Identification of Differentially Expressed miRNAs in Colorado Potato Beetles (Leptinotarsa decemlineata (Say)) Exposed to Imidacloprid
Abstract
:1. Introduction
2. Results
2.1. Small RNA Sequence Analysis
2.2. miRNA Expression in Control and Imidacloprid-Treated L. decemlineata by High-Throughput Sequencing
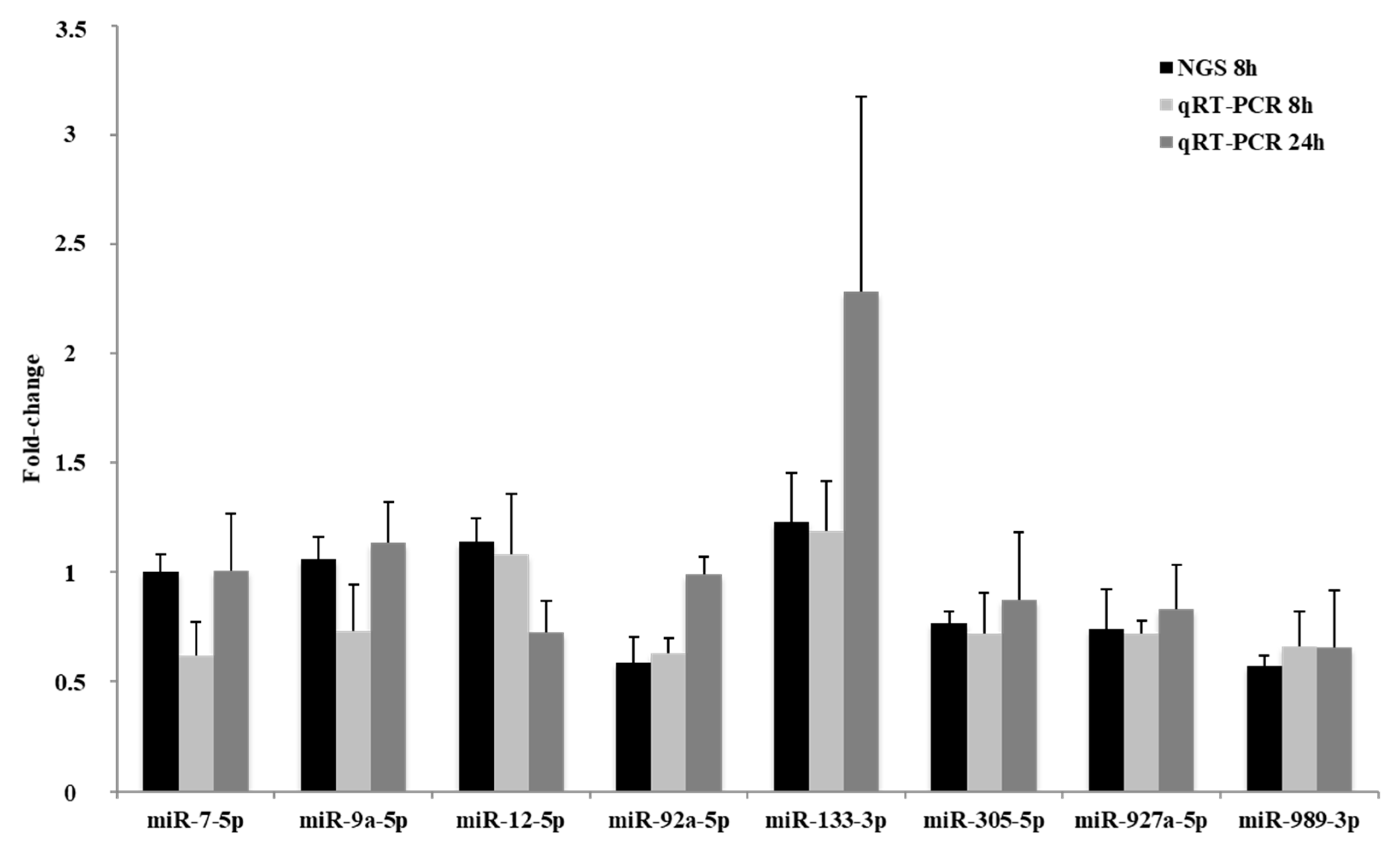
2.3. qRT-PCR Quantification of Selected miRNAs
2.4. Prediction of miRNA Targets and Functional Classification
3. Discussion
4. Materials and Methods
4.1. Insect Collection and Treatment
4.2. Small RNA Isolation
4.3. Small RNA Library Construction and Sequencing
4.4. cDNA Synthesis
4.5. PCR and qRT-PCR Amplification of miRNAs
4.6. miRNA Transcript Targets Prediction
4.7. Quantification and Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weber, D. Colorado beetle: Pest on the move. Pestic. Outlook 2003, 14, 256–259. [Google Scholar] [CrossRef]
- Radcliffe, E.B.; Lagnaoui, A. Insect pests in potato: Insects. In Potato Biology and Biotechnology: Advances and Perspectives; Vreughenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Taylor, M., MacKerron, D., Ross, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 543–567. [Google Scholar]
- Hare, D.J. Impact of defoliation by the Colorado potato beetle on potato yields. J. Econ. Entomol. 1980, 73, 369–373. [Google Scholar] [CrossRef]
- Shields, E.J.; Wyman, J.A. Effect of defoliation at specific growth stages on potato yields. J. Econ. Entomol. 1984, 77, 1194–1199. [Google Scholar] [CrossRef]
- Jiang, W.H.; Wang, Z.T.; Xiong, M.H.; Lu, W.P.; Liu, P.; Guo, W.C.; Li, G.Q. Insecticide resistance status of Colorado Potato Beetle (Coleoptera: Chrysomelidae) adults in northern Xinjiang Uygur autonomous region. J. Econ. Entomol. 2010, 103, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Alyokhin, A.; Baker, M.; Mota-Sanchez, D.; Dively, G.; Grafius, E. Colorado Potato Beetle Resistance to Insecticides. Am. J. Potato Res. 2008, 85, 395–413. [Google Scholar] [CrossRef]
- Szendrei, Z.; Grafius, E.; Byrne, A.; Ziegler, A. Resistance to neonicotinoid insecticides in field populations of the Colorado potato beetle (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2012, 68, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.Z.; Bishop, B.A.; Grafius, E.J. Inheritance and synergism of resistance to imidacloprid in the Colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2000, 93, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.; Schoville, S.; Peterson, N.; Lan, Q.; Groves, R.L. Characterizing Molecular Mechanisms of Imidacloprid Resistance in Select Populations of Leptinotarsa decemlineata in the Central Sands Region of Wisconsin. PLoS ONE 2016, 11, e0147844. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Moural, T.W.; Nelson, D.R.; Palli, S.R. A specialist herbivore pest adaptation to xenobiotics through up-regulation of multiple Cytochrome P450s. Sci. Rep. 2016, 6, 20421. [Google Scholar] [CrossRef] [PubMed]
