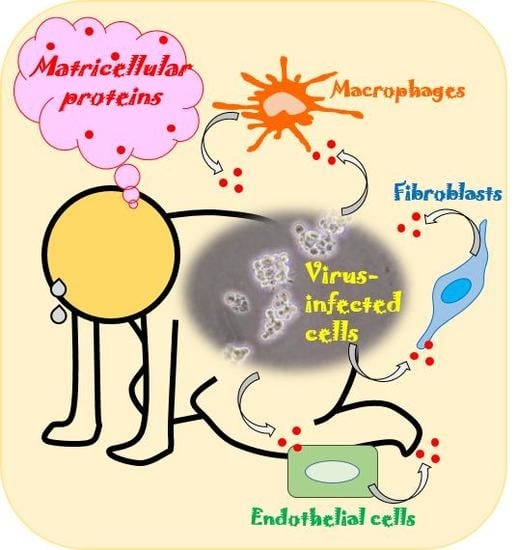
The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets
Abstract
1. Introduction
2. Current Knowledge Regarding the Roles of the Matricellular Proteins That Constitute the ECM in the Tumor Microenvironment
2.1. Osteopontin (OPN)
2.2. Tenascin-C (TNC)
2.3. Thrombospondin (TSP)
2.4. Periostin (POSTN)
2.5. Secreted Protein Acidic and Rich in Cysteine (SPARC)
3. The Involvement of Matricellular Proteins in the Tumorigenesis of Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets
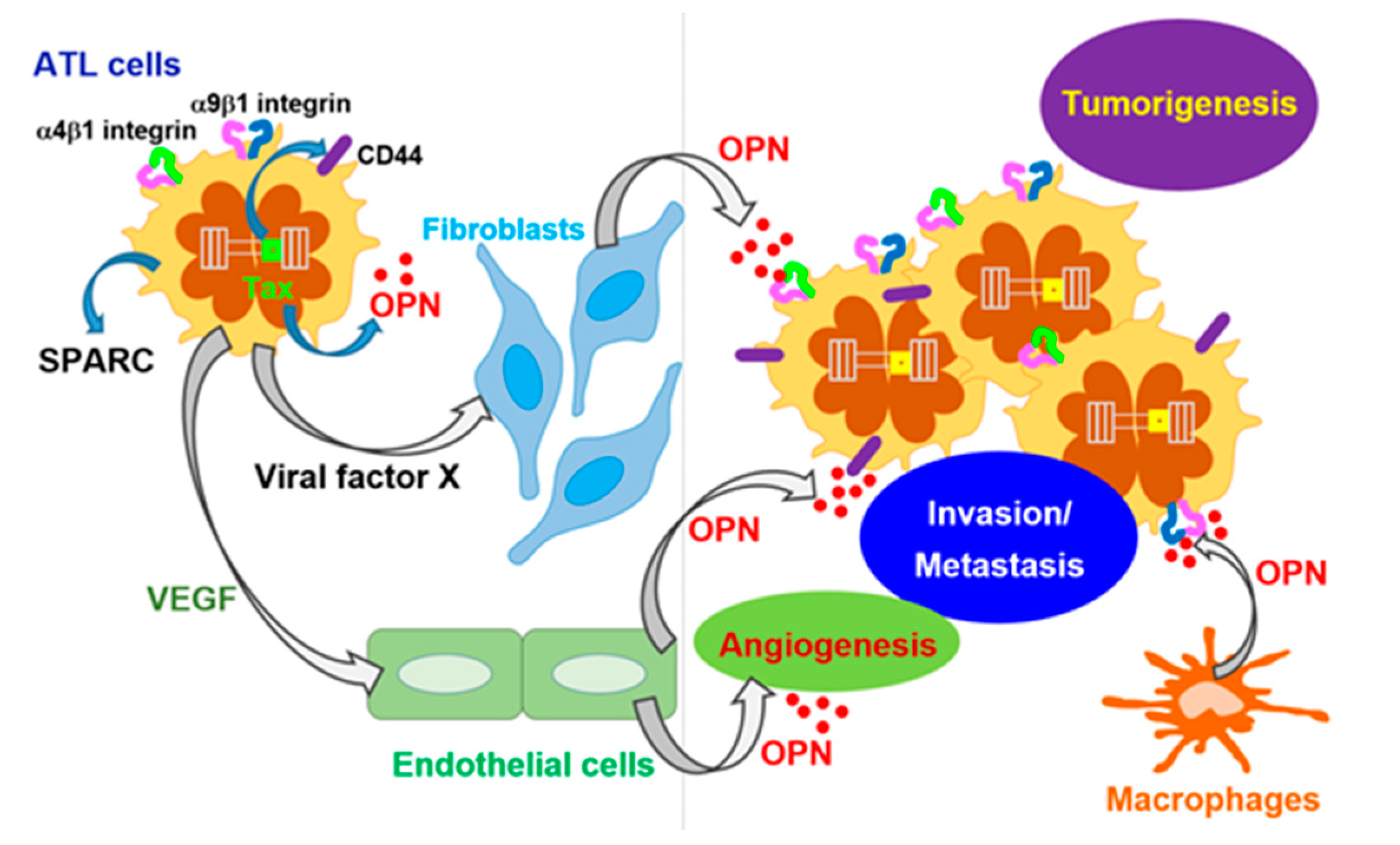
3.1. HTLV-I-Induced ATL
3.2. HBV-Related HCC
3.3. HCV-Related HCC
3.4. HPV-Induced Cervical Cancer
3.5. EBV-Related Nasopharyngeal Carcinoma
3.6. KSHV-Related KS and PEL
3.7. MCPyV-Induced MCC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| a.a. | Amino acid |
| Akt | Protein kinase B |
| ATL | Adult T-cell leukemia |
| EBER | Epstein–Barr virus-encoded small RNA |
| EBV | Epstein–Barr virus |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| EMT | Epithelial-mesenchymal transition |
| ERK | Extracellular signal-regulated kinase |
| HBV | Hepatitis B virus |
| HBx | Hepatitis B virus x protein |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HHV | Human herpesvirus |
| HPV | Human papillomavirus |
| HTLV-I | Human T-cell leukemia virus type I |
| IHC | Immunohistochemical |
| KS | Kaposi’s sarcoma |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| LMP1 | Latent membrane protein 1 |
| LOX | Lipoxygenase |
| mAb | Monoclonal antibody |
| MCPyV | Merkel cell polyomavirus |
| MCC | Merkel cell carcinoma |
| MEK | Mitogen-activated protein kinase kinase |
| miRNA | MicroRNA |
| MMP | Matrix metalloproteinase |
| NOG | NOD/Shi- scid,IL-2Rgnull |
| ODN | Oligodeoxynucleotide |
| OPN | Osteopontin |
| PEL | Primary effusion lymphoma |
| PI3K | Phosphoinositide 3-kinase |
| POSTN | Periostin |
| SPARC | Secreted protein acidic and rich in cysteine |
| sT | Small T |
| TGF-β | Transforming growth factor-beta |
| TNC | Tenascin-C |
| TSP | Thrombospondin |
| uPA | Urokinase-type plasminogen activator |
| VEGF | Vascular endothelial growth factor |
References
- Uede, T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011, 61, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Pascapurnama, D.N.; Labayo, H.K.; Dapat, I.; Nagarajegowda, D.D.; Zhao, J.; Zhang, J.; Yamada, O.; Kikuchi, H.; Egawa, S.; Oshima, Y.; et al. Induction of Osteopontin by Dengue Virus-3 Infection in THP-1 Cells: Inhibition of the Synthesis by Brefelamide and Its Derivative. Front. Microbiol. 2017, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, G.; Blumenthal, M.J.; Katz, A.A. Interaction of human tumor viruses with host cell surface receptors and cell entry. Viruses 2015, 7, 2592–2617. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Cui, Y.; Owen, S.; Li, W.; Cheng, S.; Jiang, W.G. Human osteopontin: Potential clinical applications in cancer (Review). Int. J. Mol. Med. 2017, 39, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.; Wirth, D.F.; Hynes, R.O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 1979, 16, 885–893. [Google Scholar] [CrossRef]
