Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b
Abstract
:1. Introduction
2. Results
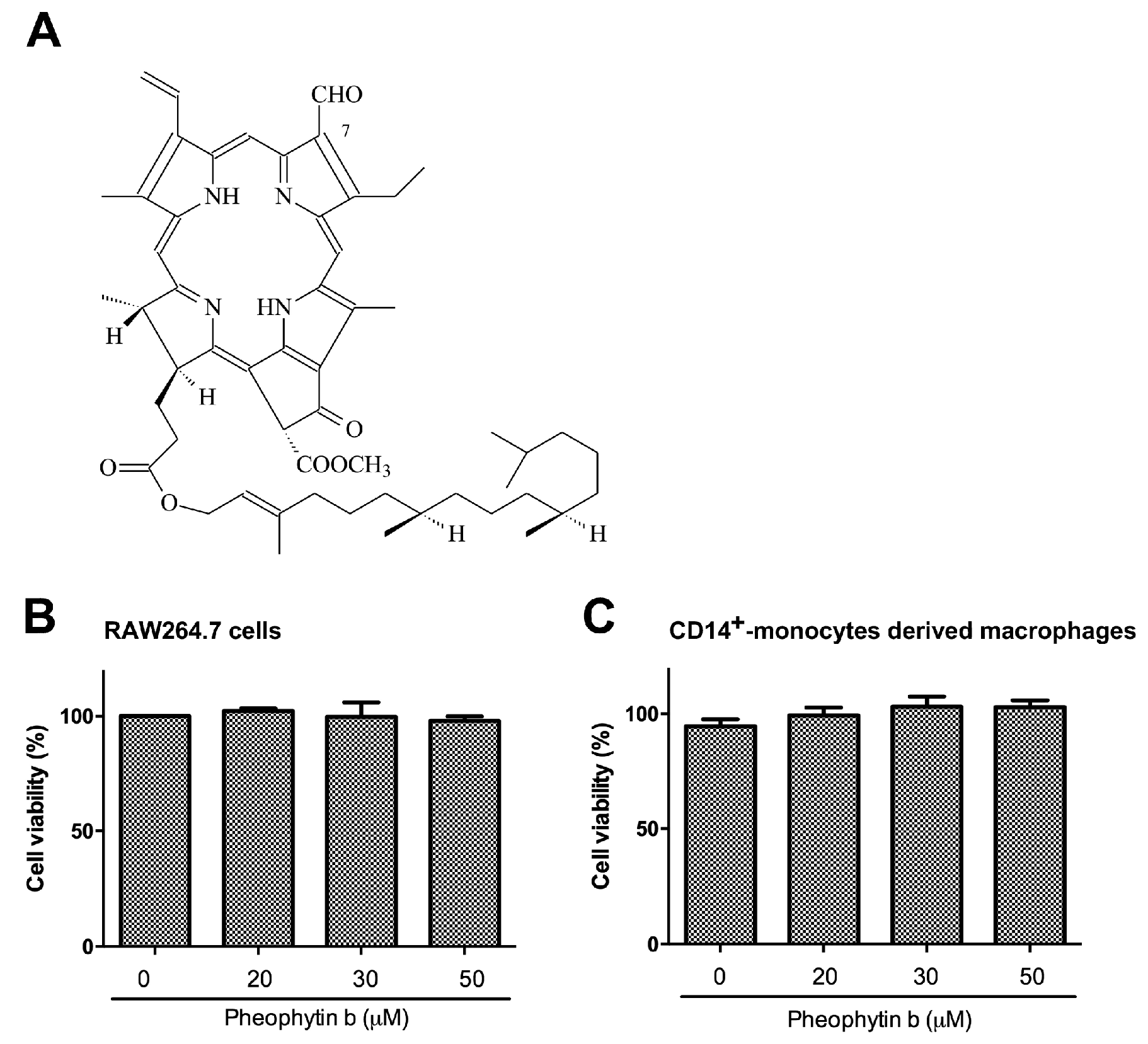
2.1. Pheophytin-b Does Not Induce Macrophage Cytotoxicity
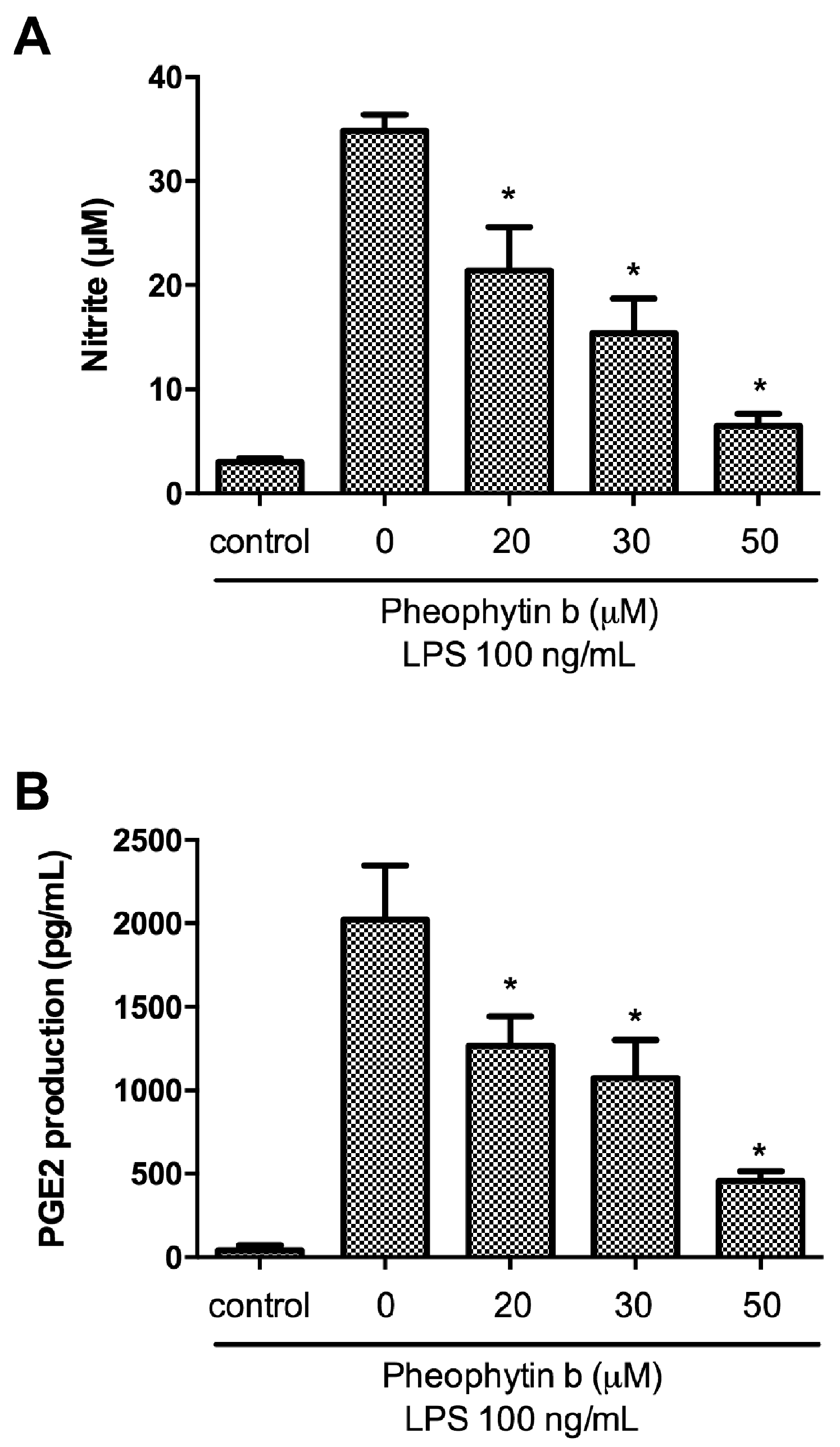
2.2. Effects of Pheophytin-b on NO and PGE2 Production in LPS-Stimulated Macrophages
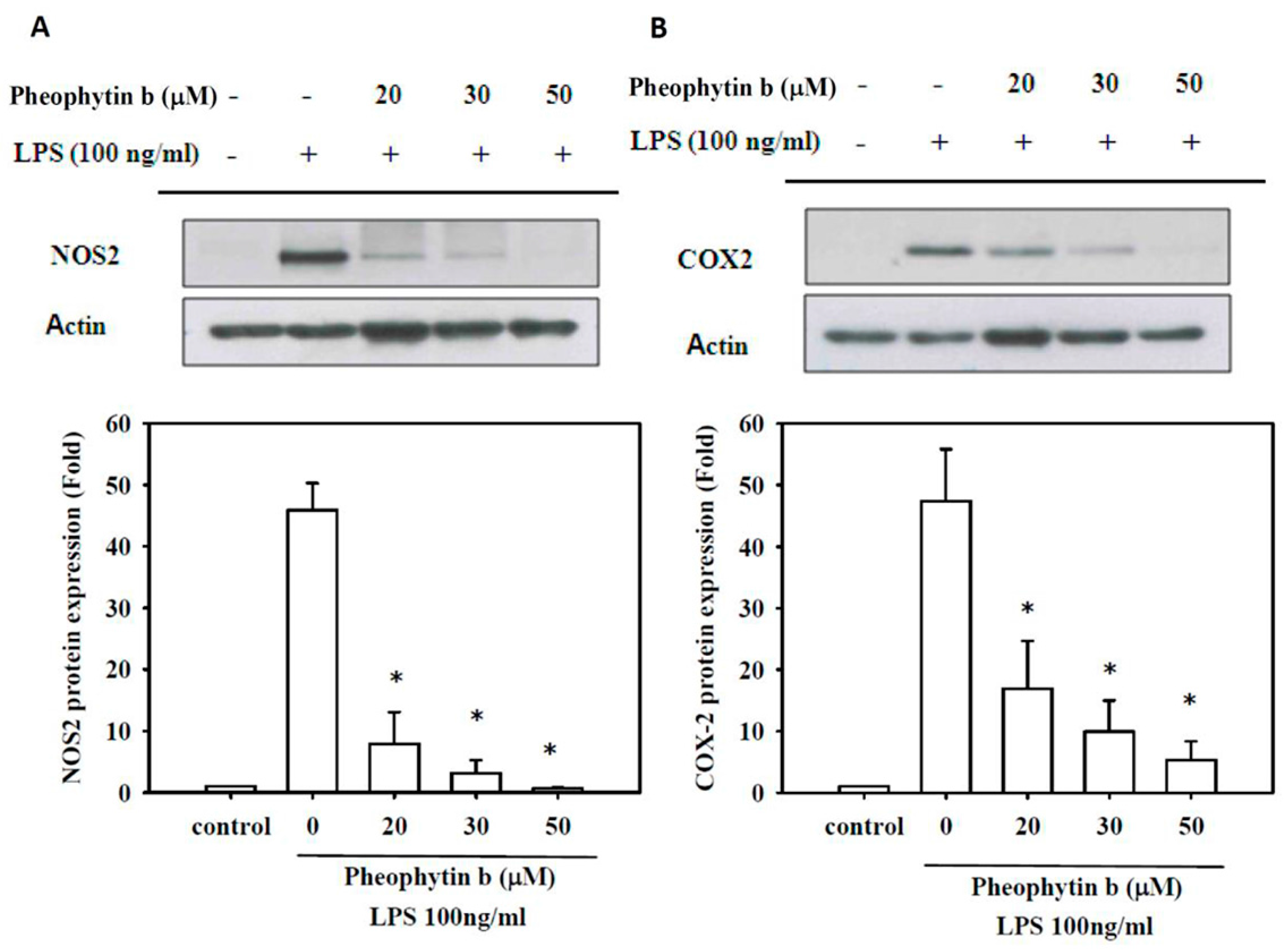
2.3. Effects of Pheophytin-b on NOS2 and COX-2 Expression
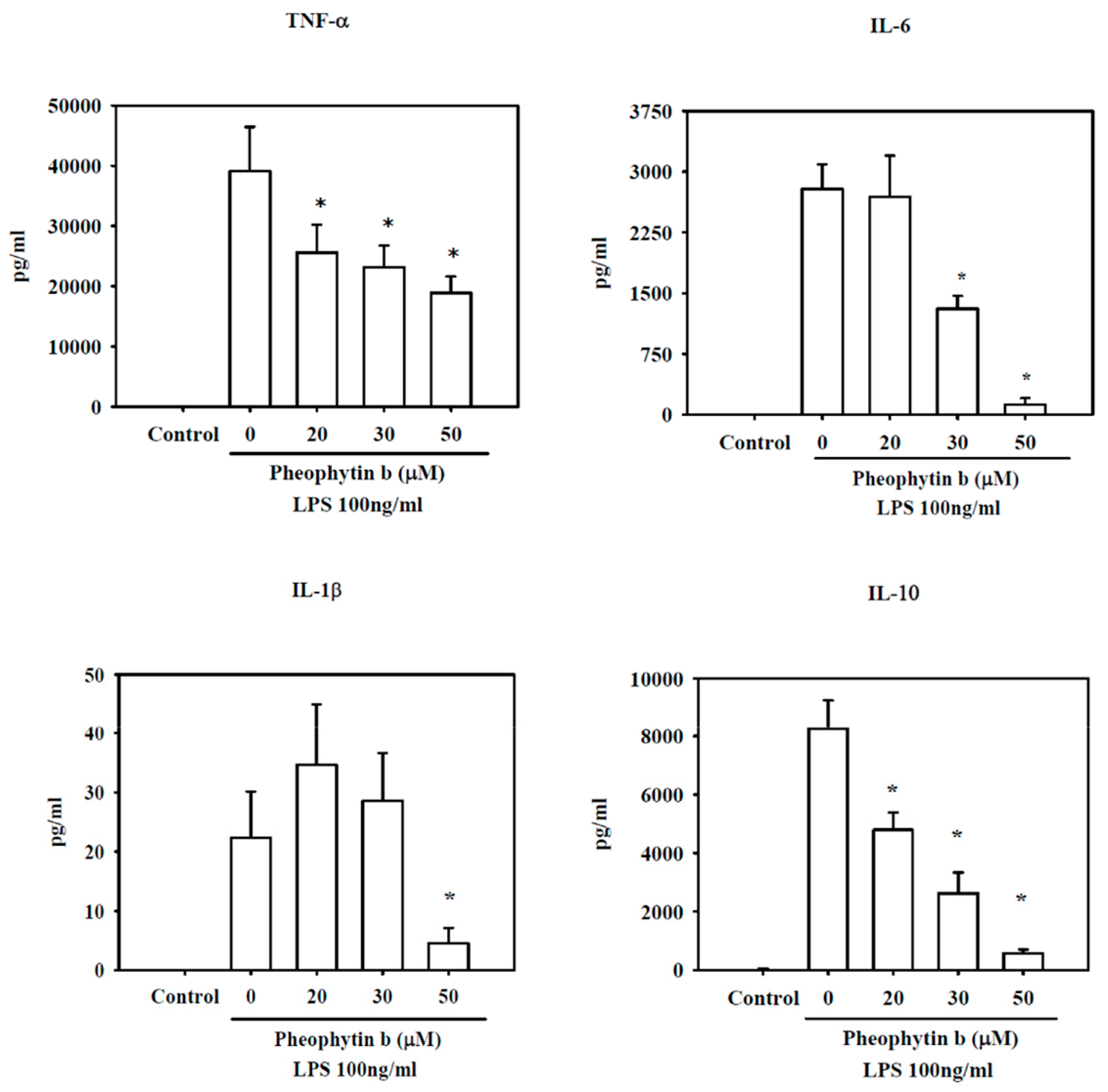
2.4. Effects of Pheophytin-b on Cytokine Production in LPS-Stimulated Macrophages
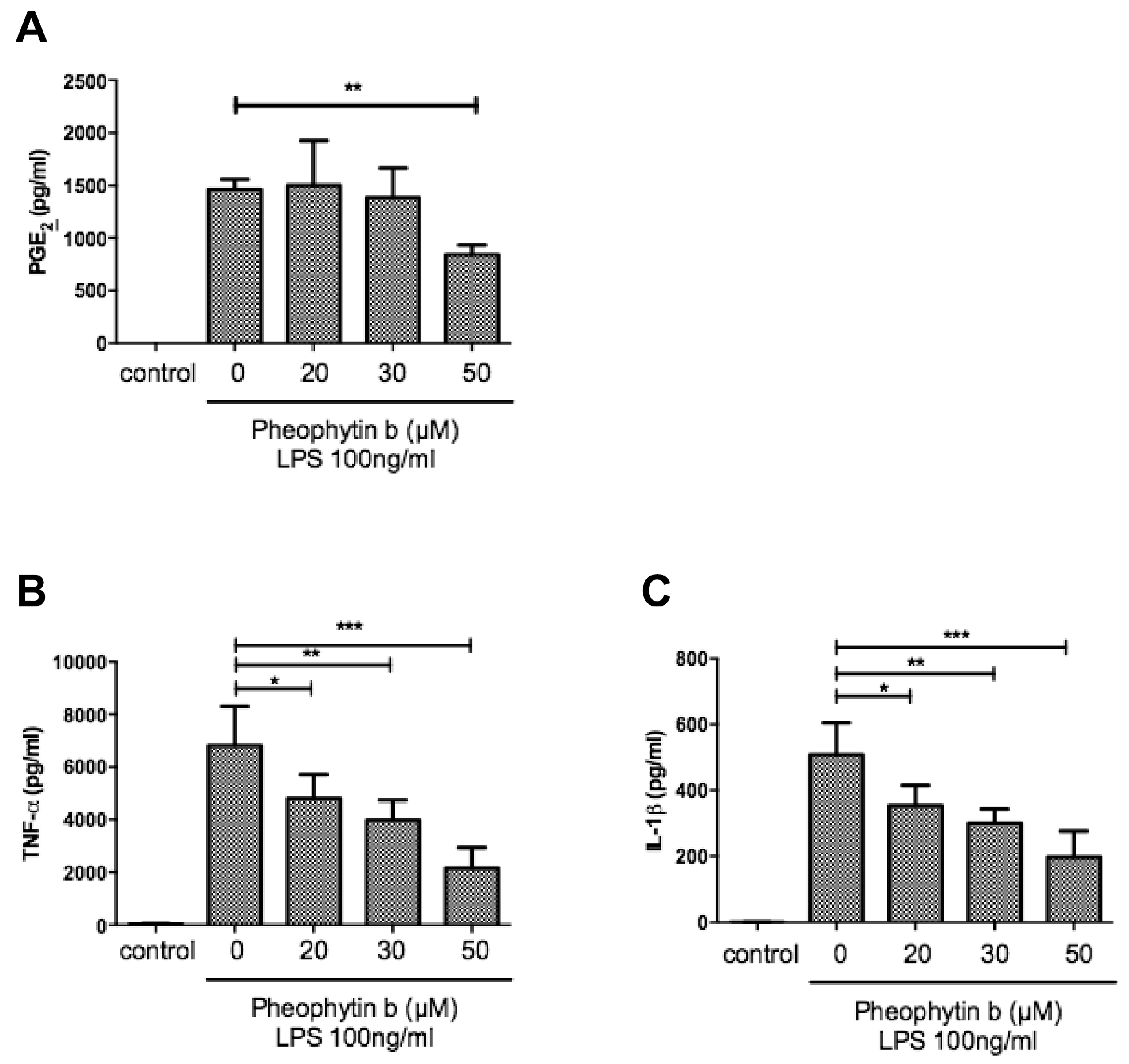
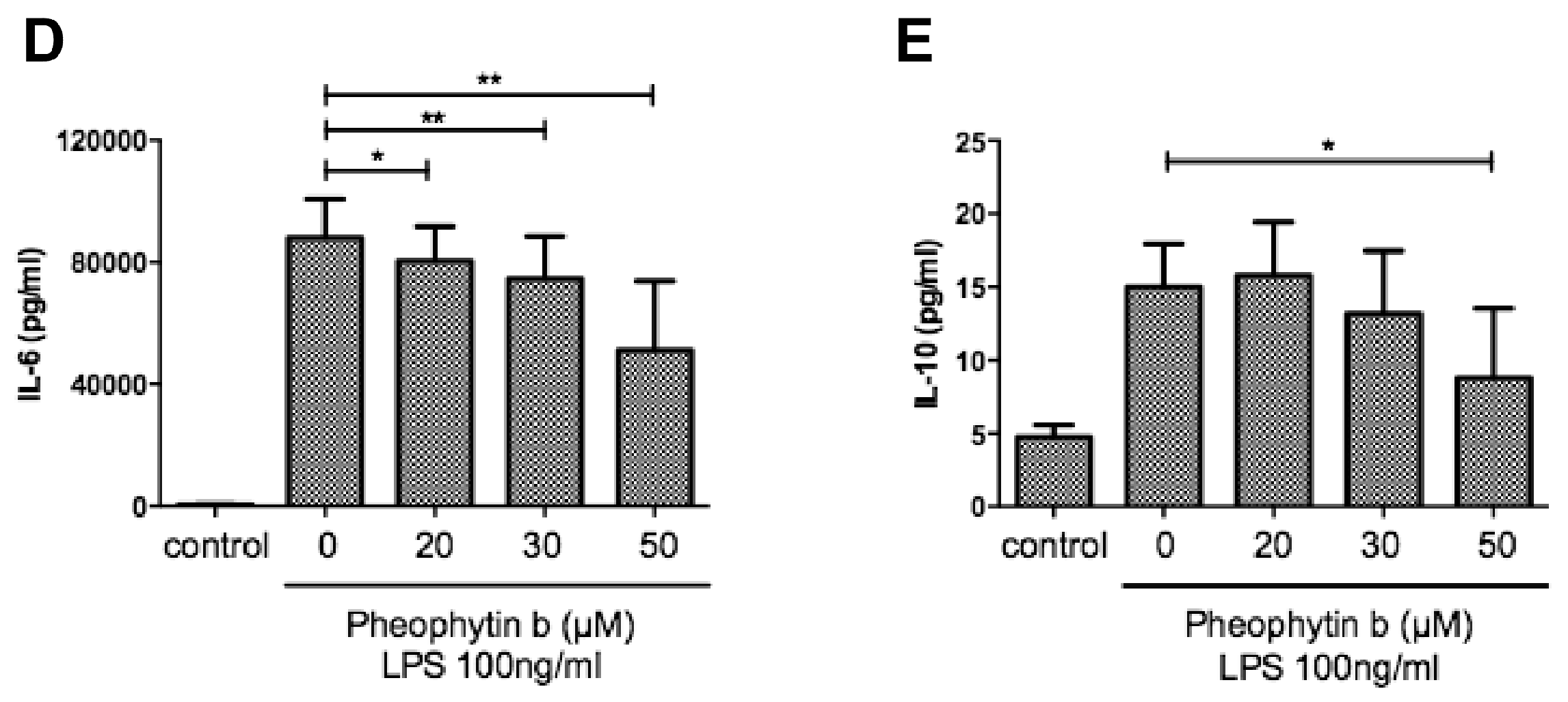
2.5. Effects of Pheophytin-b on PGE2 and Cytokine Production in CD14+ Monocyte-Derived Macrophages after LPS Stimulation
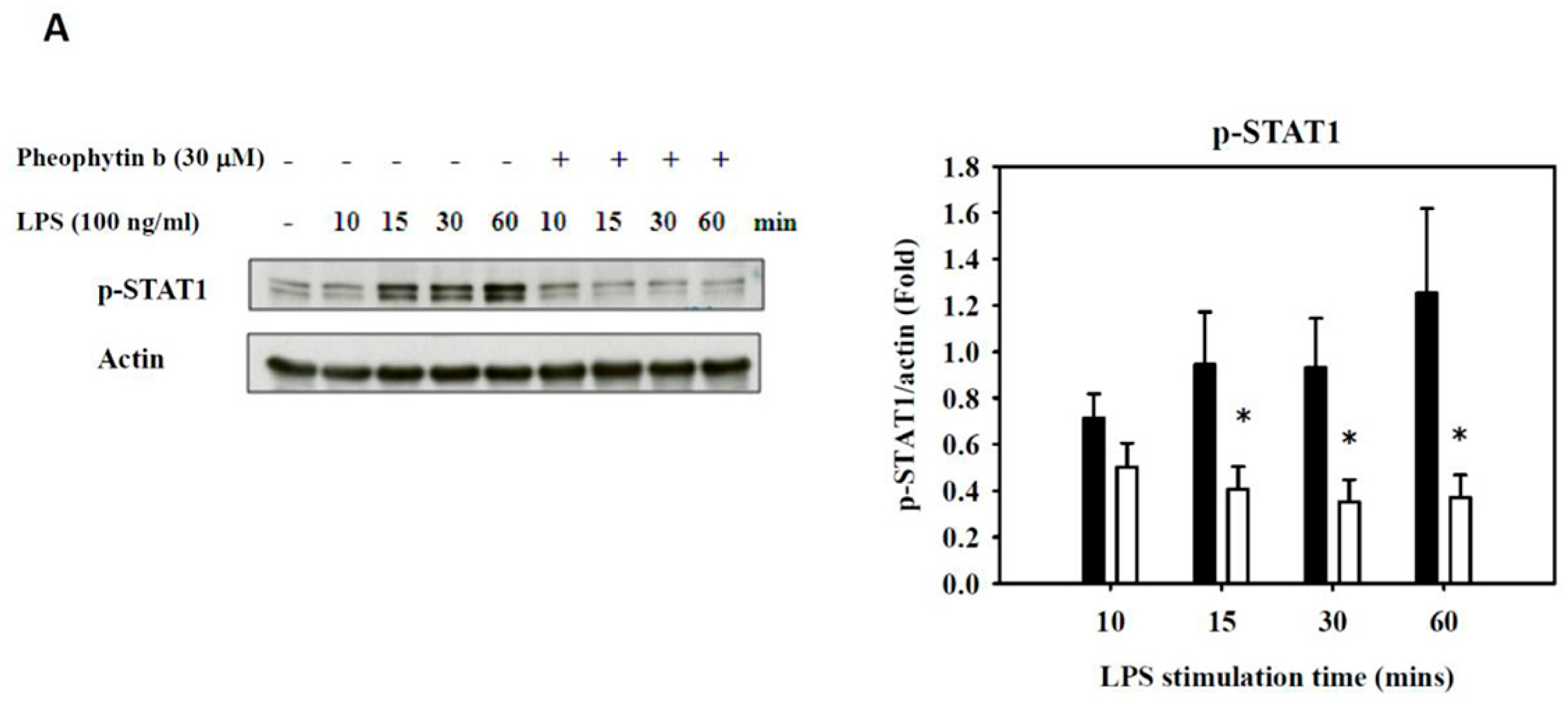
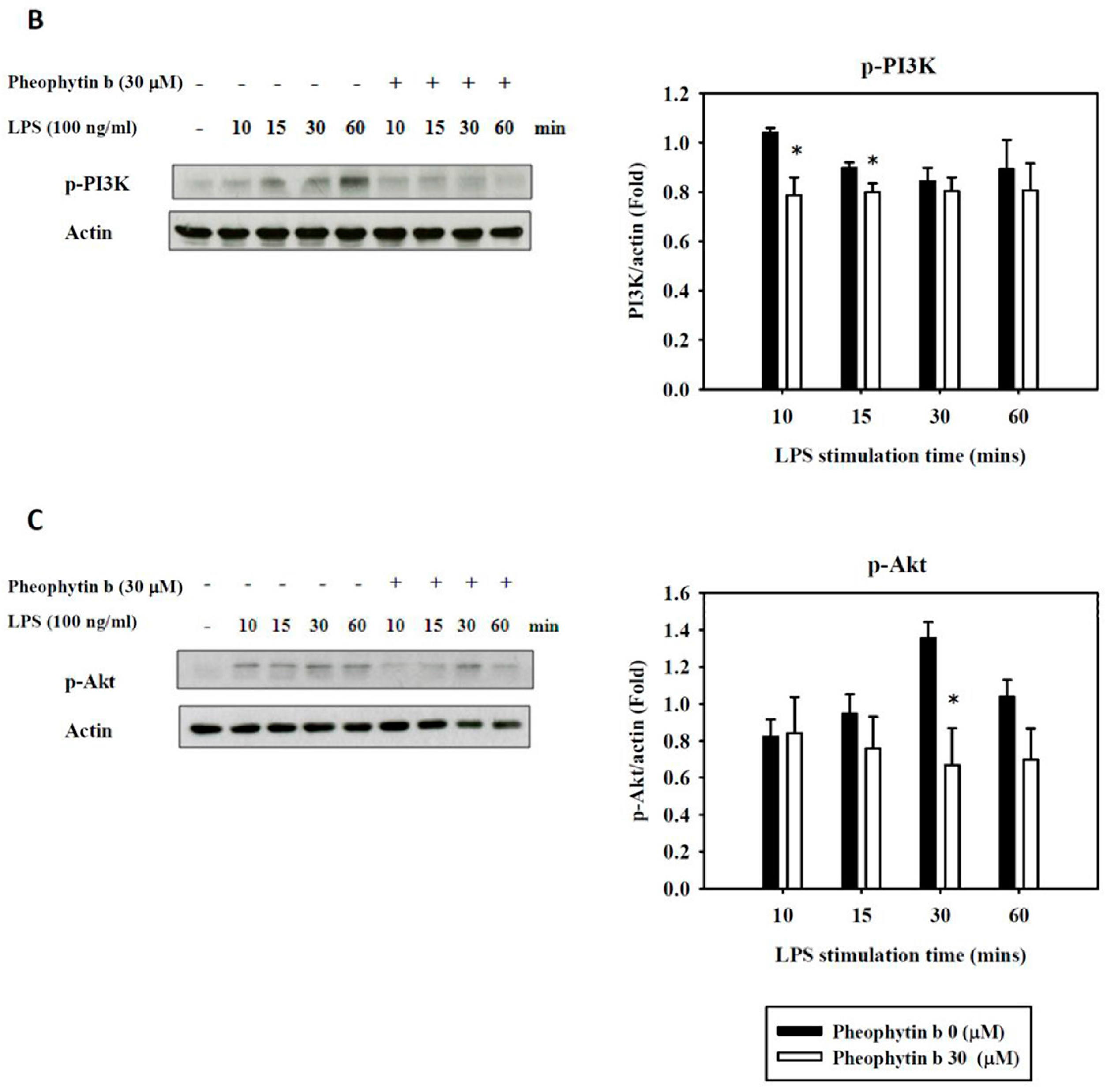
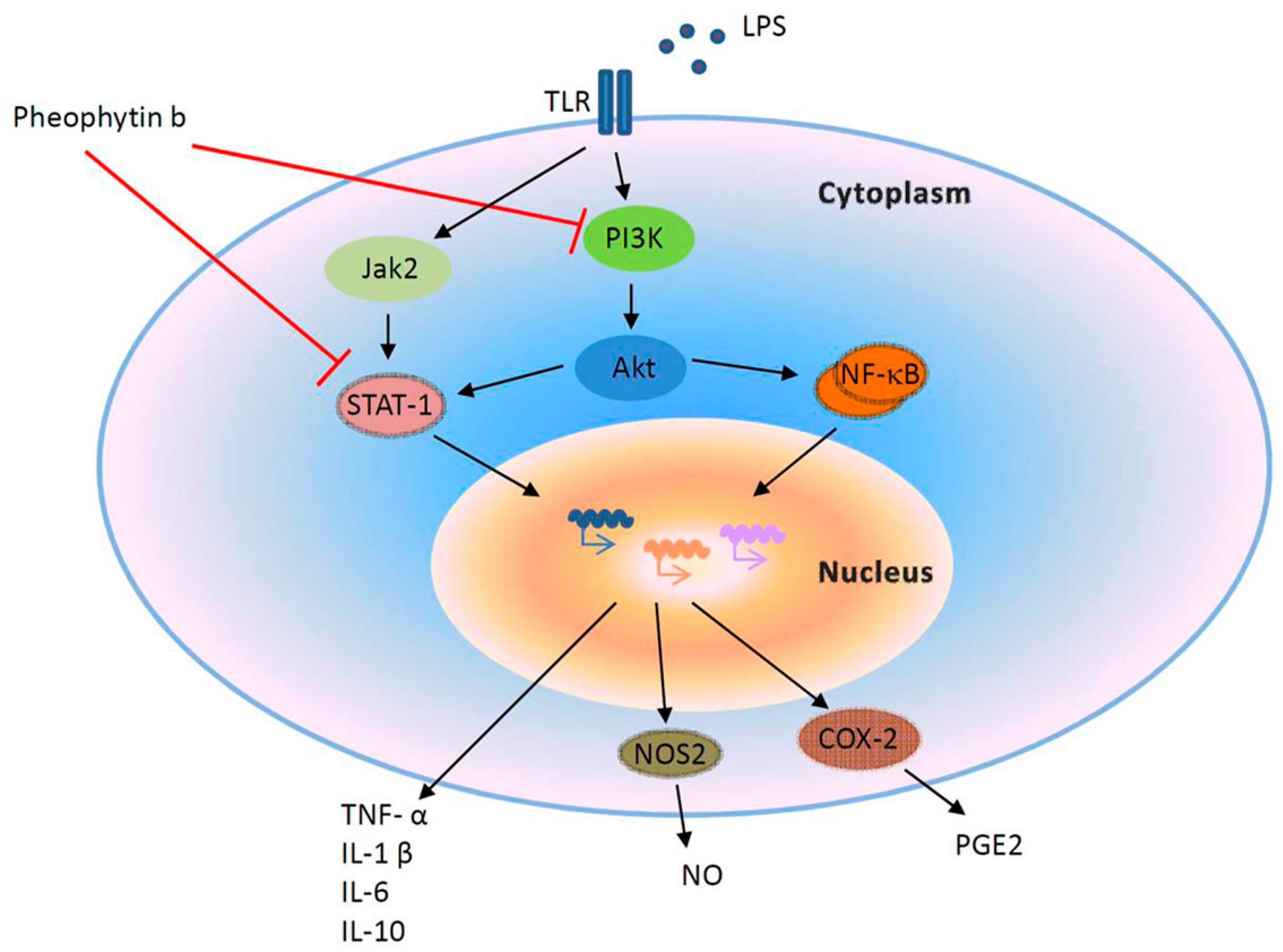
2.6. Effects of Pheophytin-b on LPS-Mediated Signal Transduction Pathways in Macrophages
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. RAW 264.7 Cell Culture
4.3. Ex Vivo Isolation of Human CD14+ Monocytes and Stimulation into Macrophages
4.4. Cytotoxicity Analysis Following Pheophytin-b Treatment
4.5. Western Blot and QRT-PCR Analysis of NO2 and COX-2 Protein in Pheophytin-b Treatment and LPS Stimulation of RAW 264.7 Cells
4.6. Measurement of Nitrite Release Indicative of NO Production