- Barrio, L.; Dekanty, A.; Milán, M. MicroRNA-mediated regulation of Dp53 in the Drosophila fat body contributes to metabolic adaptation to nutrient deprivation. Cell Rep. 2014, 8, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.J.; Storey, K.B.; Morin, P.J. Expression of miRNAs in response to freezing and anoxia stresses in the freeze tolerant fly Eurosta solidaginis. Cryobiology 2015, 71, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Guo, Q.; Wang, W.; Hu, S.; Fang, F.; Lv, Y.; Yu, J.; Zou, F.; Lei, Z.; Ma, K.; et al. Identification of differentially expressed microRNAs in Culex pipiens and their potential roles in pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 55, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Zhou, X.; Gao, X.; Liang, P. miRNAs regulated overexpression of ryanodine receptor is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Sci. Rep. 2015, 5, 14095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z.; Wu, Q.; Peng, M.; Liu, Y.; Liu, X.; Shi, L.; Shen, G.; Pan, Y.; He, L. Identification of Differentially Expressed microRNAs between the Fenpropathrin Resistant and Susceptible Strains in Tetranychus cinnabarinus. PLoS ONE 2016, 11, e0152924. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Tian, M.; Guo, Q.; Ma, L.; Zhou, D.; Shen, B.; Sun, Y.; Zhu, C. MiR-932 Regulates Pyrethroid Resistance in Culex pipiens pallens (Diptera: Culicidae). J. Med. Entomol. 2016, 53, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, B.; Hu, H.; Li, X.; Guo, Q.; Zou, F.; Liu, X.; Hu, M.; Guo, J.; Ma, L.; et al. MiR-285 targets P450 (CYP6N23) to regulate pyrethroid resistance in Culex pipiens pallens. Parasitol. Res. 2016, 115, 4511–4517. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Rueda, A.; Barturen, G.; Lebrón, R.; Gómez-Martín, C.; Alganza, Á.; Oliver, J.L.; Hackenberg, M. sRNAtoolbox: An integrated collection of small RNA research tools. Nucleic Acids Res. 2015, 43, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, H.; Zhang, Y.; Liu, C.; Liu, Z. Transcriptional Changes in nAChRs, Interactive Proteins and P450s in Locusta migratoria manilensis (Orthoptera: Acrididae) CNS in Response to High and Low Oral Doses of Imidacloprid. J. Insect Sci. 2015, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.L.; Zhang, M.; Wang, K.; Qiao, X.F.; Chen, M.H. Molecular cloning, expression pattern of multidrug resistance associated protein 1 (MRP1, ABCC1) gene, and the synergistic effects of verapamil on toxicity of two insecticides in the bird cherry-oat aphid. Arch. Insect Biochem. Physiol. 