Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment
Abstract
:1. Introduction
2. Results
2.1. Clinical Features
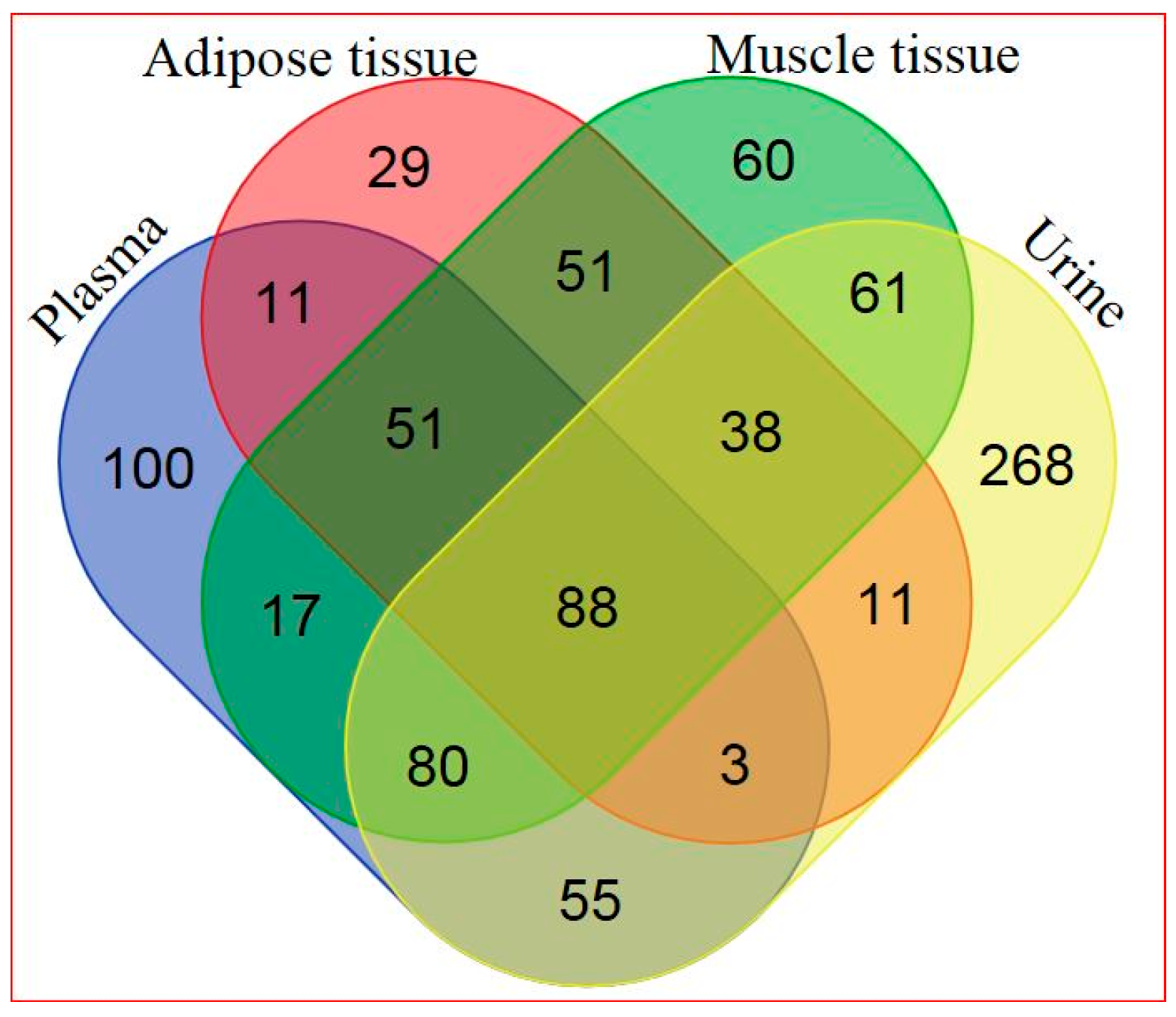
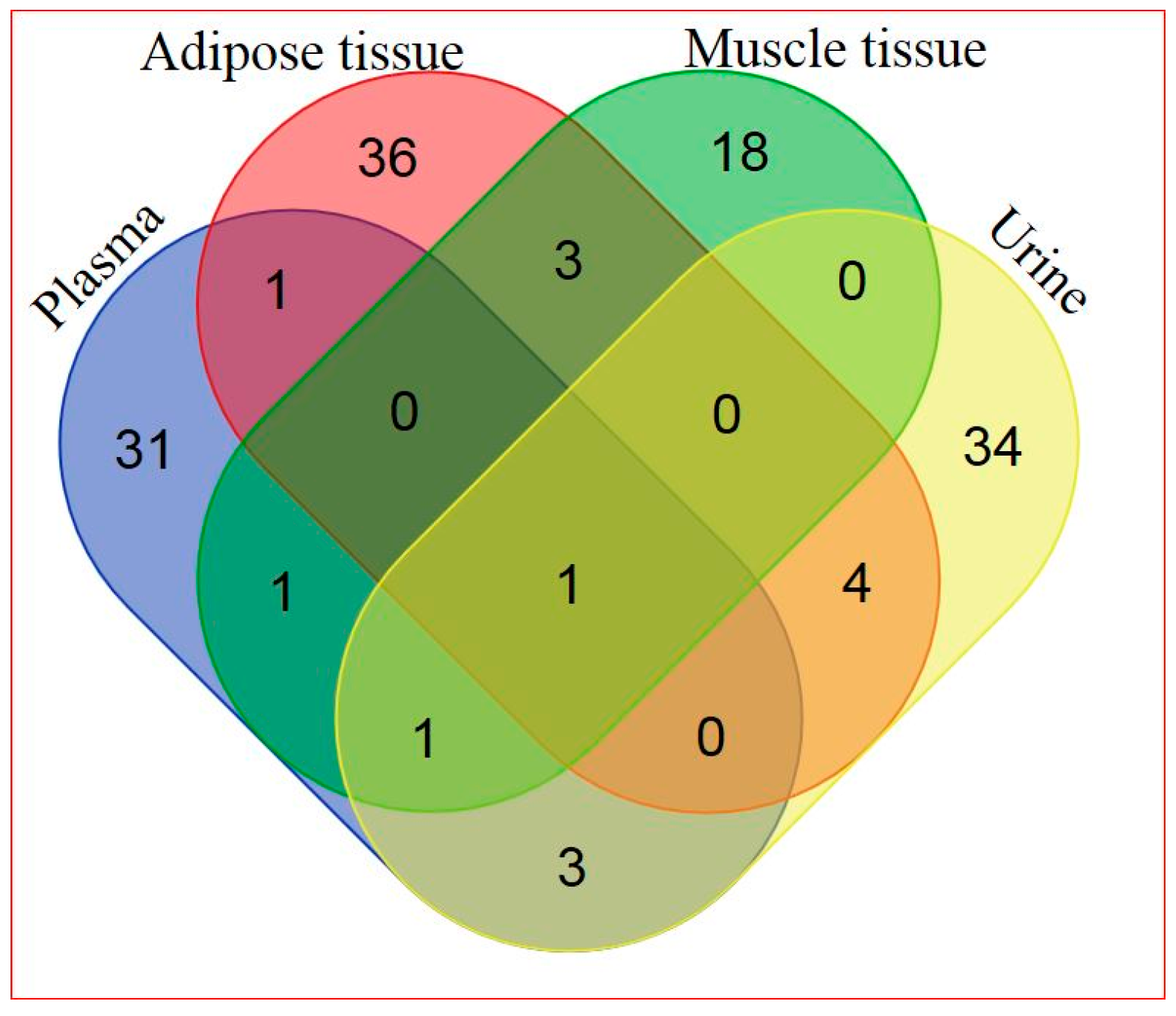
2.2. Global Metabolic Profiling
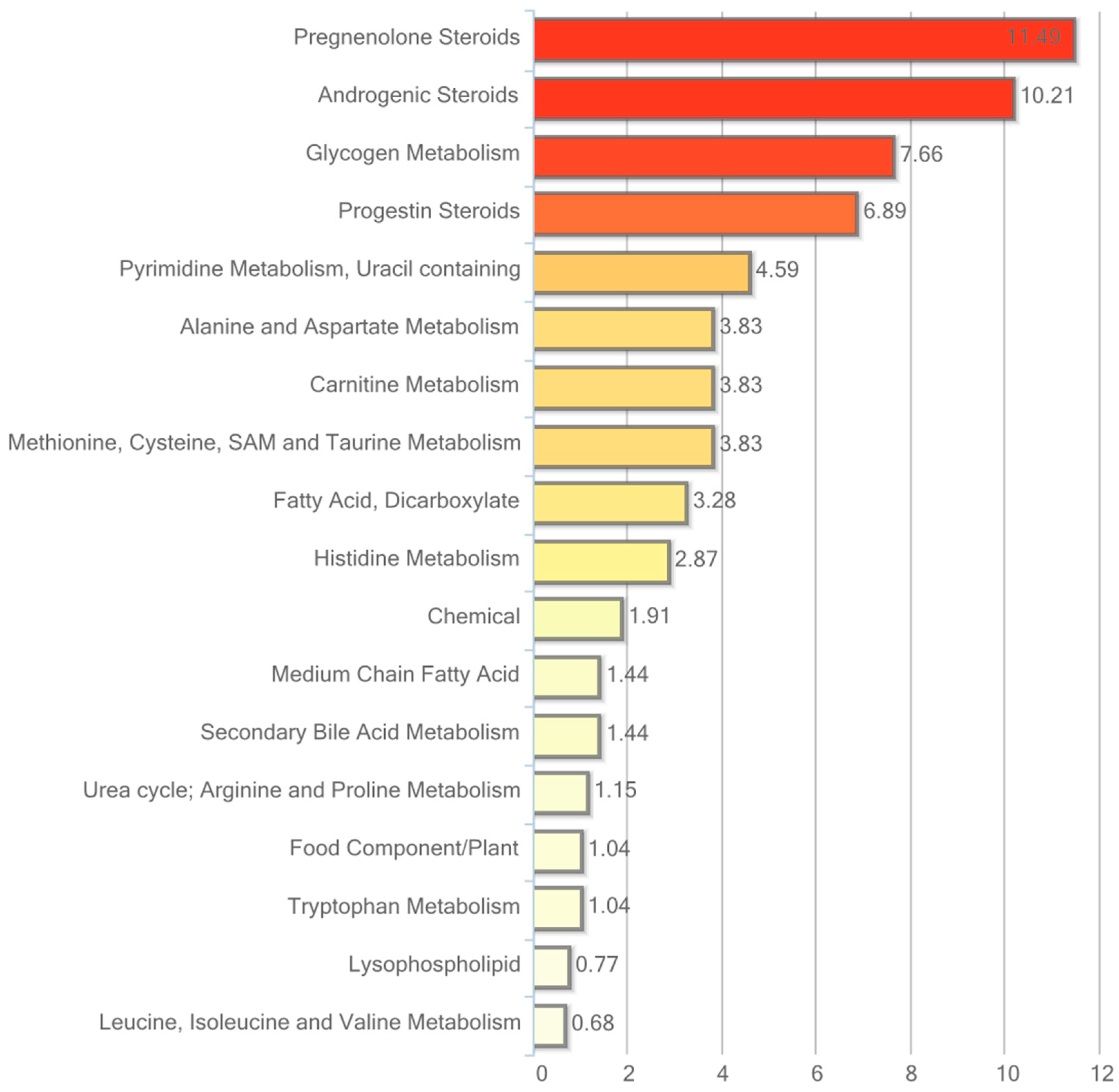
2.3. Metabolic Profiling in Adipose Tissue
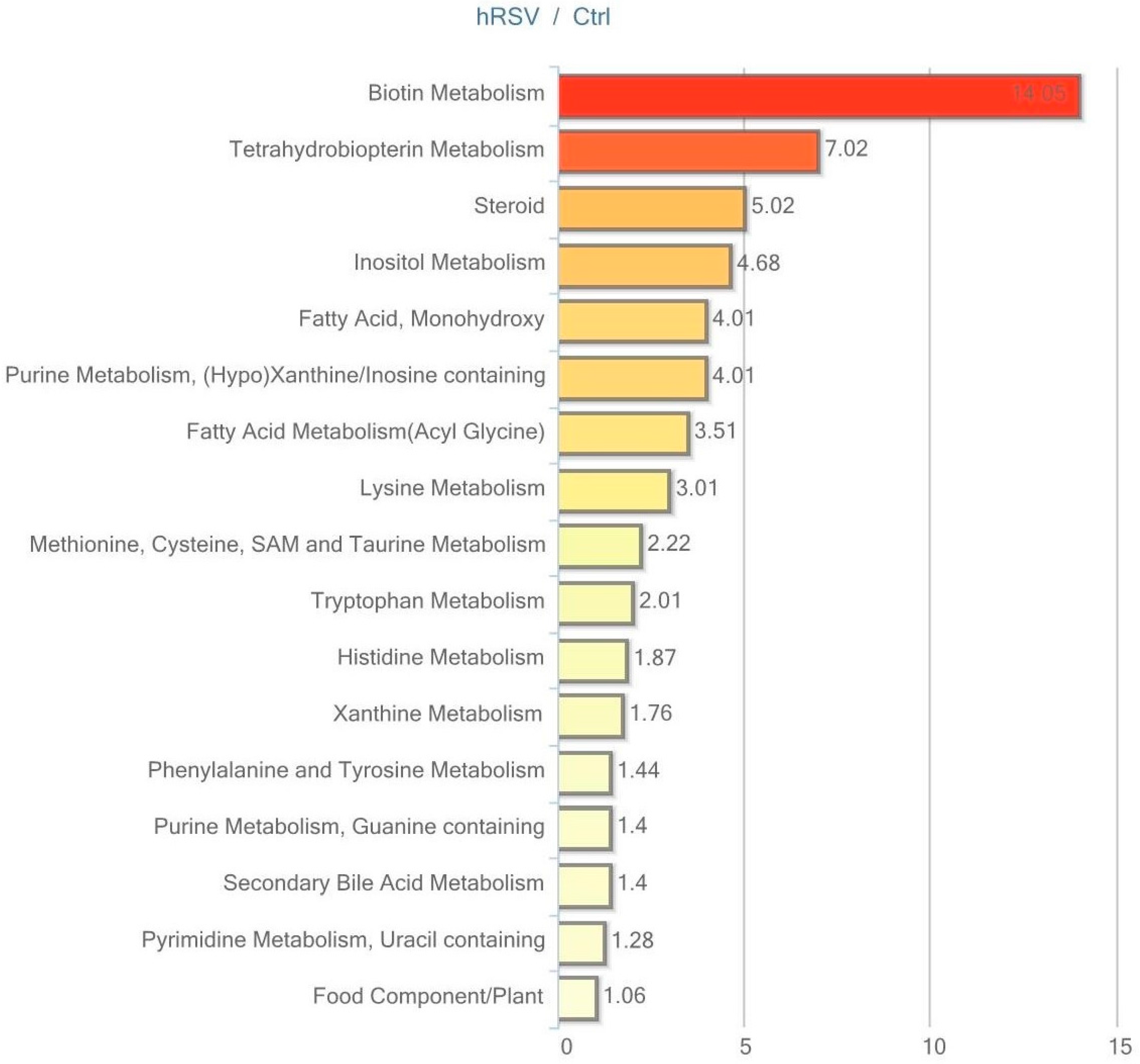
2.4. Metabolic Profiling in Plasma
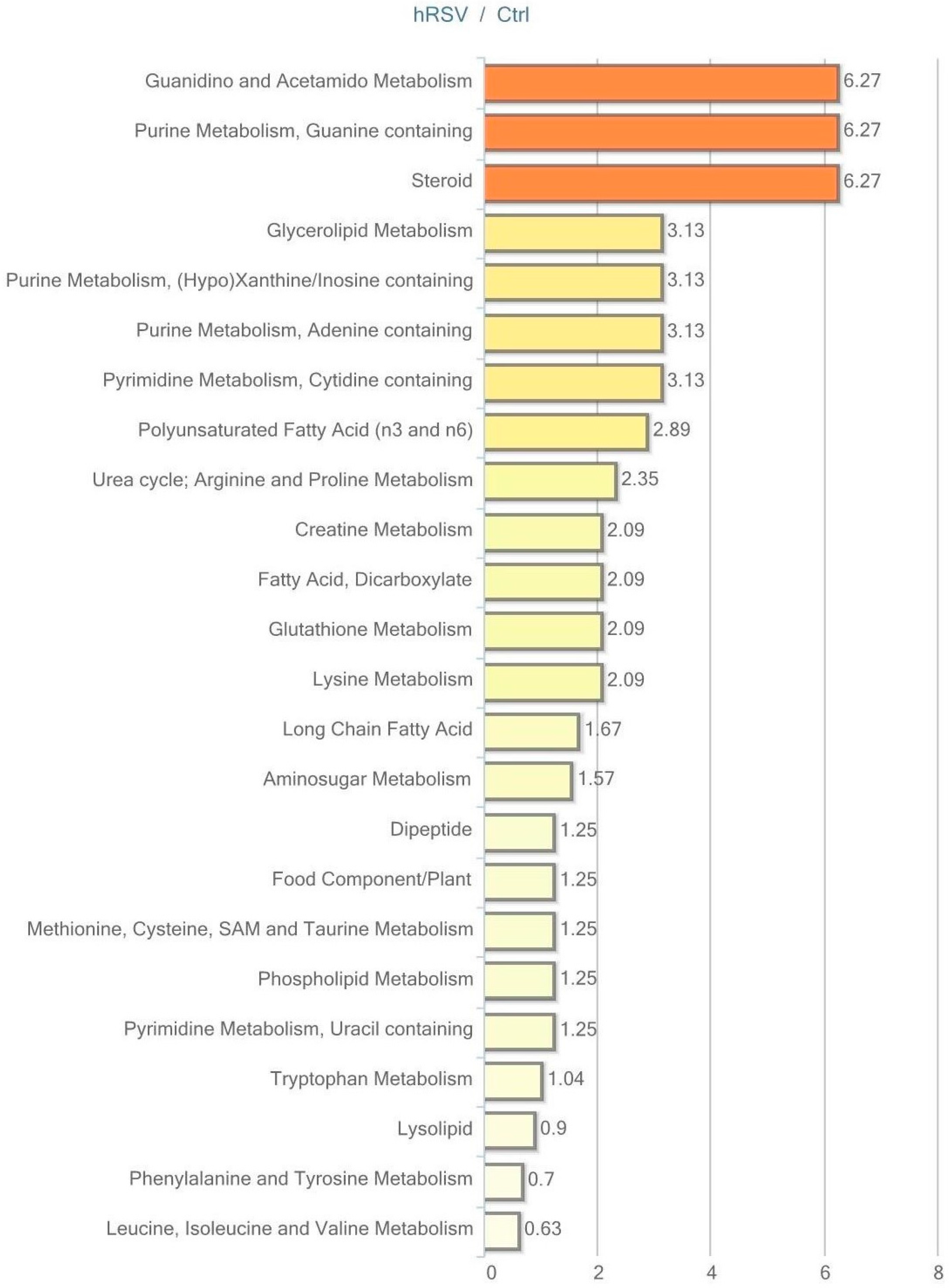
2.5. Metabolic Profiling in Skeletal Muscle Tissue
2.6. Metabolic Profiling in Urine
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Samples
4.3. Metabolomic Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| hRSV | High-dose resveratrol |
| RF | Random forest analysis |
| PEV | Pathway enrichment value |
| DHA | Docosahexaenoate |
| ALA | α-linolenic acid |
| LA | Linoleic acid |
| MetS | Metabolic syndrome |
References
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007, 2, 449–457. [Google Scholar] [CrossRef]
- Olholm, J.; Paulsen, S.K.; Cullberg, K.B.; Richelsen, B.; Pedersen, S.B. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int. J. Obes. 2010, 34, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, M.K.; Dudele, A.; Poulsen, M.M.; Ebbesen, L.H.; Radko, Y.; Christensen, L.P.; Jessen, N.; Richelsen, B.; Lund, S.; Pedersen, S.B. LPS-Enhanced Glucose-Stimulated Insulin Secretion Is Normalized by Resveratrol. PLoS ONE 2016, 11, e0146840. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.-I.; Schechtman, K.B.; Gu, C.; Kunz, I.; Fanelli, F.R.; Patterson, B.W.; et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stødkilde-Jørgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O.L. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2012, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M.; et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.N.; Kilkus, J.M.; Booth, J.N.; Bromley, L.E.; Imperial, J.G.; Penev, P.D. Effects of sleep restriction on the human plasma metabolome. Physiol. Behav. 2013, 122, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Franquesa, A.; Burkart, A.M.; Isganaitis, E.; Patti, M.-E. What Have Metabolomics Approaches Taught Us About Type 2 Diabetes? Curr. Diab. Rep. 2016, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Narath, S.H.; Mautner, S.I.; Svehlikova, E.; Schultes, B.; Pieber, T.R.; Sinner, F.M.; Gander, E.; Libiseller, G.; Schimek, M.G.; Sourij, H.; Magnes, C. An Untargeted Metabolomics Approach to Characterize Short-Term and Long-Term Metabolic Changes after Bariatric Surgery. PLoS ONE 2016, 11, e0161425. [Google Scholar] [CrossRef] [PubMed]
