Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice
Abstract
1. Introduction
2. Results and Discussion
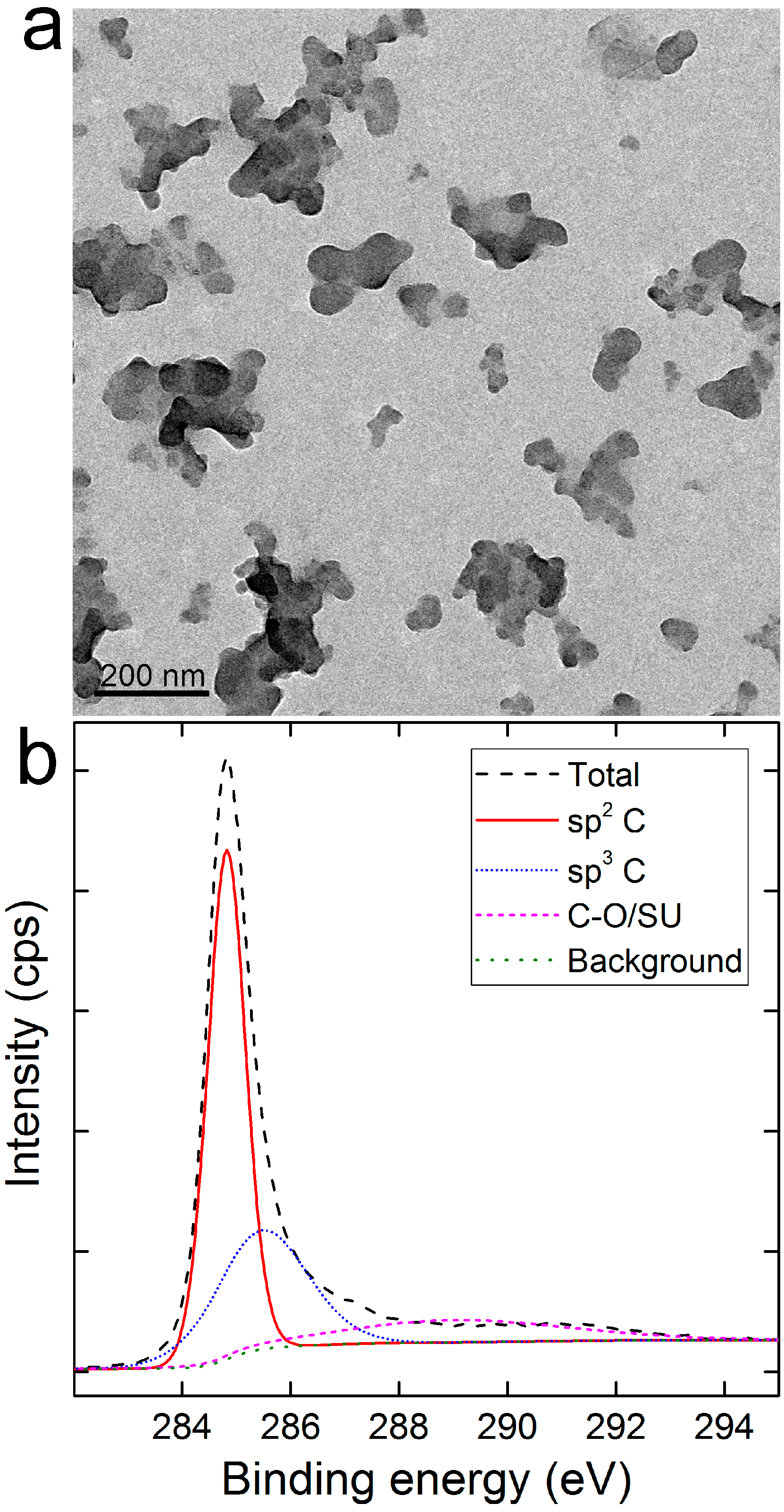
2.1. Characterization of CNSI
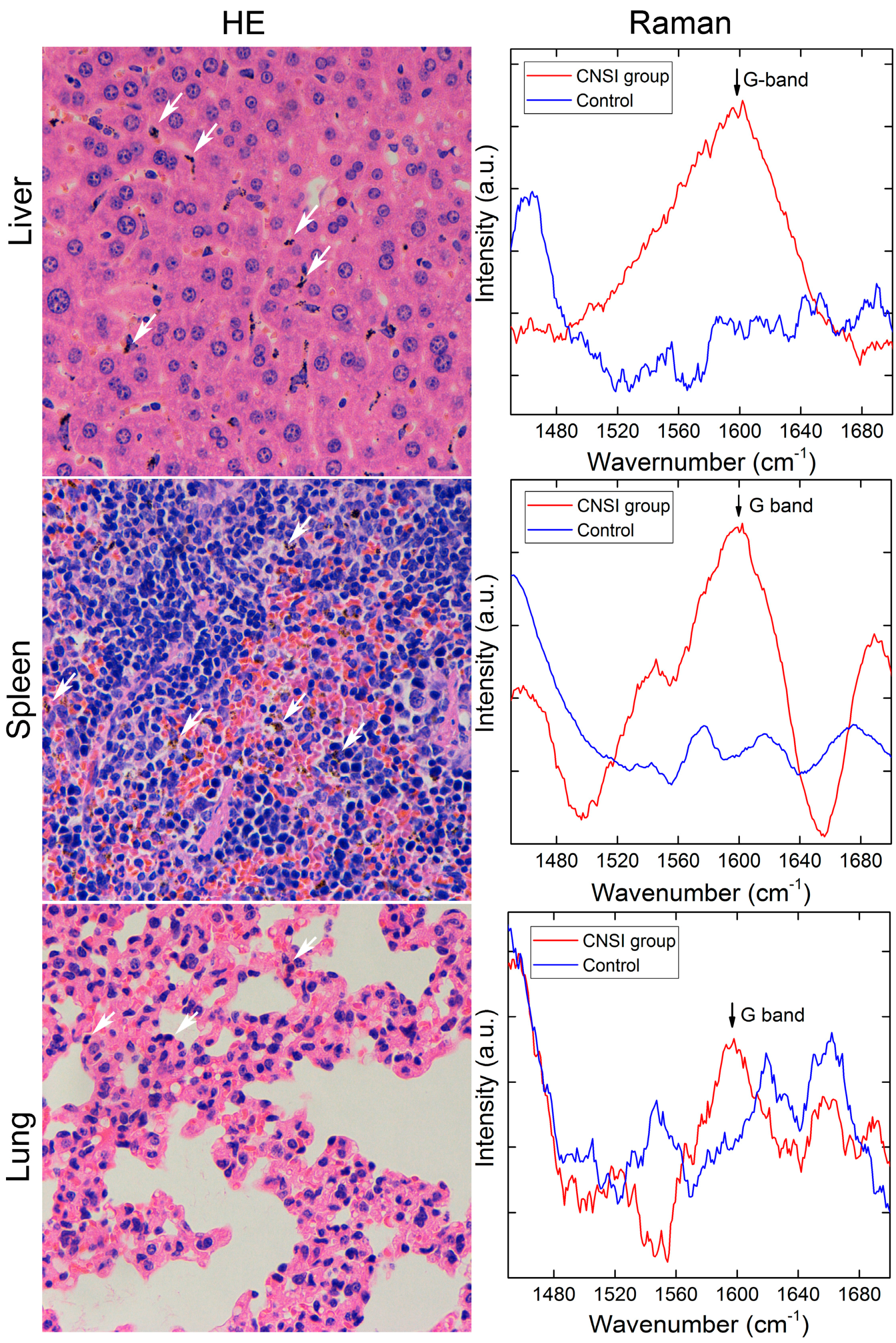
2.2. Bioaccumulation of CNSI in Reticuloendothelial System (RES)
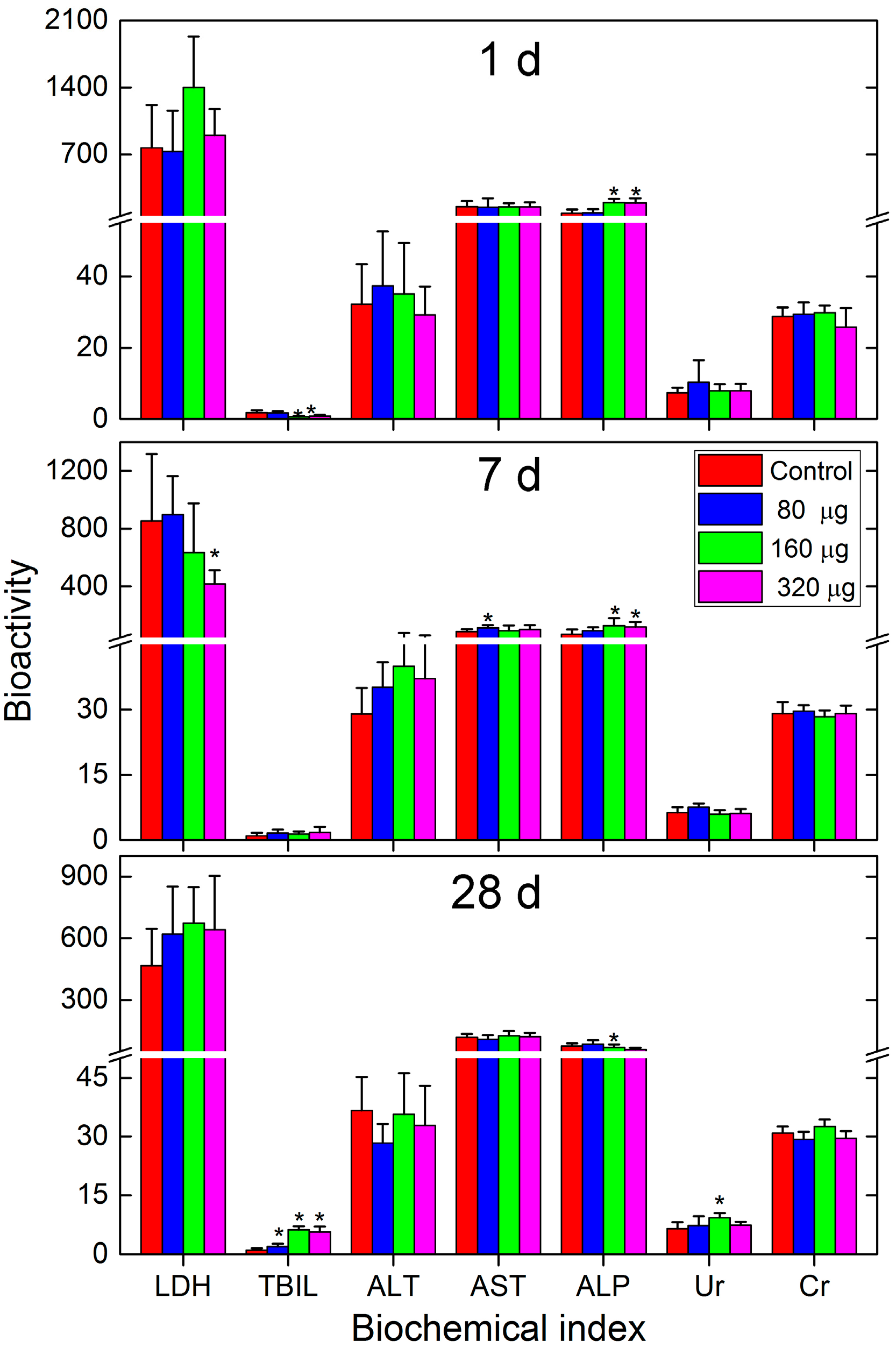
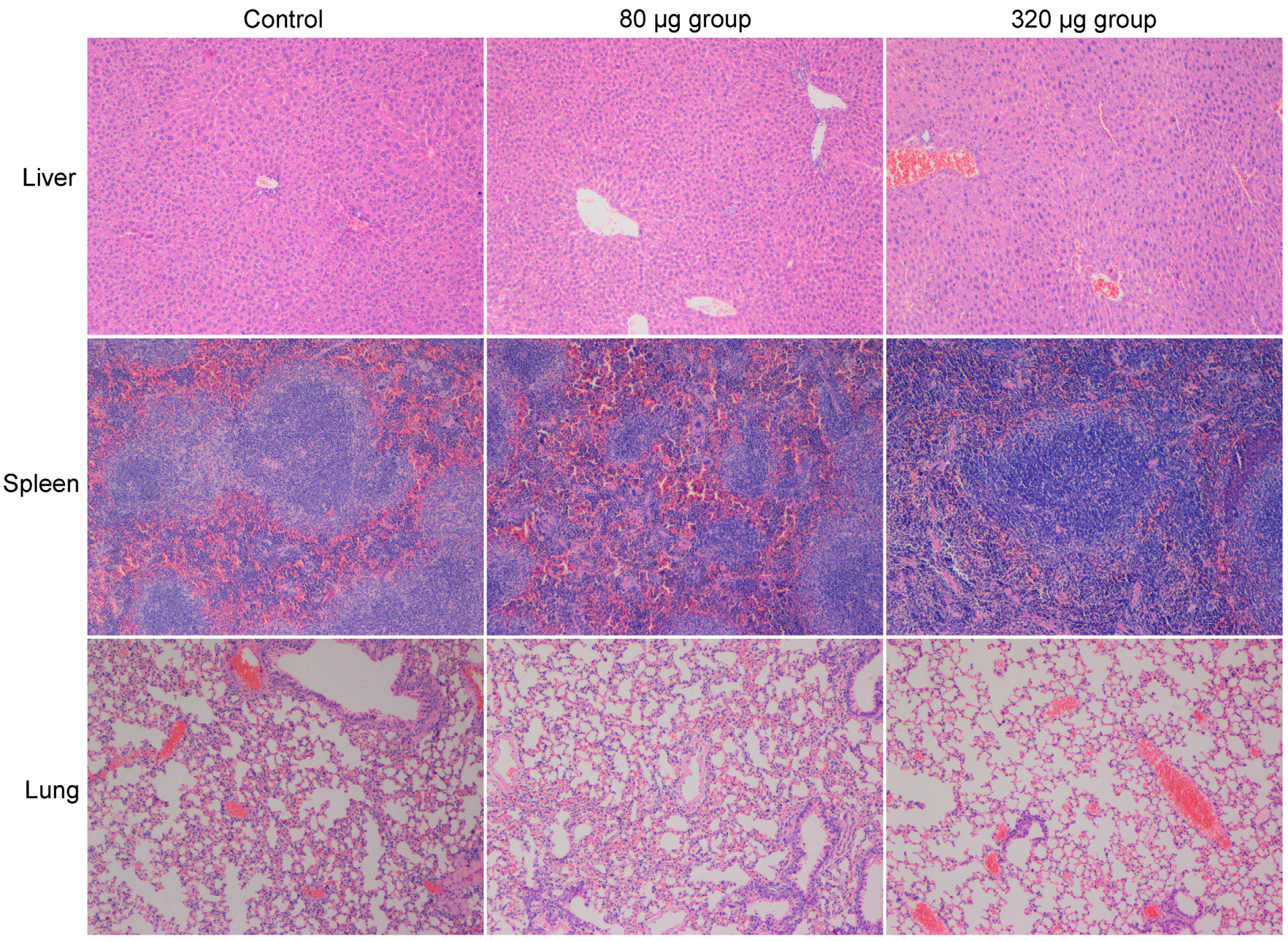
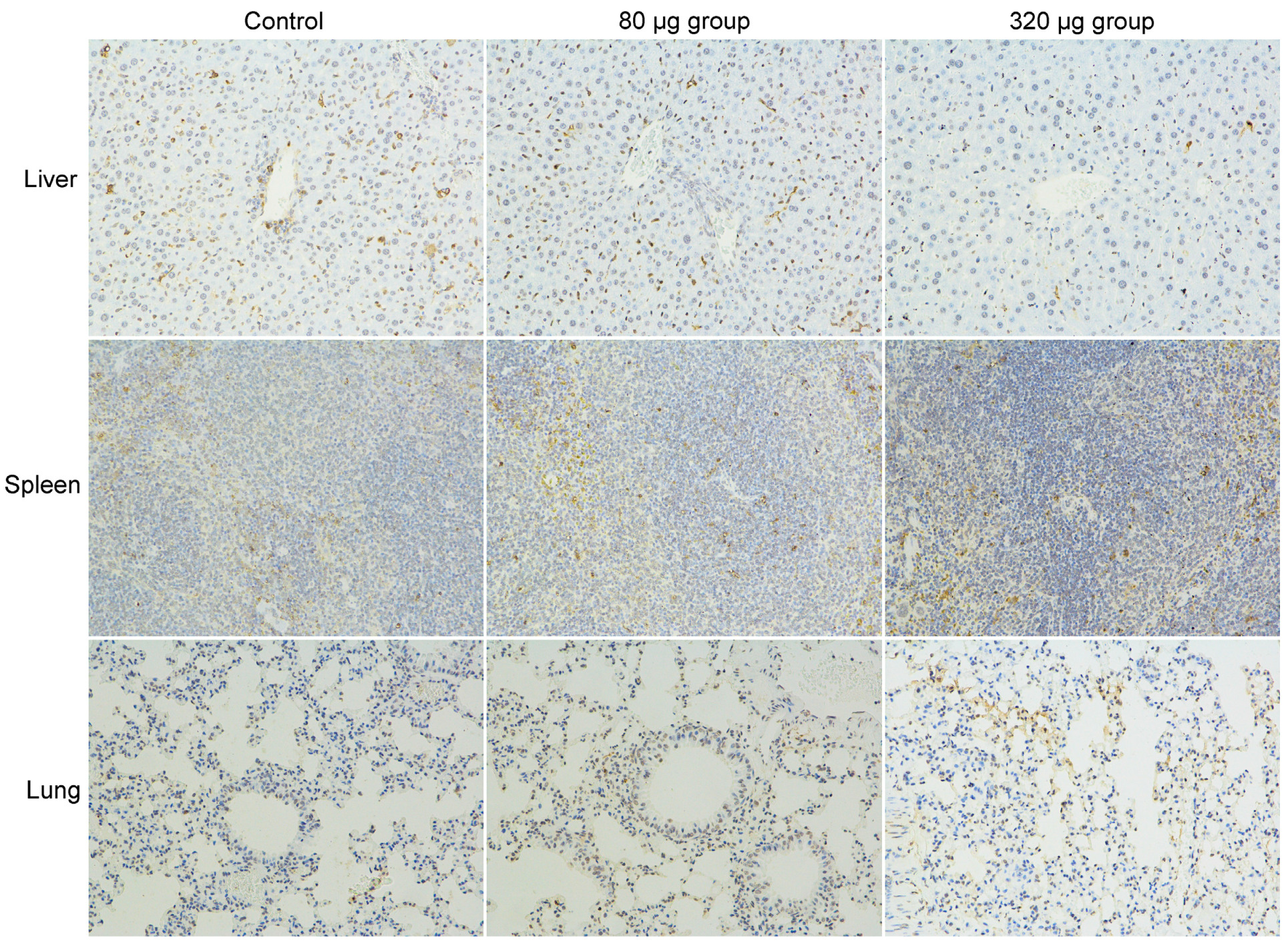
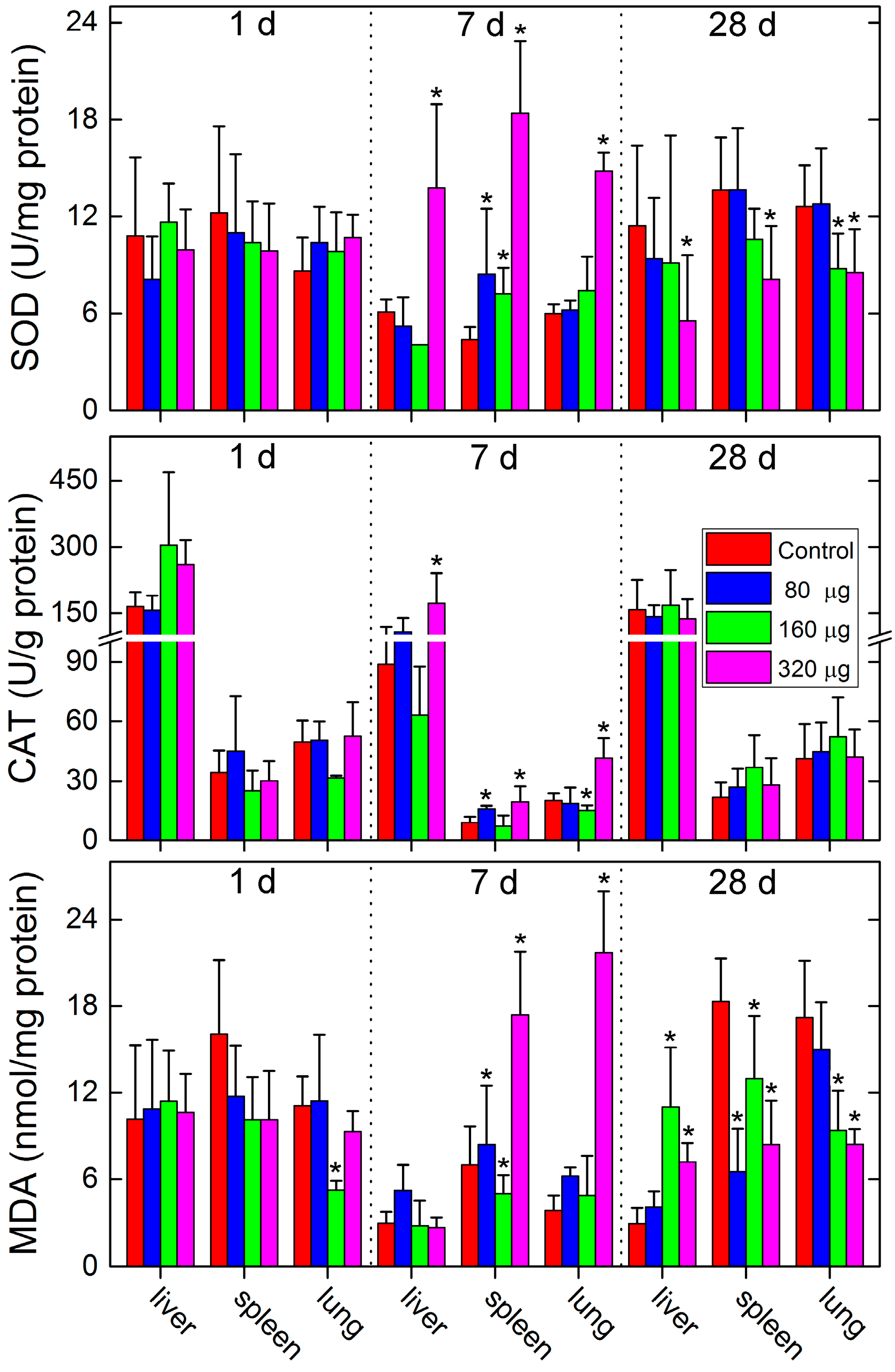
2.3. Toxicity Evaluations
3. Materials and Methods
3.1. Materials
3.2. Characterization of CNSI