2016, 92, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Pu, J.; Chen, F.; Wang, J.; Han, Z. Multiple ATP-binding cassette transporters are involved in insecticide resistance in the small brown planthopper, Laodelphax striatellus. Insect Mol. Biol. 2017, 26, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, S.; Werk, A.N.; Cascorbi, I. MicroRNAs and their relevance to ABC transporters. Br. J. Clin. Pharmacol. 2014, 77, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Hogan, E.M.; Casserly, A.P.; Scofield, M.D.; Mou, Z.; Zhao-Shea, R.; Johnson, C.W.; Tapper, A.R.; Gardner, P.D. miRNAome analysis of the mammalian neuronal nicotinic acetylcholine receptor gene family. RNA 2014, 20, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Li, X.; Hu, H.; Zhou, D.; Sun, Y.; Ma, L.; Zhu, C.; Shen, B. Pyrethroid-resistance is modulated by miR-92a by targeting CpCPR4 in Culex pipiens pallens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 203, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, X.; Liu, Y.; Gao, X.; Liang, P. Global identification of microRNAs associated with chlorantraniliprole resistance in diamondback moth Plutella xylostella (L.). Sci. Rep. 2017, 7, 40713. [Google Scholar] [CrossRef] [PubMed]
- Derecka, K.; Blythe, M.J.; Malla, S.; Genereux, D.P.; Guffanti, A.; Pavan, P.; Moles, A.; Snart, C.; Ryder, T.; Ortori, C.A.; et al. Transient exposure to low levels of insecticide affects metabolic networks of honeybee larvae. PLoS ONE 2013, 8, e68191. [Google Scholar] [CrossRef] [PubMed]
- Kugler, J.M.; Verma, P.; Chen, Y.W.; Weng, R.; Cohen, S.M. miR-989 is required for border cell migration in the Drosophila ovary. PLoS ONE 2013, 8, e67075. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Zheng, Y.; Sumathipala, N.; Jiang, H.; Arrese, E.L.; Soulages, J.L.; Zhang, W.; Sunkar, R. Deep sequencing of small RNA libraries reveals dynamic regulation of conserved and novel microRNAs and microRNA-stars during silkworm development. BMC Genom. 2010, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Vilmos, P.; Bujna, A.; Szuperák, M.; Havelda, Z.; Várallyay, É.; Szabad, J.; Kucerova, L.; Somogyi, K.; Kristó, I.; Lukácsovich, T.; et al. Viability, longevity, and egg production of Drosophila melanogaster are regulated by the miR-282 microRNA. Genetics 2013, 195, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Puinean, A.M.; Foster, S.P.; Oliphant, L.; Denholm, I.; Field, L.M.; Millar, N.S.; Williamson, M.S.; Bass, C. Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet. 2010, 6, e1000999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, T.; Pan, Y.; Gao, X.; Xi, J.; Zhang, L.; Ma, K.; Wu, Y.; Zhang, J.; Shang, Q. Reduced abundance of the CYP6CY3-targeting let-7 and miR-100 miRNAs accounts for host adaptation of Myzus persicae nicotianae. Insect Biochem. Mol. Biol. 2016, 75, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Mittner, F.; Fent, K. Molecular Effects of Neonicotinoids in HoneyBees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.A.; Chandor-Proust, A.; Dauphin-Villemant, C.; Poupardin, R.; Jones, C.M.; Strode, C.; Régent-Kloeckner, M.; David, J.P.; Reynaud, S. Molecular mechanisms associated with increased tolerance to the neonicotinoid insecticide imidacloprid in the dengue vector Aedes aegypti. Aquat. Toxicol. 