4.7. Transcription Factor (NF-κB and AP-1) Translocation Assays
4.8. Enzyme-Linked Immunosorbent Assay (ELISA) Analyses
4.9. Signal Transduction Pathway Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Conflicts of Interest
Funding
Abbreviations
| COX-2 | cyclooxygenase-2 |
| ELISA | enzyme-linked immunosorbent assay |
| IL | interleukin |
| LPS | lipopolysaccharide |
| NO | nitric oxide |
| NOS2 | NO synthase 2 |
| PGE2 | prostaglandin E2 |
| QRT-PCR | real-time quantitative PCR |
| SD | standard deviations |
| SEM | standard errors of mean |
| TNF-α | tumor necrosis factor-α |
References
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Rea, T.D.; Kahn, J.M.; Walkey, A.J.; Yealy, D.M.; Angus, D.C. Severe sepsis in pre-hospital emergency care: Analysis of incidence, care, and outcome. Am. J. Respir. Crit. Care Med. 2012, 186, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Bleiblo, F.; Michael, P.; Brabant, D.; Ramana, C.V.; Tai, T.; Saleh, M.; Parrillo, J.E.; Kumar, A.; Kumar, A. The role of immunostimulatory nucleic acids in septic shock. Int. J. Clin. Exp. Med. 2012, 5, 1–23. [Google Scholar] [PubMed]