3.3. Animal Exposure
3.4. Biodistribution of CNSI
3.5. Toxicity Evaluations
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CNSI | Carbon nanoparticles suspension injection |
| RES | Reticuloendothelial system |
| CNTs | Carbon nanotubes |
| TDLN | Tumor drainage lymph node |
| GO | Graphene oxide |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling |
| PVP | Polyvinyl pyrrolidone |
| TEM | Transmission electron microscopy |
| DLS | Dynamic light scattering |
| XPS | X-ray photoelectron spectroscopy |
| FWHM | Full width at half maximum |
| IR | Infrared spectroscopy |
| HE | Hematoxylin-eosin |
| HB | Hemoglobin |
| MCV | Mean corpuscular volume |
| MCHC | Mean corpuscular hemoglobin |
| MPV | Mean platelet volume |
| PLT | Platelet |
| WBC | White blood cell count |
| RBC | Red blood cell count |
| MCH | Mean corpuscular hemoglobin |
| RDW | Red cell distribution width |
| PDW | Platelet distribution width |
| TBIL | Total bilirubin |
| LDH | Lactate dehydrogenase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| Ur | Urea |
| Cr | Creatinine |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| ICR | Institute of Cancer Research |
References and Notes
- LeCroy, G.E.; Yang, S.-T.; Yang, F.; Yang, F.; Liu, Y.; Fernando, K.A.S.; Bunker, C.E.; Hu, Y.; Luo, P.G.; Sun, Y.-P. Functionalized carbon nanoparticles: Syntheses and applications in optical bioimaging and energy conversion. Coord. Chem. Rev. 2016, 320–321, 66–81. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.G.; Yang, F.; Yang, S.-T.; Sonkar, S.K.; Yang, L.; Broglie, J.J.; Liu, Y.; Sun, Y.-P. Carbon-based quantum dots for fluorescence imaging of cells and tissues. RSC Adv. 2014, 4, 10791–10807. [Google Scholar] [CrossRef]
- Son, K.H.; Hong, J.H.; Lee, J.W. Carbon nanotubes as cancer therapeutic carriers and mediators. Int. J. Nanomed. 2016, 11, 5163–5185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Liu, J.; Zhai, J. Recent developments of phototherapy based on graphene family nanomaterials. Curr. Med. Chem. 2017, 24, 268–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, J.; Gao, H.; Hu, Y.; Hou, X.; LeCroy, G.E.; Bunker, C.E.; Liu, Y.; Sun, Y.-P. Host–guest carbon dots as high-performance fluorescence probes. J. Mater. Chem. C 2017, 5, 6328–6335. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, X.; Zhang, X.; Liu, S.; Pei, Q.; Zheng, M.; Xie, Z. Porphyrin-based carbon dots for photodynamic therapy of hepatoma. Adv. Healthc. Mater. 2017, 6, 1600924. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef] [PubMed]
- Chongqing Lummy Pharmaceutical Co., Ltd., Chongqing, China. Internal data of carbon nanoparticles suspension injection, 2017.