2013, 126, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Kalajdzic, P.; Oehler, S.; Reczko, M.; Pavlidi, N.; Vontas, J.; Hatzigeorgiou, A.G.; Savakis, C. Use of mutagenesis, genetic mapping and next generation transcriptomics to investigate insecticide resistance mechanisms. PLoS ONE 2012, 7, e40296. [Google Scholar] [CrossRef] [PubMed]
- Sawczyn, T.; Dolezych, B.; Klosok, M.; Augustyniak, M.; Stygar, D.; Buldak, R.J.; Kukla, M.; Michalczyk, K.; Karcz-Socha, I.; Zwirska-Korczala, K. Alteration of carbohydrates metabolism and midgut glucose absorption in Gromphadorhina portentosa after subchronic exposure to imidacloprid and fenitrothion. J. Environ. Sci. Health Part A 2012, 47, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.J.; Fu, K.Y.; Lü, F.G.; Wang, X.X.; Guo, W.C.; Li, G.Q. Knocking down a putative Δ(1)-pyrroline-5-carboxylate dehydrogenase gene by RNA interference inhibits flight and causes adult lethality in the Colorado potato beetle Leptinotarsa decemlineata (Say). Pest Manag. Sci. 2015, 71, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.D.; Frigault, J.J.; Lyons, P.J.; Crapoulet, N.; Boquel, S.; Storey, K.B.; Morin, P.J. Amplification and quantification of cold-associated microRNAs in the Colorado potato beetle (Leptinotarsa decemlineata) agricultural pest. Insect Mol. Biol. 2017, 26, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.J.; Crapoulet, N.; Storey, K.B.; Morin, P.J. Identification and profiling of miRNAs in the freeze-avoiding gall moth Epiblema scudderiana via next-generation sequencing. Mol. Cell. Biochem. 2015, 410, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lyons, P.J.; Govaere, L.; Crapoulet, N.; Storey, K.B.; Morin, P.J. Characterization of cold-associated microRNAs in the freeze-tolerant gall fly Eurosta solidaginis using high-throughput sequencing. Comp. Biochem. Phys. D 2016, 20, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Kornfield, S.F.; Storey, K.B. Amplification and sequencing of mature microRNAs in uncharacterized animal models using stem-loop reverse transcription-polymerase chain reaction. Anal. Biochem. 2011, 416, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Lang-Ouellette, D.; Morin, P.J. Differential expression of miRNAs with metabolic implications in hibernating thirteen-lined ground squirrels, Ictidomys tridecemlineatus. Mol. Cell. Biochem. 2014, 394, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Ledet-Jensen, J.; Ørntoft, T. Normalization of real-time quantitative RT-PCR data: A model based variance estimation approach to identify genes suited for normalization, applied bladder- and colon-cancer data-sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Stark, A.; Johnston, W.K.; Kellis, M.; Bartel, D.P.; Lai, E.C. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 2007, 17, 1850–1864. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Biogeosciences 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