- Lin, C.Y.; Chen, T.C.; Lu, P.L.; Chen, H.J.; Bojang, K.S.; Chen, Y.H. Lower initial central venous pressure in septic patients from long-term care facilities than in those from the community. J. Microbiol. Immunol. Infect. 2014, 47, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Linde-Zwirble, W.T.; Angus, D.C. Severe sepsis epidemiology: Sampling, selection, and society. Crit. Care 2004, 8, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.A.; Li, N.; Bauer, D.; Mendonca, P.; Taka, E.; Darb, M.; Thomas, L.; Williams, H.; Soliman, K.F. Natural product HTP screening for antibacterial (E. coli 0157:H7) and anti-inflammatory agents in (LPS from E. coli O111:B4) activated macrophages and microglial cells; focus on sepsis. BMC Complement. Altern. Med. 2016, 16, 467. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar] [PubMed]
- Russell, J.A. Management of sepsis. N. Engl. J. Med. 2006, 355, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Adib-Conquy, M. Monocytes/macrophages and sepsis. Crit. Care Med. 2005, 33 (Suppl. S12), S506–S509. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, M.; Sakaeda, Y.; Yamaguchi, H.; Ohmori, Y. Anti-inflammatory cytokine interleukin-4 inhibits inducible nitric oxide synthase gene expression in the mouse macrophage cell line RAW264.7 through the repression of octamer-dependent transcription. Mediat. Inflamm. 2013, 2013, 369693. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Baasner, S.; Ince, C.; Nocken, F.; Stover, J.F.; Westphal, M. Differentiated control of deranged nitric oxide metabolism: A therapeutic option in sepsis? Crit. Care 2013, 17, 311. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Huang, W.C.; Liou, C.J. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012, 134, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thota, V.; Dee, L.; Olson, J.; Uretz, E.; Parrillo, J.E. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J. Exp. Med. 1996, 183, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Brar, R.; Wang, P.; Dee, L.; Skorupa, G.; Khadour, F.; Schulz, R.; Parrillo, J.E. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am. J. Physiol. 1999, 276, R265–R276. [Google Scholar] [PubMed]
- Zhang, H.; Wang, H.Y.; Bassel-Duby, R.; Maass, D.L.; Johnston, W.E.; Horton, J.W.; Tao, W. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2408–H2416. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, C.H.; Chang, Y.W.; Wang, H.M.; Chen, C.Y.; Chen, Y.H. Pheophytin a inhibits inflammation via suppression of LPS-induced nitric oxide synthase-2, prostaglandin E2, and interleukin-1beta of macrophages. Int. J. Mol. Sci. 2014, 15, 22819–22834. [Google Scholar] [CrossRef] [PubMed]
- Higashi-Okai, K.; Otani, S.; Okai, Y. Potent suppressive activity of pheophytin a and b from the non-polyphenolic fraction of green tea (Camellia sinensis) against tumor promotion in mouse skin. Cancer Lett. 1998, 129, 223–228. [Google Scholar] [CrossRef]