- Fazelzadeh, P.; Hangelbroek, R.W.J.; Tieland, M.; de Groot, L.C.P.G.M.; Verdijk, L.B.; van Loon, L.J.C.; Smilde, A.K.; Alves, R.D.A.M.; Vervoort, J.; Müller, M.; van Duynhoven, J.P.M.; Boekschoten, M.V. The Muscle Metabolome Differs between Healthy and Frail Older Adults. J. Proteome Res. 2016, 15, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Ornstrup, M.J.; Harsløf, T.; Kjær, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol increases bone mineral density and bone alkaline phosphatase in obese men: A randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Kjær, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Jørgensen, J.O.L.; Hougaard, D.M.; Cohen, A.S.; Neghabat, S.; Richelsen, B.; Pedersen, S.B. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate 2015, 75, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Kjær, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stødkilde-Jørgensen, H.; Jessen, N.; Jørgensen, J.O.L.; Richelsen, B.; Pedersen, S.B. No beneficial effects of resveratrol on the metabolic syndrome: A randomized placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2017. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; de Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.M.-D.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Xiao, C.; Morgantini, C.; Szeto, L.; Lewis, G.F. High-dose resveratrol treatment for 2 weeks inhibits intestinal and hepatic lipoprotein production in overweight/obese men. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; Mi, M. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis 2014, 47, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived ω-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Wootton, S.A. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Gooren, L.J.G.; Toorians, A.W.F.T.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [PubMed]
- Taouis, M.; Dagou, C.; Ster, C.; Durand, G.; Pinault, M.; Delarue, J. N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E664–E671. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.M.; Hirsch, J. Does dietary linolenic acid influence blood pressure? Am. J. Clin. Nutr. 1986, 44, 336–340. [Google Scholar] [PubMed]
- Gomez-Zorita, S.; Tréguer, K.; Mercader, J.; Carpéné, C. Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J. Physiol. Biochem. 2013, 69, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.B.; Paulsen, S.K.; Bennetzen, M.F.; Richelsen, B. Low Sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. Int. J. Obes. 2008, 32, 1250–1255. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Niu, Y.; Wang, C.; Wang, M.; Li, Y.; Sun, C. Activated glucose-6-phosphate dehydrogenase is associated with insulin resistance by upregulating pentose and pentosidine in diet-induced obesity of rats. Horm. Metab. Res. 2012, 44, 938–942. [Google Scholar] [PubMed]