- Li, Z.; Ao, S.; Bu, Z.; Wu, A.; Wu, X.; Shan, F.; Ji, X.; Zhang, Y.; Xing, Z.; Ji, J. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: A prospective randomized trial. World J. Surg. Oncol. 2016, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Q.; Chen, G.; Lu, J.; Zeng, Y.; Chen, X.; Yan, J. Sentinel lymph node detection using carbon nanoparticles in patients with early breast cancer. PLoS ONE 2015, 10, e0135714. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, X.; Zhang, H.; Chen, L.; Zhou, S.; Wu, K.; Wang, Z.; Kong, L.; Zhuang, H. Carbon nanoparticle–guided central lymph node dissection in clinically node-negative patients with papillary thyroid carcinoma. Head Neck 2016, 38, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, J.; Nie, X.; Wang, W.; Shang, J. Potential role for carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection. Int. J. Clin. Exp. Med. 2015, 8, 9640–9648. [Google Scholar] [PubMed]
- Xie, P.; Tang, X.; Li, L.; Qian, Z.; Ran, M.; Zhang, X.; Xin, Q.; Luo, H. Drug-loaded carbon nanoparticle suspension injection, Drug selection, releasing behavior, in vitro cytotoxicity and in vivo lymph node targeting. J. Nanosci. Nanotechnol. 2016, 16, 6910–6918. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Chen, J.; Tian, C.; Li, T.; Chen, Y.; Lv, C. A clinical study on regional lymphatic chemotherapy using an activated carbon nanoparticle–epirubicin in patients with breast cancer. Tumor Biol. 2012, 33, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Xin, Q.; Yang, S.-T.; He, T.; Huang, Y.; Zeng, G.; Ran, M.; Tang, X.H. Skeleton labeled 13C-carbon nanoparticles for the imaging and quantification in tumor drainage lymph nodes. Int. J. Nanomed. 2017, 12, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Yang, S.-T.; Xing, G. Molecular toxicity of nanomaterials. J. Biomed. Nanotechnol. 2014, 10, 2828–2851. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Luo, J.; Zhou, Q.; Wang, H. Pharmacokinetics, metabolism and toxicity of carbon nanotubes for biomedical purposes. Theranostics 2012, 2, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Wang, X.; Jia, G.; Gu, Y.; Wang, T.; Nie, H.; Ge, C.; Wang, H.; Liu, Y. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol. Lett. 2008, 181, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M.; et al. Carbon dots as nontoxic and high performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, T.; Wang, H.; Gu, Y.; Xu, Y.; Tang, H.; Jia, G.; Liu, Y. Biocompatibility of graphene oxide intravenously administrated in mice-effects of dose, size and exposure protocols. Toxicol. Res. 2015, 4, 83–91. [Google Scholar] [CrossRef]
- Kanakia, S.; Toussaint, J.D.; Chowdhury, S.M.; Tembulkar, T.; Lee, S.; Jiang, Y.; Lin, R.Z.; Shroyer, K.R.; Moore, S.; Sitharaman, B. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials 2014, 35, 7022–7031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Deng, X.; Ji, Z.; Dong, L.; Wu, M.; Gu, T.; Liu, Y. Long-term hepatotoxicity of polyethylene-glycol functionalized multi-walled carbon nanotubes in mice. Nanotechnology 2010, 21, 175101. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, S.-T.; Cao, A.; Liu, Y. Quantification of carbon nanomaterials in vivo. Acc. Chem. Res. 2013, 46, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Chongqing Lummy Pharmaceutical Co., Ltd., Chongqing, China. Histopathological observations of mice injected with carbon nanoparticles suspension injection at day 1 and 7, 2017.
- Snyder-Talkington, B.N.; Dong, C.; Porter, D.W.; Ducatman, B.; Wolfarth, M.G.; Andrew, M.; Battelli, L.; Raese, R.; Castranova, V.; Guo, N.L.; et al. Multiwalled carbon nanotube-induced pulmonary inflammatory and fibrotic responses and genomic changes following aspiration exposure in mice: A 1-year post-exposure study. J. Toxicol. Environ. Health A 2016, 79, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, S.; Knudsen, K.B.; Kyjovska, Z.O.; Berthing, T.; Skaug, V.; Levin, M.; Koponen, I.K.; Shivayogimath, A.; Booth, T.J.; Alonso, B.; et al. Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide. PLoS ONE 2016, 12, e0178355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Sun, Q.; Bo, J.; Huang, R.; Zhang, M.; Xia, Z.; Ju, L.; Xiang, G. Single-walled carbon nanohorn (SWNH) aggregates inhibited proliferation of human liver cell lines and promoted apoptosis, especially for hepatoma cell lines. Int. J. Nanomed. 2014, 9, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Chongqing Lummy Pharmaceutical Co., Ltd., Chongqing, China. TUNEL assays of mice injected with carbon nanoparticles suspension injection at day 1 and 7, 2017.
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmcol. 2012, 261, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y. In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.dingguo.com/Asset/emspics/UP_2012051107034911.pdf (accessed on 3 March 2017).
- Available online: http://elder.njjcbio.com/index_en.php (accessed on 3 March 2017).