- Briones-Orta, M.A.; Avendaño-Vázquez, S.E.; Aparicio-Bautista, D.I.; Coombes, J.D.; Weber, G.F.; Syn, W.K. Osteopontin splice variants and polymorphisms in cancer progression and prognosis. Biochim. Biophys. Acta 2017, 1868, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Raab-Westphal, S.; Marshall, J.F.; Goodman, S.L. Integrins as Therapeutic Tartes: Success and Cancers. Cancers 2017, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin—A promising biomarker for cancer therapy. J. Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Cappellano, G.; Orilieri, E.; Woldetsadik, A.D.; Boggio, E.; Soluri, M.F.; Comi, C.; Sblattero, D.; Chiocchetti, A.; Dianzani, U. Anti-cytokine autoantibodies in autoimmune diseases. Am. J. Clin. Exp. Immunol. 2012, 1, 136–146. [Google Scholar] [PubMed]
- Ying, X.; Zhao, Y.; Wang, J.L.; Zhou, X.; Zhao, J.; He, C.C.; Guo, X.J.; Jin, G.H.; Wang, L.J.; Zhu, Q.; et al. Serum anti-osteopontin autoantibody as a novel diagnostic and prognostic biomarker in patients with hepatocellular carcinoma. Oncol. Rep. 2014, 32, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, B.; Shi, J.; Peng, L.; Zhang, D.; Qian, W.; Hou, S.; Zhao, L.; Gao, J.; Cao, Z.; et al. A humanized anti-osteopontin antibody inhibits breast cancer growth and metastasis in vivo. Cancer Immunol. Immunother. 2010, 59, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, F.; Scott, N.; Kang, X.; Lappin, P.B.; Fitzgerald, A.A.; Karlicek, S.; Simmons, B.H.; Wu, A.; Lee, J.H.; Bergqvist, S.; et al. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J. Exp. Clin. Cancer Res. 2012, 31, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Dai, J.; Wang, H.; Wei, H.; Zhao, J.; Guo, Y.; Fan, K. Anti-osteopontin monoclonal antibody prevents ovariectomy-induced osteoporosis in mice by promotion of osteoclast apoptosis. Biochem. Biophys. Res. Commun. 2014, 452, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Cen, C.; Aziz, M.; Yang, W.L.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Osteopontin Blockade Attenuates Renal Injury after Ischemia Reperfusion by Inhibiting NK Cell Infiltration. Shock 2017, 47, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Chiovaro, F.; Chiquet-Ehrismann, R.; Chiquet, M. Transcriptional regulation of tenascin genes. Cell Adh. Migr. 2015, 9, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.P.; Chiquet-Ehrismann, R. Tenascin-C: Its functions as an integrin ligand. Int. J. Biochem. Cell Biol. 2015, 65, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Chiquet-Ehrismann, R.; Tucker, R.P. Tenascins and the importance of adhesion modulation. Cold Spring Harb. Perspect. Biol. 2011, 3, a004960. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K.; Aoki, H. Tenascin-C and mechanotrasnduction in the development and diseases of cardiovascular system. Front. Physiol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Akatsuka, T.; Imanaka-Yoshida, K. Tenascin-C and integrins in cancer. Cell Adh. Migr. 2015, 9, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Schumann, C.; Lepper, P.M.; Frank, H.; Schneiderbauer, R.; Wibmer, T.; Kropf, C.; Stoiber, K.M.; Rüdiger, S.; Kruska, L.; Krahn, T.; et al. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 2010, 15, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Page, T.H.; Charles, P.J.; Piccinini, A.M.; Nicolaidou, V.; Taylor, P.C.; Midwood, K.S. Raised circulating tenascin-C in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R260. [Google Scholar] [CrossRef] [PubMed]