- Olesen, J.; Gliemann, L.; Biensø, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Polley, K.R.; Jenkins, N.; O’Connor, P.; McCully, K. Influence of exercise training with resveratrol supplementation on skeletal muscle mitochondrial capacity. Appl. Physiol. Nutr. Metab. 2015, 41, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Scribbans, T.D.; Ma, J.K.; Edgett, B.A.; Vorobej, K.A.; Mitchell, A.S.; Zelt, J.G. E.; Simpson, C.A.; Quadrilatero, J.; Gurd, B.J. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl. Physiol. Nutr. Metab. 2014, 39, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bies, E.; Tung, B.T. Resveratrol primes the effects of physical activity in old mice. Br. J. Nutr. 2016, 116, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Profiling: Metabolomics Based Approach to Unravel Compounds Affecting Human Health. Front. Microbiol. 2016, 7, 1144. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, K.; Pappan, K.L.; Gandhi, A.V.; Saxena, S.; Kang, C.Y.; McMahon, H.; Chipitsyna, G.I.; Tichansky, D.S.; Arafat, H.A. Conserved Metabolic Changes in Nondiabetic and Type 2 Diabetic Bariatric Surgery Patients: Global Metabolomic Pilot Study. J. Diabetes Res. 2016, 2016, 3467403. [Google Scholar] [CrossRef]
- Holmes, E.; Li, J.V.; Marchesi, J.R.; Nicholson, J.K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012, 16, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Heebøll, S.; Thomsen, K.L.; Pedersen, S.B.; Vilstrup, H.; George, J.; Grønbæk, H. Effects of resveratrol in experimental and clinical non-alcoholic fatty liver disease. World J. Hepatol. 2014, 6, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, A.; Tan, K.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr. 2009, 5, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G. M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Placebo (n = 24) | hRSV (n = 21) | p-Value | ||
|---|---|---|---|---|---|
| Baseline | 4 Months | Baseline | 4 Months | ||
| Age, years | 47.8 ± 1.3 | 51.9 ± 1.3 | p < 0.05 | ||
| Body mass index, kg/m2 | 34.1 ± 0.8 | 33.4 ± 0.2 | 33.8 ± 0.7 | 33.7 ± 0.2 | NS |
| HOMA-IR | 4.36 ± 0.5 | 4.19 ± 0.3 | 3.87 ± 0.5 | 4.50 ± 0.3 | NS |
| Systolic blood pressure, mmHg | 150 ± 3 | 142 ± 3 | 146 ± 2 | 140 ± 3 | NS |
| Diastolic blood pressure, mmHg | 91.3 ± 2.1 | 86.0 ± 1.3 | 89.3 ± 1.7 | 87.8 ± 1.4 | NS |