| Types of RNAs | Unique Reads Control/Treated | Percent Control/Treated | Read Count Control/Treated | Percent Control/Treated |
|---|---|---|---|---|
| Total reads | 8,825,610/6,211,853 | 100%/100% | 22,534,030/13,763,160 | 100%/100% |
| miRNAs | 26,447/24,221 | 0.30%/0.39% | 1,558,719/1,511,201 | 6.92%/10.98% |
| tRNAs | 40,144/22,115 | 0.45%/0.36% | 326,281/125,085 | 1.45%/0.91% |
| snRNAs | 4705/2937 | 0.05%/0.05% | 11,161/6302 | 0.05%/0.05% |
| snoRNAs | 4735/3616 | 0.05%/0.06% | 10,813/6910 | 0.05%/0.05% |
| rRNAs | 102,238/64,189 | 1.16%/1.03% | 619,781/172,969 | 2.75%/1.26% |
| miRNAs | miRNA Sequences | Normalized Expression |
|---|---|---|
| miR-14-3p | UCAGUCUUUUUCUCUCUCCUAU | 88,717.57 |
| miR-8-3p | UAAUACUGUCAGGUAAAGAUGUC | 73,723.79 |
| miR-276-3p | UAGGAACUUCAUACCGUGCUCU | 54,681.67 |
| miR-317-3p | UGAACACAGCUGGUGGUAUCUCAGU | 39,445.82 |
| miR-1-3p | UGGAAUGUAAAGAAGUAUGGAG | 16,976.97 |
| miR-2a-3p | UAUCACAGCCAGCUUUGAUGAGC | 16,697.46 |
| miR-281-5p | AAGAGAGCUAUCCGUCGACAGU | 16,606.36 |
| miR-1175-3p | UGAGAUUCAACUCCUCCAUCUC | 14,289.62 |
| miR-13b-3p | UAUCACAGCCAUUUUGACGAGU | 14,017.42 |
| bantam-3p | UGAGAUCAUUGUGAAAGCUGAUU | 13,166.75 |
| miRNAs | miRNA Sequences | Normalized Expression Treated/Control | Log2 Fold-Change Treated/Control | |
|---|---|---|---|---|
| miR-iab-8-3p | AGGAUACAUUCAGUAUACG | 27.77 | 53.71 | 0.95 |
| miR-252a-3p | CCUGCUGCUCAAGUGCUUAUC | 12.40 | 21.64 | 0.80 |
| miR-282-5p | UAGCCUCUCCUAGGCUUUGUCU | 13.23 | 20.32 | 0.62 |
| miR-iab-8-5p | UUACGUAUACUGAAGGUAUACCGGAC | 30.54 | 44.06 | 0.53 |
| miR-1000-5p | AUAUUGUCCUGUCACAGC | 63.29 | 86.10 | 0.44 |
| miR-3849-5p | UGACAUUUUAACCAUAGUGCU | 59.97 | 81.16 | 0.44 |
| miR-193-3p | UACUGGCCUGUUAAGUCCCAAGU | 57.31 | 77.14 | 0.43 |
| miR-7-3p | CAAGGAAUCACUAAUCAUCCCAC | 36.79 | 49.25 | 0.42 |
| miR-124-3p | UAAGGCACGCGGUGAAUGCCAAG | 183.64 | 239.11 | 0.38 |
| miR-970-3p | UCAUAAGACACACGCGGCUAU | 337.13 | 427.79 | 0.34 |
| miR-276-5p | AGCGAGGUAUAGAGUUCCUACGUG | 2120.16 | 2672.97 | 0.33 |
| let-7-5p | UGAGGUAGUAGGUUGUAUAG | 4613.21 | 5776.29 | 0.32 |
| miR-1-3p | UGGAAUGUAAAGAAGUAUGGAG | 15,177.88 | 18,776.07 | 0.31 |
| miR-133-3p | UUGGUCCCCUUCAACCAGCUGU | 1771.68 | 2187.69 | 0.30 |
| miR-263b-5p | CUUGGCACUGGAAGAAUUCAC | 95.91 | 76.77 | −0.32 |
| miR-281-5p | AAGAGAGCUAUCCGUCGACAGU | 18,510.91 | 14,701.80 | −0.33 |
| miR-2944c-3p | UAUCACAGCCAGUAGUUACC | 4468.29 | 3543.74 | −0.33 |
| miR-1175-5p | AAGUGGAGCAGUGGUCUCUUCAC | 286.32 | 224.91 | −0.35 |