- Balasenthil, S.; Huang, Y.; Liu, S.; Marsh, T.; Chen, J.; Stass, S.A.; KuKuruga, D.; Brand, R.; Chen, N.; Frazier, M.L.; et al. A Plasma Biomarker Panel to Identify Surgically Resectable Early-Stage Pancreatic Cancer. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Mock, A.; Warta, R.; Geisenberger, C.; Bischoff, R.; Schulte, A.; Lamszus, K.; Stadler, V.; Felgenhauer, T.; Schichor, C.; Schwartz, C.; et al. Printed peptide arrays identify prognostic TNC serumantibodies in glioblastoma patients. Oncotarget 2015, 6, 13579–13590. [Google Scholar] [CrossRef] [PubMed]
- Odaka, K.; Uehara, T.; Arano, Y.; Adachi, S.; Tadokoro, H.; Yoshida, K.; Hasegawa, H.; Imanaka-Yoshida, K.; Yoshida, T.; Hiroe, M.; et al. Noninvasive detection of cardiac repair after acute myocardial infarction in rats by 111In Fab fragment of monoclonal antibody specific for tenascin-C. Int. Heart J. 2008, 49, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Odaka, K.; Uehara, T.; Imanaka-Yoshida, K.; Kato, Y.; Oyama, H.; Tadokoro, H.; Akizawa, H.; Tanada, S.; Hiroe, M.; et al. Toward in vivo imaging of heart disease using a radiolabeled single-chain Fv fragment targeting tenascin-C. Anal. Chem. 2011, 83, 9123–9130. [Google Scholar] [CrossRef] [PubMed]
- Kirk, J.A.; Cingolani, O.H. Thrombospondins in the transition from myocardial infarction to heart failure. J. Mol. Cell. Cardiol. 2016, 90, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Lawler, J.W.; Slayter, H.S.; Coligan, J.E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J. Biol. Chem. 1978, 253, 8609–8616. [Google Scholar] [PubMed]
- Chen, H.; Herndon, M.E.; Lawler, J. The cell biology of thrombospondin-1. Matrix Biol. 2000, 19, 597–614. [Google Scholar] [CrossRef]
- Lawler, P.R.; Lawler, J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef] [PubMed]
- Dudek, A.Z.; Mahaseth, H. Circulating angiogenic cytokines in patients with advanced non-small cell lung cancer: Correlation with treatment response and survival. Cancer Investig. 2005, 23, 193–200. [Google Scholar] [CrossRef]
- Cymbaluk-Płoska, A.; Chudecka-Głaz, A.; Pius-Sadowska, E.; Machaliński, B.; Menkiszak, J. Thrombospondin-I concentrations behavior in plasma of patients with ovarian cancer. Cancer Biomark. 2017, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, H.; Shimada, M.; Yoshikawa, K.; Higashijima, J.; Tokunaga, T.; Nishi, M.; Takasu, C.; Ishikawa, D. Correlation Between Thrombospondin-1 Expression in Non-cancer Tissue and Gastric Carcinogenesis. Anticancer Res. 2017, 37, 3547–3552. [Google Scholar] [PubMed]
- Jeanne, A.; Schneider, C.; Martiny, L.; Dedieu, S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front. Pharmacol. 2015, 6, 252. [Google Scholar] [CrossRef] [PubMed]
- Coronella, J.; Li, L.; Johnson, K.; Pirie-Shepherd, S.; Roxas, G.; Levin, N. Selective activity against proliferating tumor endothelial cells by CVX-22, a thrombospondin-1 mimetic CovX-Body. Anticancer Res. 2009, 29, 2243–2252. [Google Scholar] [PubMed]