| Superpathway | Sub Pathway | Biochemical Name | hRSV Ctrl | p-Value | q-Value |
|---|---|---|---|---|---|
| Lipid | Long-Chain Fatty Acid | Myristate (14:0) | 1.10 | 0.003 | 0.111 |
| Myristoleate (14:1n5) | 1.67 | 0.017 | 0.182 | ||
| Pentadecanoate (15:0) | 1.01 | 0.977 | 0.816 | ||
| Palmitate (16:0) | 1.10 | 0.033 | 0.240 | ||
| Palmitoleate (16:1n7) | 1.41 | 0.003 | 0.109 | ||
| Margarate (17:0) | 0.98 | 0.500 | 0.672 | ||
| 10-heptadecenoate (17:1n7) | 1.12 | 0.086 | 0.309 | ||
| Stearate (18:0) | 1.02 | 0.610 | 0.731 | ||
| Oleate (18:1n9) | 1.17 | 0.109 | 0.328 | ||
| cis-vaccenate (18:1n7) | 1.17 | 0.304 | 0.538 | ||
| Nonadecanoate (19:0) | 1.00 | 0.866 | 0.794 | ||
| 10-nonadecenoate (19:1n9) | 1.08 | 0.569 | 0.703 | ||
| Arachidate (20:0) | 0.94 | 0.472 | 0.660 | ||
| Eicosenoate (20:1n9 or 11) | 1.12 | 0.335 | 0.566 | ||
| Erucate (22:1n9) | 0.96 | 0.657 | 0.734 | ||
| Polyunsaturated Fatty Acid (n3 and n6) | Stearidonate (18:4n3) | 1.50 | 0.045 | 0.240 | |
| Eicosapentaenoate (EPA; 20:5n3) | 1.25 | 0.093 | 0.311 | ||
| Docosapentaenoate (n3 DPA; 22:5n3) | 1.31 | 0.012 | 0.177 | ||
| Docosahexaenoate (DHA; 22:6n3) | 1.38 | 0.042 | 0.240 | ||
| Linoleate (18:2n6) | 1.24 | 0.021 | 0.203 | ||
| Linolenate [α or γ; (18:3n3 or 6)] | 1.21 | 0.060 | 0.253 | ||
| Dihomo-linolenate (20:3n3 or n6) | 1.19 | 0.065 | 0.261 | ||
| Arachidonate (20:4n6) | 1.17 | 0.107 | 0.328 | ||
| Adrenate (22:4n6) | 1.69 | 0.010 | 0.177 | ||
| Docosapentaenoate (n6 DPA; 22:5n6) | 1.63 | 0.056 | 0.253 | ||
| Docosadienoate (22:2n6) | 1.10 | 0.275 | 0.498 | ||
| Dihomo-linoleate (20:2n6) | 1.11 | 0.216 | 0.443 | ||
| Mead acid (20:3n9) | 1.33 | 0.045 | 0.240 | ||
| Steroid | Dehydroisoandrosterone sulfate (DHEA-S) | 0.40 | 0.013 | 0.177 | |
| 4-androsten-3β,17β-diol disulfate (1) | 0.37 | 0.009 | 0.177 | ||
| Carbohydrate | Glycolysis, Gluconeogenesis, and pyruvate metabolism pathway | 1,5-anhydroglucitol (1,5-AG) | 0.86 | 0.235 | 0.464 |
| Glucose | 0.98 | 0.594 | 0.722 | ||
| Glucose-6-phosphate (G6P) | 1.23 | 0.059 | 0.253 | ||
| Isobar: fructose 1,6-diphosphate, glucose 1,6-diphosphate, myo-inositol 1,4 or 1,3-Diphosphate | 1.16 | 0.159 | 0.394 | ||
| Dihydroxyacetone phosphate (DHAP) | 1.30 | 0.080 | 0.303 | ||
| 3-phosphoglycerate | 1.75 | 0.057 | 0.253 | ||
| Phosphoenolpyruvate (PEP) | 1.39 | 0.128 | 0.357 | ||
| Lactate | 1.04 | 0.396 | 0.599 | ||
| Glycerate | 1.08 | 0.771 | 0.777 | ||
| Pentose phosphate pathway | Sedoheptulose-7-phosphate | 1.27 | 0.051 | 0.253 |
| Superpathway | Sub Pathway | Biochemical Name | hRSV Ctrl | p-Value | q-Value |