| miR-92a-3p | AUUGCACUAGUCCCGGCCUA | 62.52 | 48.60 | −0.36 |
| miR-305-5p | AUUGUACUUCAUCAGGUGCUC | 7239.92 | 5605.19 | −0.37 |
| miR-iab-4-3p | CGGUAUACCUUCAGUAUACGUAAC | 24.56 | 18.65 | −0.40 |
| miR-927a-5p | UUUAGAAUUCCUACGCUUUA | 20.10 | 14.98 | −0.42 |
| miR-9c-5p | UCUUUGGUGAUCUAGCCGUGUG | 405.18 | 297.72 | −0.44 |
| miR-34-3p | CGACCACUAUCCAUACUCCCUCC | 29.11 | 21.28 | −0.45 |
| miR-316-5p | UGUCUUUUUCCGCUUUGCUGC | 11,436.58 | 8348.15 | −0.45 |
| miR-998-3p | UAGCACCAUGGGAUUCAGCUCA | 87.90 | 59.03 | −0.57 |
| miR-100-5p | AACCCGUAGAUCCGAACUUGUGGG | 5341.40 | 3528.46 | −0.60 |
| miR-750-3p | CCAGAUCUAACUCUUCCAUAUGACG | 6089.67 | 3795.84 | −0.68 |
| miR-995-3p | UAGCACCACAUGAUUCAGCUUACG | 551.70 | 340.13 | −0.70 |
| miR-2796-5p | AGGGGUUUCUUUCGGCCUCCAGCG | 41.19 | 24.70 | −0.74 |
| miR-92a-5p | AGUCCGUGAUGCGUGACAAUAU | 197.04 | 117.23 | −0.75 |
| miR-315-5p | UUUUGAUUGUUGCUCAGAAAGC | 21.80 | 12.80 | −0.77 |
| miR-989-3p | UGUGAUGUGACGUAGUGG | 4764.90 | 2748.54 | −0.79 |
| miRNAs | TargetScanFly Targets | MiRanda Targets |
|---|---|---|
| miR-282-5p | Ero1L, Fus, CG14435, CG9515, CcapR | Meso18E, Rogdi, Resilin, Nkd, Kraken |
| miR-1000-5p | CG34355, Nplp1, CG10804, Net, CG13384 | Nplp1, CG10804, Kon, CG13384, Net |
| miR-193-3p | P38b, Cp7Fb, Ana, CG11041, CG11313 | CG34394, RYBP, CG6707, Pb, CG32736 |
| miR-124-3p | Sinu, Gli, CG12977, Pk92B, Axs | Pk92B, CG14299, Sinu, Gli, Cp110 |
| miR-970-3p | Btsz, CG15097, Ru, Ace, Rab6 | CG15097, Ubc-E2H, CG32372, CG42256, StmA |
| let-7-5p | Ab, CG18265, A3-3, Apt, Cpr49Aa | Ab, CG5026, CG34118, CG9548, Slam |
| miR-1-3p | CG4297, Tub, CG30457, Hmu, CG6490 | Par-6, CG18542, Pen, CG5053, Pdm2 |
| miR-133-3p | Fili, CG17193, CG2774, CG33324, CG9541 | CG30409, SkpA, Wts, Pde1c, CG32351 |
| miR-263b-5p | Qkr54B, Bx, Wls, CG32062, CG34339 | CG2371, Wls, Qkr54B, Bx, GATAe |
| miR-92a-3p | Sha, Khc-73, CrebA, CG4297, Tusp | CrebA, Cpr50Ca, CG8128, CG3077, CG14274 |
| miR-305-5p | CG33174, CG11997, CG3287, Gr98d, Nerfin-1 | CG3287, CG31855, Mi-2, NfI, Eya |
| miR-927a-5p | Gprs, CG32245, Kr-h1, CG8485, Growl | CG8485, EcR, CG9850, Aef1, Sfl |
| miR-9c-5p | Nerfin-1, Rbp9, CG11206, CG32333, Bru-2 | Sinu, CadN, CG5746, CG11206, CG9426 |
| miR-316-5p | Hr39, Rbp9, CG32121, Numb, Bsg25D | Nkd, Numb, Aret, Cib, RdgC |
| miR-100-5p | E(Pc), Gogo, CG17985, CG31772, DopR2 | CG3630, CG10979, Gogo, Pc, CG13326 |
| miR-315-5p | Eip93F, CG15465, CG32333, CG32137, CG32206 | CG12424, CG14989, CG32137, Rtnl1, CG34126 |
| miR-989-3p | Tankyrase, Fal, Lac, PGRP-SD, Qkr54B | CG12772, Chrw, CG34449, Nedd4, Kni |
| GO Biological Process | Genes Targeted | miRNAs | p-Value |
|---|---|---|---|
| Sensory perception of pain | 13 | 11 | 6.0 × 10−3 |
| Regulation of transcription, DNA-templated | 12 | 10 | 7.2 × 10−4 |
| Transcription, DNA-templated | 11 | 9 | 1.6 × 10−3 |