- Li, L.; Leedom, T.A.; Do, J.; Huang, H.; Lai, J.; Johnson, K.; Osothprarop, T.F.; Rizzo, J.D.; Doppalapudi, V.R.; Bradshaw, C.W.; et al. Antitumor efficacy of a thrombospondin 1 mimetic CovX-body. Transl. Oncol. 2011, 4, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Kikuno, R.; Tezuka, K.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Idolazzi, L.; Ridolo, E.; Fassio, A.; Gatti, D.; Montagni, M.; Caminati, M.; Martignago, I.; Incorvaia, C.; Senna, G. Periostin: The bone and beyond. Eur. J. Intern. Med. 2017, 38, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, P.V.; Buzzatti, G.; Ricci, F.; Rubagotti, A.; Argellati, F.; Zinoli, L.; Boccardo, F. Periostin: A novel prognostic and therapeutic target for genitourinary cancer? Clin. Genitourin. Cancer 2014, 12, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αvβ3 and αvβ5 integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar] [PubMed]
- Baril, P.; Gangeswaran, R.; Mahon, P.C.; Caulee, K.; Kocher, H.M.; Harada, T.; Zhu, M.; Kalthoff, H.; Crnogorac-Jurcevic, T.; Lemoine, N.R. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: Role of the β4 integrin and the PI3k pathway. Oncogene 2007, 26, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Moniuszko, T.; Wincewicz, A.; Koda, M.; Domysławska, I.; Sulkowski, S. Role of periostin in esophageal, gastric and colon cancer. Oncol. Lett. 2016, 12, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, B.A. Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J. Gastroenterol. 2011, 17, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, P.V.; Rubagotti, A.; Argellati, F.; Di Meglio, A.; Zanardi, E.; Zinoli, L.; Comite, P.; Mussap, M.; Boccardo, F. Prognostic Value of Preoperative Serum Levels of Periostin (PN) in Early Breast Cancer (BCa). Int. J. Mol. Sci. 2015, 16, 17181–17192. [Google Scholar] [CrossRef] [PubMed]
- Thuwajit, C.; Thuwajit, P.; Jamjantra, P.; Pairojkul, C.; Wongkham, S.; Bhudhisawasdi, V.; Ono, J.; Ohta, S.; Fujimoto, K.; Izuhara, K. Clustering of patients with intrahepatic cholangiocarcinoma based on serum periostin may be predictive of prognosis. Oncol. Lett. 2017, 14, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.H.; Wang, W.; Lin, Y.; Qian, L.H.; Zhang, X.W.; Wang, Q.B.; Yu, L.K. Diagnostic and prognostic value of serum periostin in patients with non-small cell lung cancer. Oncotarget 2017, 8, 18746–18753. [Google Scholar] [CrossRef] [PubMed]
- Field, S.; Uyttenhove, C.; Stroobant, V.; Cheou, P.; Donckers, D.; Coutelier, J.P.; Simpson, P.T.; Cummings, M.C.; Saunus, J.M.; Reid, L.E.; et al. Novel highly specific anti-periostin antibodies uncover the functional importance of the fascilin 1-1 domain and highlight preferential expression of periostin in aggressive breast cancer. Int. J. Cancer 2016, 138, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Kyutoku, M.; Taniyama, Y.; Katsuragi, N.; Shimizu, H.; Kunugiza, Y.; Iekushi, K.; Koibuchi, N.; Sanada, F.; Oshita, Y.; Morishita, R. Role of periostin in cancer progression and metastasis: Inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int. J. Mol. Med. 2011, 28, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Termine, J.D.; Kleinman, H.K.; Whitson, S.W.; Conn, K.M.; McGarvey, M.L.; Martin, G.R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell 1981, 26, 99–105. [Google Scholar] [CrossRef]