|---|---|---|---|---|---|
| Lipid | Steroid | Pregnenolone sulfate | 0.63 | 0.001 | 0.018 |
| 21-hydroxypregnenolone disulfate | 0.41 | <0.001 | <0.001 | ||
| 5α-pregnan-3β,20α-diol disulfate | 0.42 | <0.001 | <0.001 | ||
| 5α-pregnan-3α,20β-diol disulfate 1 | 0.93 | 0.140 | 0.762 | ||
| Pregnen-diol disulfate | 0.53 | 0.001 | 0.015 | ||
| Pregn steroid monosulfate | 0.72 | 0.046 | 0.515 | ||
| Pregnanediol-3-glucuronide | 0.92 | 0.644 | 0.927 | ||
| Cortisol | 0.92 | 0.745 | 0.941 | ||
| Cortisone | 0.93 | 0.285 | 0.824 | ||
| Dehydroisoandrosterone sulfate (DHEA-S) | 0.57 | <0.001 | 0.013 | ||
| Epiandrosterone sulfate | 0.42 | <0.001 | 0.004 | ||
| Androsterone sulfate | 0.40 | <0.001 | 0.004 | ||
| 4-androsten-3β,17β-diol disulfate 1 | 0.51 | <0.001 | 0.016 | ||
| 4-androsten-3β,17β-diol disulfate 2 | 0.49 | <0.001 | <0.001 | ||
| 5α-androstan-3β,17α-diol disulfate | 0.37 | <0.001 | 0.001 | ||
| 5α-androstan-3α,17β-diol disulfate | 0.71 | 0.071 | 0.659 | ||
| 5α-androstan-3β,17β-diol disulfate | 0.37 | <0.001 | 0.002 | ||
| Andro steroid monosulfate 2 | 0.31 | <0.001 | <0.001 | ||
| Estrone 3-sulfate | 0.87 | 0.132 | 0.762 | ||
| Fatty Acid, Dicarboxylate | 2-hydroxyglutarate | 0.98 | 0.660 | 0.934 | |
| Sebacate (decanedioate) | 0.73 | 0.101 | 0.762 | ||
| Dodecanedioate | 0.61 | <0.001 | 0.001 | ||
| Tetradecanedioate | 0.75 | 0.021 | 0.278 | ||
| Hexadecanedioate | 0.85 | 0.206 | 0.762 | ||
| Octadecanedioate | 0.88 | 0.255 | 0.811 | ||
| 3-carboxy-4-methyl-5-propyl-2-furanpropanoate (CMPF) | 0.95 | 0.955 | 0.964 | ||
| Amino Acid | Histidine Metabolism | Histidine | 1.16 | 0.008 | 0.147 |
| 3-methylhistidine | 1.93 | 0.056 | 0.570 | ||
| N-acetyl-3-methylhistidine | 1.23 | 0.084 | 0.721 | ||
| trans-urocanate | 1.38 | 0.128 | 0.762 |
| Superpathway | Sub Pathway | Biochemical Name | hRSV Ctrl | p-Value | q-Value |
|---|---|---|---|---|---|
| Lipid | Steroid | Cortisol | 1.40 | 0.211 | 0.962 |
| Cortisone | 1.14 | 0.778 | 0.987 | ||
| Dehydroisoandrosterone sulfate (DHEA-S) | 0.48 | <0.001 | 0.025 | ||
| Epiandrosterone sulfate | 0.29 | <0.001 | 0.001 | ||
| Androsterone sulfate | 0.24 | <0.001 | 0.001 | ||
| 4-androsten-3β,17β-diol disulfate (1) | 0.46 | 0.002 | 0.125 | ||
| Short-Chain Fatty Acid | Valerate | 0.86 | <0.001 | 0.026 | |
| Medium-Chain Fatty Acid | Caproate (6:0) | 1.09 | 0.442 | 0.962 | |
| Heptanoate (7:0) | 0.82 | <0.001 | 0.032 | ||
| Caprylate (8:0) | 1.08 | 0.619 | 0.962 | ||
| Undecanoate (11:0) | 0.89 | 0.002 | 0.125 | ||
| Long-Chain Fatty Acid | Myristoleate (14:1n5) | 1.78 | 0.252 | 0.962 | |
| Palmitate (16:0) | 0.98 | 0.449 | 0.962 | ||
