2. Cell Spiking
4.3. Tumour Cell Enrichment from Whole Blood
4.4. Cell Enumeration
4.5. Cellular RNA Extraction
4.6. Digital Droplet PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caixeiro, N.J.; Kienzle, N.; Lim, S.H.; Spring, K.J.; Tognela, A.; Scott, K.F.; Souza, P.D.; Becker, T.M. Circulating tumour cells—A bona fide cause of metastatic cancer. Cancer Metastasis Rev. 2014, 33, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Joosse, S.A.; Gorges, T.M.; Pantel, K. Biology, detection, and clinical implications of circulating tumor cells. EMBO Mol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.M.; Caixeiro, N.J.; Lim, S.H.; Tognela, A.; Kienzle, N.; Scott, K.F.; Spring, K.J.; de Souza, P. New frontiers in circulating tumor cell analysis: A reference guide for biomolecular profiling toward translational clinical use. Int. J. Cancer 2014, 134, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells—Biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.W.M.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; Nadal, R.; Paller, C.J.; Denmeade, S.R.; Carducci, M.A.; et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015, 1, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Luk, A.; Young, F.P.; Lynch, D.; Chua, W.; Balakrishnar, B.; de Souza, P.; Becker, T.M. Droplet digital PCR based androgen receptor variant 7 (AR-V7) detection from prostate cancer patient blood biopsies. Int. J. Mol. Sci. 2016, 17, 1264. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J. Clin. Oncol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Kleinerman, J.; Saidel, G.M. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974, 34, 997–1004. [Google Scholar] [PubMed]
- Butler, T.P.; Gullino, P.M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975, 35, 512–516. [Google Scholar] [PubMed]
- Chang, Y.S.; Tomaso, E.D.; McDonald, D.M.; Jones, R.; Jain, R.K.; Munn, L.L. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 2000, 97, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, X.; Zhou, J.; Qiu, S.; Fan, J.; Xu, Y. Circulating tumor cells: Advances in detection methods, biological issues, and clinical relevance. J. Cancer Res. Clin. Oncol. 2011, 137, 1151–1173. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Stott, S.; Toner, M.; Maheswaran, S.; Haber, D.A. Circulating tumor cells: Approaches to isolation and characterization. J. Cell Biol. 2011, 192, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 2014, 14, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.Y.L.; Rainer, T.H.; Chiu, R.W.K.; Lo, Y.M.D. EDTA is a better anticoagulant than heparin or citrate for delayed blood processing for plasma DNA analysis. Clin. Chem. 2004, 50, 256–257. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Ludovici, G.; de Marchis, M.L.; Savonarola, A.; Leone, B.; Spila, A.; de Angelis, F.; Morte, D.D.; Ferroni, P.; Guadagni, F. Preanalytical procedures for DNA studies: The experience of the interinstitutional multidisciplinary BioBank (BioBIM). Biopreserv. Biobank. 2011, 9, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fehm, T.; Solomayer, E.F.; Meng, S.; Tucker, T.; Lane, N.; Wang, J.; Gebauer, G. Methods for isolating circulating epithelial cells and criteria for their classification as carcinoma cells. Cytotherapy 2005, 7, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Chen, K.; Norton, S.; Krzyzanowski, G.; Bourne, D.; Hunsley, B.; Ryan, W.L.; Bassett, C. A new methodology to preserve the original proportion and integrity of cell-free fetal DNA in maternal plasma during sample processing and storage. Prenat. Diagn. 2010, 30, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Fernando, M.R.; Norton, S.E.; Luna, K.K.; Lechner, J.M.; Qin, J. Stabilization of cell-free RNA in blood samples using a new collection device. Clin. Biochem. 2012, 45, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Warrino, D.E.; DeGennaro, L.J.; Hanson, M.; Swindells, S.; Pirruccello, S.J.; Ryan, W.L. Stabilization of white blood cells and immunologic markers for extended analysis using flow cytometry. J. Immunol. Methods 2005, 305, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.S.; Lieberman, D.B.; Blanchard, T.; Rader, J.; Zhao, J.; Troxel, A.B.; DeSloover, D.; Fox, A.J.; Daber, R.D.; Kakrecha, B.; et al. A Novel approach for next-generation sequencing of circulating tumor cells. Mol. Genet. Genom. Med. 2016, 4, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Alt, J.R.; Hunsley, B.A.; Williams, T.L.; Fernando, M.R. Stabilization of circulating tumor cells in blood using a collection device with a preservative reagent. Cancer Cell Int. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Williams, T.L.; Fernando, M.R. A novel blood collection device stabilizes cell-free RNA in blood during sample shipping and storage. BMC Res. Notes 2013, 6, 380. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Norton, S.E.; Alt, J.R.; Krzyzanowski, G.D.; Williams, T.L.; Fernando, M.R. Stabilization of cellular RNA in blood during storage at room temperature: A comparison of cell-free RNA BCT with K3EDTA tubes. Mol. Diagn. Ther. 2014, 18, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Flores, L.M.; Kindelberger, D.W.; Ligon, A.H.; Capelletti, M.; Fiorentino, M.; Loda, M.; Cibas, E.S.; Jänne, P.A.; Krop, I.E. Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br. J. Cancer 2010, 102, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Tsui, N.B.Y.; Ng, E.K.O.; Lo, Y.M.D. Stability of endogenous and added RNA in blood specimens, serum, and plasma. Clin. Chem. 2002, 48, 1647. [Google Scholar] [PubMed]
- Denis, M.G.; Knol, A.; Théoleyre, S.; Vallée, A.; Dréno, B. Efficient detection of BRAF mutation in plasma of patients after long-term storage of blood in cell-free DNA blood collection tubes. Clin. Chem. 2015, 61, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, G.; Hrebien, S.; Garcia-Murillas, I.; Cutts, R.J.; Pearson, A.; Tarazona, N.; Fenwick, K.; Kozarewa, I.; Lopez-Knowles, E.; Ribas, R.; et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl. Med. 2015, 7, 313ra182. [Google Scholar] [CrossRef] [PubMed]
- Toro, P.V.; Erlanger, B.; Beaver, J.A.; Cochran, R.L.; VanDenBerg, D.A.; Yakim, E.; Cravero, K.; Chu, D.; Zabransky, D.J.; Wong, H.Y.; et al. Comparison of cell stabilizing blood collection tubes for circulating plasma tumor DNA. Clin. Biochem. 2015, 48, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Diaz, I.M.; Nocon, A.; Mehnert, D.H.; Fredebohm, J.; Diehl, F.; Holtrup, F. Performance of streck cfDNA blood collection tubes for liquid biopsy testing. PLoS ONE 2016, 11, e0166354. [Google Scholar]
- Kang, Q.; Henry, N.L.; Paoletti, C.; Jiang, H.; Vats, P.; Chinnaiyan, A.M.; Hayes, D.F.; Merajver, S.D.; Rae, J.M.; Tewari, M. Comparative analysis of circulating tumor DNA stability in K3EDTA, Streck, and CellSave blood collection tubes. Clin. Biochem. 2016, 49, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.L.; Corcoran, C.; Brown, H.; Sharpe, A.D.; Musilova, M.; Kohlmann, A. Optimised pre-analytical methods improve KRAS mutation detection in circulating tumour DNA (ctDNA) from patients with non-small cell lung cancer (NSCLC). PLoS ONE 2016, 11, e0150197. [Google Scholar] [CrossRef] [PubMed]
| Patient | Hormone Sensitivity Status 1 | CTC Count/mL Blood | AR-V7 Copies/mL Blood | Total AR Copies/mL Blood | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <4 h * | <4 h * | 4 h | 24 h | 48 h | <4 h * | 4 h | 24 h | 48 h | ||
| 1 | CRPC | 9 * | 2 * | 7 | 5 | 3 | 96 * | 155 | 49 | 84 |
| 2 | CRPC | 2 * | 110 * | 126 | 128 | 74 | 19,140 * | 7963 | 6083 | 4191 |
| 3 | CRPC | 6 * | 45 * | 210 | 135 | 102 | 2610 * | 11,392 | 5994 | 3848 |
| Gene | Primers (5′→3′) | Probes (5′→3′) |
|---|---|---|
| Total AR | F: GGA ATT CCT GTG CAT GAA AGC R: CGA TCG AGT TCC TTG ATG TAG TTC | [HEX] CTT CAG CAT TAT TCC AGT G [BHQ1] |
| AR-V7 | F: CGG AAA TGT TAT GAA GCA GGG ATG A R: CTG GTC ATT TTG AGA TGC TTG CAA T | [6FAM] TCT GGG AGA AAA ATT CCG [BHQ1] |
| EpCAM | F: CGT CAA TGC CAG TGT ACT TCA R: TTT CTG CCT TCA TCA CCA AA | [HEX] TAC TGT CAT TTG CTC AAA GC [BHQ1] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luk, A.W.S.; Ma, Y.; Ding, P.N.; Young, F.P.; Chua, W.; Balakrishnar, B.; Dransfield, D.T.; Souza, P.d.; Becker, T.M. CTC-mRNA (AR-V7) Analysis from Blood Samples—Impact of Blood Collection Tube and Storage Time. Int. J. Mol. Sci. 2017, 18, 1047. https://doi.org/10.3390/ijms18051047
Luk AWS, Ma Y, Ding PN, Young FP, Chua W, Balakrishnar B, Dransfield DT, Souza Pd, Becker TM. CTC-mRNA (AR-V7) Analysis from Blood Samples—Impact of Blood Collection Tube and Storage Time. International Journal of Molecular Sciences. 2017; 18(5):1047. https://doi.org/10.3390/ijms18051047
Chicago/Turabian StyleLuk, Alison W. S., Yafeng Ma, Pei N. Ding, Francis P. Young, Wei Chua, Bavanthi Balakrishnar, Daniel T. Dransfield, Paul de Souza, and Therese M. Becker. 2017. "CTC-mRNA (AR-V7) Analysis from Blood Samples—Impact of Blood Collection Tube and Storage Time" International Journal of Molecular Sciences 18, no. 5: 1047. https://doi.org/10.3390/ijms18051047
APA StyleLuk, A. W. S., Ma, Y., Ding, P. N., Young, F. P., Chua, W., Balakrishnar, B., Dransfield, D. T., Souza, P. d., & Becker, T. M. (2017). CTC-mRNA (AR-V7) Analysis from Blood Samples—Impact of Blood Collection Tube and Storage Time. International Journal of Molecular Sciences, 18(5), 1047. https://doi.org/10.3390/ijms18051047