| Regulation of glucose metabolic process | 9 | 7 | 1.5 × 10−4 |
| Positive regulation of transcription RNA pol II promoter | 8 | 7 | 3.8 × 10−3 |
| Border follicle cell migration | 6 | 6 | 5.7 × 10−3 |
| Axon guidance | 6 | 5 | 2.3 × 10−2 |
| Imaginal disc-derived wing morphogenesis | 6 | 5 | 4.1 × 10−2 |
| Muscle organ development | 5 | 6 | 5.9 × 10−3 |
| Negative regulation of transcription, DNA-templated | 5 | 5 | 2.1 × 10−2 |
| Negative regulation of transcription RNA pol II promoter | 5 | 5 | 4.1 × 10−2 |
| Primer | Sequence | Eff. | Temp. |
|---|---|---|---|
| miR-1-3p | 5′-ACACTCCAGCTGGGTGGAATGTAAAGAAGTA-3′ | 92.0% | 62.5 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGCTCCATAC-3′ | |||
| miR-7-5p | 5′-ACACTCCAGCTGGGTGGAAGACTAGTGAT-3′ | 96.0% | 60.0 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGCACAACAA-3′ | |||
| miR-9a-5p | 5′-ACACTCCAGCTGGGTCTTTGGTTATCTAG-3′ | 101.4% | 58.8 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGTCATACAG-3′ | |||
| miR-12-5p | 5′-ACACTCCAGCTGGGTGAGTATTACATCAGGT-3′ | 95.6% | 64.5 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGCAGTACCT-3′ | |||
| miR-92a-5p | 5′-ACACTCCAGCTGGGAGTCCGTGATGCGTGAC-3′ | 88.5% | 56.5°C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGATATTGTC-3′ | |||
| miR-133-3p | 5′-ACACTCCAGCTGGGTTGGTCCCCTTCAACCA-3′ | 84.3% | 64.5 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAG-3′ | |||
| miR-305-5p | 5′-ACACTCCAGCTGGGATTGTACTTCATCAGGT-3′ | 91.3% | 63.4 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGGAGCACCT-3′ | |||
| miR-927a-5p | 5′-ACACTCCAGCTGGGTTTAGAATTCCTACGCT-3′ | 105.3% | 60.9 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGTAAAGCGT-3′ | |||
| miR-989-3p | 5′-ACACTCCAGCTGGGTGTGATGTGACGTAGTG-3′ | 99.5% | 56.9 °C |
| 5′-CTCACAGTACGTTGGTATCCTTGTGATGTTCGATGCCATATTGTACTGTGAGCCACTACG-3′ | |||
| Universal | 5′-CTCACAGTACGTTGGTATCCTTGTG-3′ | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morin, M.D.; Lyons, P.J.; Crapoulet, N.; Boquel, S.; Morin, P.J. Identification of Differentially Expressed miRNAs in Colorado Potato Beetles (Leptinotarsa decemlineata (Say)) Exposed to Imidacloprid. Int. J. Mol. Sci. 2017, 18, 2728. https://doi.org/10.3390/ijms18122728
Morin MD, Lyons PJ, Crapoulet N, Boquel S, Morin PJ. Identification of Differentially Expressed miRNAs in Colorado Potato Beetles (Leptinotarsa decemlineata (Say)) Exposed to Imidacloprid. International Journal of Molecular Sciences. 2017; 18(12):2728. https://doi.org/10.3390/ijms18122728
Chicago/Turabian StyleMorin, Mathieu D., Pierre J. Lyons, Nicolas Crapoulet, Sébastien Boquel, and Pier Jr Morin. 2017. "Identification of Differentially Expressed miRNAs in Colorado Potato Beetles (Leptinotarsa decemlineata (Say)) Exposed to Imidacloprid" International Journal of Molecular Sciences 18, no. 12: 2728. https://doi.org/10.3390/ijms18122728