| Margarate (17:0) | 0.95 | 0.268 | 0.962 | ||
| 10-heptadecenoate (17:1n7) | 1.09 | 0.029 | 0.655 | ||
| Stearate (18:0) | 0.97 | 0.209 | 0.962 | ||
| Oleate (18:1n9) | 1.01 | 0.994 | 0.996 | ||
| cis-vaccenate (18:1n7) | 1.02 | 0.904 | 0.995 | ||
| 10-nonadecenoate (19:1n9) | 0.97 | 0.882 | 0.995 | ||
| Arachidate (20:0) | 0.84 | 0.003 | 0.168 | ||
| Eicosenoate (20:1n9 or 11) | 1.01 | 0.958 | 0.996 | ||
| Erucate (22:1n9) | 0.88 | 0.193 | 0.962 | ||
| Polyunsaturated Fatty Acid (n3 and n6) | Stearidonate (18:4n3) | 1.45 | 0.015 | 0.474 | |
| Eicosapentaenoate (EPA; 20:5n3) | 0.98 | 0.554 | 0.962 | ||
| Docosapentaenoate (n3 DPA; 22:5n3) | 1.04 | 0.511 | 0.962 | ||
| Docosahexaenoate (DHA; 22:6n3) | 1.07 | 0.344 | 0.962 | ||
| Linoleate (18:2n6) | 1.02 | 0.714 | 0.980 | ||
| Linolenate [α or γ; (18:3n3 or 6)] | 1.16 | 0.065 | 0.962 | ||
| Dihomo-linolenate (20:3n3 or n6) | 1.02 | 0.873 | 0.995 | ||
| Arachidonate (20:4n6) | 1.00 | 0.990 | 0.996 | ||
| Docosapentaenoate (n6 DPA; 22:5n6) | 0.99 | 0.994 | 0.996 | ||
| Docosadienoate (22:2n6) | 0.93 | 0.680 | 0.962 | ||
| Dihomo-linoleate (20:2n6) | 0.83 | 0.287 | 0.962 | ||
| Mead acid (20:3n9) | 0.93 | 0.619 | 0.962 |
| Superpathway | Sub Pathway | Biochemical Name | hRSV Ctrl | p-Value | q-Value |
|---|---|---|---|---|---|
| Lipid | Steroid | 21-hydroxypregnenolone disulfate | 0.50 | 0.012 | 0.313 |
| Pregnen-diol disulfate | 0.79 | 0.421 | 0.861 | ||
| Pregn steroid monosulfate | 2.10 | 0.008 | 0.281 | ||
| Pregnanediol-3-glucuronide | 0.87 | 0.910 | 0.938 | ||
| Cortisol | 1.29 | 0.734 | 0.915 | ||
| Cortisol glucuronide | 1.36 | 0.600 | 0.893 | ||
| Cortisone | 1.13 | 0.757 | 0.915 | ||
| Tetrahydrocortisone | 1.54 | 0.270 | 0.762 | ||
| Dehydroisoandrosterone sulfate (DHEA-S) | 3.95 | <0.001 | 0.001 | ||
| 16a-hydroxy DHEA 3-sulfate | 1.04 | 0.201 | 0.735 | ||
| Epiandrosterone | 0.87 | 0.967 | 0.938 | ||
| Epiandrosterone sulfate | 1.43 | 0.036 | 0.575 | ||
| Androsterone sulfate | 1.22 | 0.171 | 0.724 | ||
| 4-androsten-3β,17β-diol monosulfate (1) | 3.75 | <0.001 | 0.001 | ||
| 4-androsten-3β,17β-diol monosulfate (2) | 3.50 | 0.003 | 0.149 | ||
| 4-androsten-3α,17α-diol monosulfate (2) | 6.78 | <0.001 | 0.001 | ||
| 4-androsten-3β,17β-diol disulfate (1) | 0.72 | 0.804 | 0.920 | ||
| 4-androsten-3β,17β-diol disulfate (2) | 0.89 | 0.736 | 0.915 | ||
| 5α-androstan-3β,17α-diol disulfate | 0.20 | 0.002 | 0.127 | ||
| 5α-androstan-3β,17β-diol disulfate | 0.68 | 0.414 | 0.861 | ||
| Andro steroid monosulfate (1) | 0.45 | 0.004 | 0.170 | ||
| Testosterone sulfate | 3.74 | 0.009 | 0.281 | ||