- Okai, Y.; Higashi-Okai, K. Potent suppressing activity of the non-polyphenolic fraction of green tea (Camellia sinensis) against genotoxin-induced umu C gene expression in Salmonella typhimurium (TA 1535/pSK 1002)—Association with pheophytins a and b. Cancer Lett. 1997, 120, 117–123. [Google Scholar] [CrossRef]
- Higashi-Okai, K.; Yamazaki, M.; Nagamori, H.; Okai, Y. Identification and antioxidant activity of several pigments from the residual green tea (Camellia sinensis) after hot water extraction. J. UOEH 2001, 23, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, J.B.; Misukonis, M.A.; Shami, P.J.; Mason, S.N.; Sauls, D.L.; Dittman, W.A.; Wood, E.R.; Smith, G.K.; McDonald, B.; Bachus, K.E. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): Analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 1995, 86, 1184–1195. [Google Scholar] [PubMed]
- Tammi, R.H.; Passi, A.G.; Rilla, K.; Karousou, E.; Vigetti, D.; Makkonen, K.; Tammi, M.I. Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J. 2011, 278, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Ruland, J. Return to homeostasis: Downregulation of NF-kappaB responses. Nat. Immunol. 2011, 12, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Ward, S. The Nobel Prize in Medicine 2015: Two drugs that changed global health. Sci. Transl. Med. 2015, 7, 316ed14. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Denis, M. Human monocytes/macrophages: NO or no NO? J. Leukoc. Biol. 1994, 55, 682–684. [Google Scholar] [PubMed]
- Mateeva, N.; Gangapuram, M.; Mazzio, E.; Eyunni, S.; Soliman, K.F.; Redda, K.K. Biological evaluation of synthetic chalcone and flavone derivatives as anti-inflammatory agents. Med. Chem. Res. 2015, 24, 1672–1680. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2015, 20, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Da Costa-Silva, T.A.; Grecco, S.S.; de Sousa, F.S.; Lago, J.H.; Martins, E.G.; Terrazas, C.A.; Varikuti, S.; Owens, K.L.; Beverley, S.M.; Satoskar, A.R.; et al. Immunomodulatory and Antileishmanial Activity of Phenylpropanoid Dimers Isolated from Nectandra leucantha. J. Nat. Prod. 2015, 78, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Menk, M.; Graw, J.A.; von Haefen, C.; Sifringer, M.; Schwaiberger, D.; Unger, T.; Steckelings, U.; Spies, C.D. Stimulation of the Angiotensin II AT2 Receptor is Anti-inflammatory in Human Lipopolysaccharide-Activated Monocytic Cells. Inflammation 2015, 38, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.H.; Khalil, W.K.; Salem, H.A.; Kenawy, S.A.; El-Sayeh, B.M. Sulforaphane increases the survival rate in rats with fulminant hepatic failure induced by d-galactosamine and lipopolysaccharide. Nutr. Res. 2014, 34, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Chen, C.C.; Wang, J.K. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol. Pharmacol. 1999, 55, 481–488. [Google Scholar] [PubMed]
- Kleinert, H.; Pautz, A.; Linker, K.; Schwarz, P.M. Regulation of the expression of inducible nitric oxide synthase. Eur. J. Pharmacol. 2004, 500, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Jones, B.W.; Toshchakov, V.; Vogel, S.N.; Fenton, M.J. Toll-like receptors 2 and 4 activate STAT1 serine phosphorylation by distinct mechanisms in macrophages. J. Biol. Chem. 2003, 278, 22506–22512. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Lee, C.H.; Wang, H.M.; Chang, Y.W.; Lin, C.Y.; Chen, C.Y.; Chen, Y.H. 6-Dehydrogingerdione restrains lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages. J. Agric. Food Chem. 2014, 62, 9171–9179. [Google Scholar] [CrossRef] [PubMed]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell. Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Van Grevenynghe, J.; Rion, S.; Le Ferrec, E.; Le Vee, M.; Amiot, L.; Fauchet, R.; Fardel, O. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J. Immunol. 2003, 170, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Chen, S.T.; Yang, A.H.; Lin, W.W.; Lin, Y.L.; Chen, N.J.; Tsai, I.S.; Li, L.; Hsieh, S.L. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 2013, 121, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Bandow, K.; Kakimoto, K.; Kusuyama, J.; Matsuguchi, T. Long-time treatment by low-dose N-acetyl-l-cysteine enhances proinflammatory cytokine expressions in LPS-stimulated macrophages. PLoS ONE 2014, 9, e87229. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Wang, W.-H.; Chen, S.-H.; Chang, Y.-W.; Hung, L.-C.; Chen, C.-Y.; Chen, Y.-H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. https://doi.org/10.3390/ijms18122637
Lin C-Y, Wang W-H, Chen S-H, Chang Y-W, Hung L-C, Chen C-Y, Chen Y-H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. International Journal of Molecular Sciences. 2017; 18(12):2637. https://doi.org/10.3390/ijms18122637
Chicago/Turabian StyleLin, Chun-Yu, Wen-Hung Wang, Shin-Huei Chen, Yu-Wei Chang, Ling-Chien Hung, Chung-Yi Chen, and Yen-Hsu Chen. 2017. "Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b" International Journal of Molecular Sciences 18, no. 12: 2637. https://doi.org/10.3390/ijms18122637
APA StyleLin, C.-Y., Wang, W.-H., Chen, S.-H., Chang, Y.-W., Hung, L.-C., Chen, C.-Y., & Chen, Y.-H. (2017). Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. International Journal of Molecular Sciences, 18(12), 2637. https://doi.org/10.3390/ijms18122637