- Said, N. Roles of SPARC in urothelial carcinogenesis, progression and metastasis. Oncotarget 2016, 7, 67574–67585. [Google Scholar] [CrossRef] [PubMed]
- Trombetta-Esilva, J.; Bradshaw, A.D. The Function of SPARC as a Mediator of Fibrosis. Open Rheumatol. J. 2012, 6, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Frierson, H.F.; Sanchez-Carbayo, M.; Brekken, R.A.; Theodorescu, D. Ross of SPARC in bladder cancer enhances carcinogenesis and progression. J. Clin. Investig. 2013, 123, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Jiang, K.; Fu, Y.; Fang, R.; Liu, X.I.; Chen, J. Overexpression of SPARC correlates with poor prognosis in patients with cervical carcinoma and regulates cancer cell epithelial-mesenchymal transition. Oncol. Lett. 2016, 11, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.; Ansari, D.; Sasor, A.; Andersson, R. SPARC: A Potential Prognostic and Therapeutic Target in Pancreatic Cancer. Pancreas 2015, 44, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Mateo, F.; Meca-Cortés, O.; Celià-Terrassa, T.; Fernández, Y.; Abasolo, I.; Sánchez-Cid, L.; Bermudo, R.; Sagasta, A.; Rodríguez-Carunchio, L.; Pons, M.; et al. SPARC mediates metastatic cooperation between CSC and non-CSC prostate cancer cell subpopulations. Mol. Cancer 2014, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.L.; Fishbein, M.C.; Hong, L.S.; Krysan, K.; Minna, J.D.; Shay, J.W.; Walser, T.C.; Dubinett, S.M. A novel molecular pathway for Snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis. Cancer Prev. Res. 2014, 7, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Chiodoni, C.; Sangaletti, S.; Colombo, M.P. Matricellular proteins tune myeloid-derived suppressor Cell recruitment and function in breast cancer. J. Leukoc. Biol. 2017, 102, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Fan, H.; Yoshikai, Y. Oncogenesis by retroviruses: Old and new paradigms. Rev. Med. Virol. 2008, 18, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, H.; Ishitsuka, K. Treatment advances and prognosis for patients with adult T-cell leukemia-lymphoma. J. Clin. Exp. Hematop. 2017, 17008. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Adult T-cell leukemia: Molecular basis for clonal expansion and transformation of HTLV-1-infected T cells. Blood 2017, 129, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Kogure, Y.; Kataoka, K. Genetic alterlations in adult T-cell leukemia/lymphoma. Cancer Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yamada, O.; Matsushita, Y.; Chagan-Yasutan, H.; Hattori, T. Transactivation of human osteopontin promoter by human T-cell leukemia virus type 1-encoded Tax protein. Leuk. Res. 2010, 34, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Ohashi, T.; Chagan-Yasutan, H.; Hattori, T.; Takahashi, Y.; Harigae, H.; Hasegawa, H.; Yamada, Y.; Fujii, M.; Maenaka, K.; et al. Osteopontin-integrin interaction as a novel molecular target for antibody-mediated immunotherapy in adult T-cell leukemia. Retrovirology 2015, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Tsukasaki, K.; Takahashi, Y.; Oguma, S.; Harigae, H.; Ishii, N.; Zhang, J.; Fukumoto, M.; Hattori, T. Involvement of osteopontin and its signaling molecule CD44 in clinicopathological features of adult T cell leukemia. Leuk. Res. 2011, 35, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Peng, L.; Fan, K.; Wang, H.; Wei, R.; Ji, G.; Cai, J.; Lu, B.; Li, B.; Zhang, D.; et al. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene 2009, 28, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Jain, S.; Kundu, G.C. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008, 68, 152–161. [Google Scholar] [CrossRef] [PubMed]
- El-Sabban, M.E.; Merhi, R.A.; Haidar, H.A.; Arnulf, B.; Khoury, H.; Basbous, J.; Nijmeh, J.; de Thé, H.; Hermine, O.; Bazarbachi, A. Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood 2002, 99, 3383–3389. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Abou Merhi, R.; Gessain, A.; Talhouk, R.; El-Khoury, H.; Nasr, R.; Gout, O.; Sulahian, R.; Homaidan, F.; de Thé, H.; et al. Human T-cell lymphotropic virus type I-infected cells extravasate through the endothelial barrier by a local angiogenesis-like mechanism. Cancer Res. 2004, 64, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Watters, K.M.; Dean, J.; Gautier, V.; Hall, W.W.; Sheehy, N. Tax 1-independent induction of vascular endothelial growth factor in adult T-cell leukemia caused by human T-cell leukemia virus type 1. J. Virol. 2010, 84, 5222–5228. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, J.H.; Hamamura, R.; Kobayashi, C.; Zhang, Y.; Ohyashiki, K. A network biology approach evaluating the anticancer effects of bortezomib identifies SPARC as a therapeutic target in adult T-cell leukemia cells. Adv. Appl. Bioinform. Chem. 2008, 1, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Koh, S.S.; Lee, C.G. Hepatitis B Virus X Protein and Hepatocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 940. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yu, J.; Wong, V.W. Mechanism and prediction of HCC development in HBV infection. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, L.H.; Zhang, X.D. A mutant of hepatitis B virus X protein (HBxΔ127) enhances hepatoma cell migration via osteopontin involving 5-lipoxygenase. Acta Pharmacol. Sin. 2010, 31, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; You, X.; Wang, Q.; Zhang, T.; Du, Y.; Lv, N.; Zhang, Z.; Zhang, S.; Shan, C.; Ye, L.; et al. Hepatitis B virus X protein drives multiple cross-talk cascade loops involving NF-κB, 5-LOX, OPN and Capn4 to promote cell migration. PLoS ONE 2012, 7, e31458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, T.; Wang, Y.; Pan, Y.; Ning, H.; Hui, X.; Xie, H.; Wang, J.; Han, Y.; Liu, Z.; et al. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int. J. Clin. Pract. 2008, 62, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.N.; Plymoth, A.; Santos-Silva, D.; Ortiz-Cuaran, S.; Camey, S.; Guilloreau, P.; Sangrajrang, S.; Khuhaprema, T.; Mendy, M.; Lesi, O.A.; et al. Osteopontin and latent-TGF β binding-protein 2 as potential diagnostic markers for HBV-related hepatocellular carcinoma. Int. J. Cancer 2015, 136, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Song, J.; Du, R.; Liu, K.; Wang, J.; Tang, H.; Bai, F.; Liang, J.; Lin, T.; Liu, J.; et al. Prognostic significance of osteopontin in hepatitis B virus-related hepatocellular carcinoma. Dig. Liver Dis. 2007, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Chen, J.; He, J.; Lu, C.; Wei, Y.; Wang, L.; Xu, X.; Li, L.; Uede, T.; Diao, H. Osteopontin promotes dendritic cell maturation and function in response to HBV antigens. Drug Des. Dev. Ther. 2015, 9, 3003–3016. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Deng, B.; Wu, X.; Liu, J.; Feng, X. Identification of Enolase 1 and Thrombospondin-1 as serum biomarkers in HBV hepatic fibrosis by proteomics. Proteome Sci. 2013, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Bastos, J.C.; Padilla, M.A.; Caserta, L.C.; Miotto, N.; Vigami, A.G.; Arns, C.W. Hepatitis C virus: Promising discoveries and new treatments. World J. Gastroenterol. 2016, 22, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Vescovo, T.; Refolo, G.; Vitagliano, G.; Fimia, G.M.; Piacentini, M. Molecular mechanisms of hepatitis C virus-induced hepatocellular carcinoma. Clin. Microbiol. Infect. 2016, 22, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Presser, L.D.; Haskett, A.; Waris, G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-β1: Role of TGF-β1 in HCV replication. Virology 2011, 412, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Benzoubir, N.; Lejamtel, C.; Battaglia, S.; Testoni, B.; Benassi, B.; Gondeau, C.; Perrin-Cocon, L.; Desterke, C.; Thiers, V.; Samuel, D.; et al. HCV core-mediated activation of latent TGF-β via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J. Hepatol. 2013, 59, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Meyer, K.; Di Bisceglie, A.M.; Ray, R.B.; Ray, R. Hepatitis C virus induces epithelial-mesenchymal transition in primary human hepatocytes. J. Virol. 2012, 86, 13621–13628. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.C.; Bose, S.K.; Steele, R.; Meyer, K.; Di Bisceglie, A.M.; Ray, R.B.; Ray, R. Promotion of Cancer Stem-Like Cell Properties in Hepatitis C Virus-Infected Hepatocytes. J. Virol. 2015, 89, 11549–11556. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Devhare, P.; Ray, R.B.; Ray, R. Hepatitis C virus induced tumor initiating cancer stem-like cells activate stromal fibroblasts in xenograft tumor model. Hepatology 2017. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, G.; Huang, M.; Lou, G.; Liu, Y.; Wang, S. Plasma osteopontin concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected subjects. Clin. Chim. Acta 2010, 411, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Abu El Makarem, M.A.; Abdel-Aleem, A.; Ali, A.; Saber, R.; Shatat, M.; Rahem, D.A.; Sayed, D. Diagnostic significance of plasma osteopontin in hepatitis C virus-related hepatocellular carcinoma. Ann. Hepatol. 2011, 10, 296–305. [Google Scholar] [PubMed]
- Matsue, Y.; Tsutsumi, M.; Hayashi, N.; Saito, T.; Tsuchishima, M.; Toshikuni, N.; Arisawa, T.; George, J. Serum osteopontin predicts degree of hepatic fibrosis and serves as a biomarker in patients with hepatitis C virus infection. PLoS ONE 2015, 10, e0118744. [Google Scholar] [CrossRef] [PubMed]