| 11-ketoetiocholanolone glucuronide | 0.98 | 0.793 | 0.920 | ||
| Etiocholanolone glucuronide | 0.85 | 0.766 | 0.915 | ||
| 17α-hydroxypregnanolone glucuronide | 1.10 | 0.216 | 0.747 | ||
| 5β-pregnan-3α,21-diol-11,20-dione 21-Glucosiduronate | 1.51 | 0.604 | 0.893 | ||
| 11-ketoetiocholanolone sulfate | 1.23 | 0.957 | 0.938 | ||
| Dehydroepiandrosterone glucuronide | 0.83 | 0.585 | 0.881 | ||
| Amino Acid | Tryptophan Metabolism | Tryptophan | 0.99 | 0.908 | 0.938 |
| N-acetyltryptophan | 1.37 | 0.194 | 0.726 | ||
| Tryptamine | 0.37 | <0.001 | 0.010 | ||
| Indolelactate | 1.56 | 0.050 | 0.613 | ||
| Indoleacetate | 0.58 | 0.201 | 0.735 | ||
| 3-indoxyl sulfate | 1.39 | 0.124 | 0.724 | ||
| Kynurenine | 1.29 | 0.637 | 0.905 | ||
| Kynurenate | 1.17 | 0.325 | 0.793 | ||
| Anthranilate | 1.16 | 0.446 | 0.861 | ||
| 3-hydroxykynurenine | 1.43 | 0.480 | 0.874 | ||
| 3-hydroxyanthranilate | 1.59 | 0.057 | 0.638 | ||
| Xanthurenate | 1.23 | 0.231 | 0.748 | ||
| Picolinate | 1.16 | 0.180 | 0.724 | ||
| 5-hydroxytryptophan | 1.11 | 0.383 | 0.848 | ||
| 5-hydroxyindoleacetate | 1.02 | 0.563 | 0.881 | ||
| Serotonin (5HT) | 1.03 | 0.782 | 0.920 | ||
| Indoleacetylglutamine | 0.64 | 0.824 | 0.920 | ||
| Tryptophan betaine | 1.14 | 0.976 | 0.940 | ||
| Indole-3-carboxylic acid | 0.93 | 0.696 | 0.914 | ||
| C-glycosyltryptophan | 1.03 | 0.693 | 0.914 | ||
| N-acetylkynurenine (2) | 2.14 | 0.047 | 0.613 | ||
| Phenylalanine and Tyrosine Metabolism | Phenylalanine | 1.11 | 0.588 | 0.881 | |
| N-acetylphenylalanine | 1.31 | 0.185 | 0.726 | ||
| Phenyllactate (PLA) | 1.86 | 0.148 | 0.724 | ||
| 4-hydroxyphenylacetate | 1.02 | 0.915 | 0.938 | ||
| 3-hydroxyphenylacetate | 1.52 | 0.096 | 0.724 | ||
| Phenylacetylglycine | 1.13 | 0.801 | 0.920 | ||
| Phenylacetylglutamine | 0.97 | 0.720 | 0.914 | ||
| Tyrosine | 1.06 | 0.693 | 0.914 | ||
| N-acetyltyrosine | 1.47 | 0.051 | 0.613 | ||
| 4-hydroxycinnamate | 1.83 | 0.172 | 0.724 | ||
| Tyramine | 0.60 | 0.001 | 0.075 | ||
| m-tyramine | 0.99 | 0.923 | 0.938 | ||
| 4-hydroxyphenylpyruvate | 2.23 | 0.012 | 0.313 | ||
| 3-(4-hydroxyphenyl)lactate | 1.33 | 0.280 | 0.762 | ||
| Phenol sulfate | 1.74 | 0.016 | 0.365 | ||
| p-cresol sulfate | 0.87 | 0.465 | 0.874 | ||
| o-cresol sulfate | 1.41 | 0.301 | 0.782 | ||
| Dopamine | 0.37 | 0.998 | 0.947 | ||
| Vanillylmandelate (VMA) | 1.08 | 0.392 | 0.858 | ||
| 3-methoxytyrosine | 1.08 | 0.967 | 0.938 | ||
| 3-methoxytyramine | 1.07 | 0.226 | 0.748 | ||
| 3-methoxytyramine sulfate | 0.96 | 0.301 | 0.782 | ||
| 3,4-dihydroxyphenylacetate | 1.32 | 0.107 | 0.724 | ||