| Control (g) | 80 µg Group (g) | 160 µg Group (g) | 320 µg Group (g) | |
|---|---|---|---|---|
| Day 1 | 26.5 ± 1.3 | 25.5 ± 0.7 | 26.5 ± 1.5 | 26.3 ± 1.3 |
| Day 7 | 28.7 ± 2.5 | 28.5 ± 1.9 | 29.3 ± 2.8 | 28.3 ± 2.4 |
| Day 28 | 32.5 ± 3.1 | 33.4 ± 3.7 | 34.2 ± 4.9 | 33.5 ± 3.4 |
| 1 d | 7 d | 28 d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 80 μg | 160 μg | 320 μg | Control | 80 μg | 160 μg | 320 μg | Control | 80 μg | 160 μg | 320 μg | |
| PLT (109/L) | 474 ± 63 | 486 ± 81 | 478 ± 126 | 459 ± 98 | 422 ± 116 | 578 ± 135 | 457 ± 138 | 421 ± 69 | 444 ± 108 | 332 ± 113 | 377 ± 63 | 382 ± 52 |
| MCHC (g/L) | 353 ± 52 | 321 ± 23 | 364 ± 38 | 339 ± 33 | 360 ± 50 | 326 ± 24 | 316 ± 30 | 313 ± 3 * | 297 ± 15 | 304 ± 33 | 313 ± 22 | 305 ± 23 |
| HB (g/L) | 145 ± 7 | 155 ± 6 * | 150 ± 7 | 156 ± 9 * | 153 ± 7 | 154 ± 6 | 152 ± 4 | 149 ± 12 | 148 ± 17 | 142 ± 37 | 149 ± 4 | 152 ± 6 |
| MCV (fL) | 49 ± 5 | 56 ± 3 * | 49 ± 5 | 52 ± 5 | 49 ± 6 | 52 ± 4 | 56 ± 4 | 57 ± 1 * | 56 ± 5 | 58 ± 8 | 53 ± 5 | 56 ± 6 |
| WBC (109/L) | 4.4 ± 1.9 | 5.0 ± 0.8 | 6.3 ± 2.5 | 4.5 ± 1.0 | 8.0 ± 3.0 | 5.4 ± 3.4 | 5.1 ± 1.9 | 5.0 ± 2 | 7.6 ± 2.8 | 6.1 ± 2.7 | 6.0 ± 2 | 6.9 ± 2.7 |
| RBC (1012/L) | 8.5 ± 0.6 | 8.6 ± 0.4 | 8.5 ± 0.4 | 9.0 ± 0.2 | 8.8 ± 0.6 | 9.1 ± 0.3 | 8.7 ± 0.3 | 8.4 ± 0.7 | 9.0 ± 0.9 | 8.2 ± 1.9 | 9.0 ± 0.4 | 8.9 ± 0.6 |
| MCH (pg) | 17.2 ± 0.9 | 18.0 ± 0.7 | 17.7 ± 0.3 | 16.9 ± 0.6 | 17.4 ± 0.6 | 17.0 ± 0.6 | 17.5 ± 0.4 | 17.8 ± 0.4 | 16.5 ± 1.0 | 17.3 ± 0.7 | 16.6 ± 0.4 | 17.2 ± 1.1 |
| RDW (%) | 18.1 ± 1.4 | 17.1 ± 1.8 | 17.9 ± 2.4 | 18.4 ± 2.2 | 17.5 ± 1.4 | 19.5 ± 1.3 | 16.4 ± 1.1 | 15.7 ± 1.6 | 16.6 ± 2.9 | 14.0 ± 1.2 | 16.0 ± 1.8 | 15.3 ± 1.6 |
| MPV (fL) | 7.7 ± 0.3 | 7.8 ± 0.3 | 8.0 ± 0.5 | 7.5 ± 0.2 | 7.6 ± 0.4 | 7.3 ± 0.2 | 7.4 ± 0.3 | 7.4 ± 0.2 | 7.4 ± 0.1 | 7.7 ± 0.4 | 7.8 ± 0.4 * | 7.6 ± 0.3 |
| PDW (%) | 9.1 ± 0.6 | 9.0 ± 0.5 | 9.5 ± 0.8 | 8.7 ± 0.5 | 8.8 ± 0.8 | 8.3 ± 0.3 | 8.4 ± 0.5 | 8.4 ± 0.5 | 8.3 ± 0.2 | 8.8 ± 0.6 | 9.0 ± 0.8 | 8.7 ± 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Yang, S.-T.; He, T.; Yang, S.; Tang, X.-H. Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice. Int. J. Mol. Sci. 2017, 18, 2562. https://doi.org/10.3390/ijms18122562
Xie P, Yang S-T, He T, Yang S, Tang X-H. Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice. International Journal of Molecular Sciences. 2017; 18(12):2562. https://doi.org/10.3390/ijms18122562
Chicago/Turabian StyleXie, Ping, Sheng-Tao Yang, Tiantian He, Shengnan Yang, and Xiao-Hai Tang. 2017. "Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice" International Journal of Molecular Sciences 18, no. 12: 2562. https://doi.org/10.3390/ijms18122562
APA StyleXie, P., Yang, S.-T., He, T., Yang, S., & Tang, X.-H. (2017). Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice. International Journal of Molecular Sciences, 18(12), 2562. https://doi.org/10.3390/ijms18122562