| Homovanillate (HVA) | 1.33 | 0.040 | 0.583 | ||
| Homovanillate sulfate | 0.98 | 0.565 | 0.881 | ||
| Gentisate | 1.37 | 0.125 | 0.724 | ||
| Phenylpropionylglycine | 0.95 | 0.946 | 0.938 | ||
| 3-[3-(sulfooxy)phenyl]propanoic acid | 0.66 | 0.602 | 0.893 | ||
| 3-(3-hydroxyphenyl)propionate | 1.01 | 0.903 | 0.938 | ||
| 5-hydroxymethyl-2-furoic acid | 0.77 | 0.442 | 0.861 | ||
| 2-hydroxyphenylacetate | 1.31 | 0.075 | 0.721 | ||
| Phenylacetylcarnitine | 0.82 | 0.542 | 0.881 | ||
| Dopamine sulfate (1) | 0.48 | 0.963 | 0.938 | ||
| Dopamine sulfate (2) | 0.46 | 0.893 | 0.938 | ||
| p-cresol-glucuronide | 0.95 | 0.704 | 0.914 | ||
| Tyramine O-sulfate | 0.69 | 0.202 | 0.735 | ||
| Vanillic alcohol sulfate | 0.91 | 0.271 | 0.762 | ||
| 4-hydroxycinnamate sulfate | 0.47 | 0.240 | 0.748 | ||
| 3,4-dihydroxyphenylacetate sulfate | 0.69 | 0.548 | 0.881 | ||
| Histidine Metabolism | Histidine | 1.10 | 0.402 | 0.861 | |
| N-acetylhistidine | 1.12 | 0.152 | 0.724 | ||
| 1-methylhistidine | 1.09 | 0.712 | 0.914 | ||
| 3-methylhistidine | 2.02 | 0.080 | 0.724 | ||
| N-acetyl-3-methylhistidine | 2.10 | 0.037 | 0.576 | ||
| N-acetyl-1-methylhistidine | 1.07 | 0.482 | 0.874 | ||
| Hydantoin-5-propionic acid | 1.01 | 0.954 | 0.938 | ||
| trans-urocanate | 1.14 | 0.280 | 0.762 | ||
| cis-urocanate | 1.11 | 0.632 | 0.905 | ||
| Formiminoglutamate | 1.03 | 0.984 | 0.943 | ||
| Imidazole propionate | 1.67 | 0.010 | 0.290 | ||
| Imidazole lactate | 1.31 | 0.280 | 0.762 | ||
| 1-methylimidazoleacetate | 1.13 | 0.424 | 0.861 | ||
| 4-imidazoleacetate | 1.16 | 0.703 | 0.914 | ||
| N-acetylhistamine | 0.80 | 0.554 | 0.881 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korsholm, A.S.; Kjær, T.N.; Ornstrup, M.J.; Pedersen, S.B. Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment. Int. J. Mol. Sci. 2017, 18, 554. https://doi.org/10.3390/ijms18030554
Korsholm AS, Kjær TN, Ornstrup MJ, Pedersen SB. Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment. International Journal of Molecular Sciences. 2017; 18(3):554. https://doi.org/10.3390/ijms18030554
Chicago/Turabian StyleKorsholm, Anne Sofie, Thomas Nordstrøm Kjær, Marie Juul Ornstrup, and Steen Bønløkke Pedersen. 2017. "Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment" International Journal of Molecular Sciences 18, no. 3: 554. https://doi.org/10.3390/ijms18030554
APA StyleKorsholm, A. S., Kjær, T. N., Ornstrup, M. J., & Pedersen, S. B. (2017). Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment. International Journal of Molecular Sciences, 18(3), 554. https://doi.org/10.3390/ijms18030554