- Cabiati, M.; Gaggini, M.; Cesare, M.M.; Caselli, C.; De Simone, P.; Filipponi, F.; Basta, G.; Gastaldelli, A.; Del Ry, S. Osteopontin in hepatocellular carcinoma: A possible biomarker for diagnosis and follow-up. Cytokine 2017, 99, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.X.; Xia, Y.H.; Xue, T.C.; Zhang, H.; Ye, S.L. Down-regulation of osteopontin inhibits metastasis of hepatocellular carcinoma cells via a mechanism involving MMP-2 and uPA. Oncol. Rep. 2011, 25, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; McRae, S.; Banaudha, K.; Mai, T.; Waris, G. Mechanism of hepatitis C virus (HCV)-induced osteopontin and its role in epithelial to mesenchymal transition of hepatocytes. J. Biol. Chem. 2013, 288, 36994–37009. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef]
- Senapati, R.; Senapati, N.N.; Dwibedi, B. Molecular mechanisms of HPV mediated neoplastic progression. Infect. Agent. Cancer 2016, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Bequet-Romero, M.; López-Ocejo, O. Angiogenesis modulators expression in culture cell lines positives for HPV-16 oncoproteins. Biochem. Biophys. Res. Commun. 2000, 277, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Toussaint-Smith, E.; Donner, D.B.; Roman, A. Expression of human papillomavirus type 16 E6 and E7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene 2004, 23, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, F.; Mead, L.; White, H.; Walker, J.; Ingram, D.A.; Roman, A. Human papillomavirus causes an angiogenic switch in keratinocytes which is sufficient to alter endothelial cell behavior. Virology 2007, 367, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Si, Q.; Jia, L.; Ren, X.; Ma, R.; Wang, Y. Detection of human papillomavirus and expression of osteopontin in cervical cancer specimens. Mol. Med. Rep. 2015, 11, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.M.; Lowy, D.R.; Schiller, J.T.; Day, P.M. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Behera, R.; Lohite, K.; Karnik, S.; Kundu, G.C. p38 kinase is crucial for osteopontin-induced furin expression that supports cervical cancer progression. Cancer Res. 2010, 70, 10381–10391. [Google Scholar] [CrossRef] [PubMed]
- Tiitta, O.; Wahlström, T.; Paavonen, J.; Linnala, A.; Sharma, S.; Gould, V.E.; Virtanen, I. Enhanced tenascin expression in cervical and vulvar koilocytotic lesions. Am. J. Pathol. 1992, 141, 907–913. [Google Scholar] [PubMed]
- Pöllänen, R.; Soini, Y.; Vuopala, S.; Läärä, E.; Lehto, V.P. Tenascin in human papillomavirus associated lesions of the uterine cervix. J. Clin. Pathol. 1996, 49, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; Lambert, P.F. Human Papillomavirus and the Stroma: Bidirectional Crosstalk during the Virus Life Cycle and Carcinogenesis. Viruses 2017, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Al-Shraim, M.; Al-Hakami, A.M.; Jones, I.M. Epstein-Barr Virus: Clinical and Epidemiological Revisits and Genetic Basis of Oncogenesis. Open Virol. J. 2015, 9, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Jha, H.C.; Pei, Y.; Robertson, E.S. Epstein-Barr Virus: Diseases Linked to Infection and Transformation. Front. Microbiol. 2016, 7, 1602. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Morgan, D.R.; Meyers, M.O.; Dominguez, R.L.; Martinez, E.; Kakudo, K.; Kuan, P.F.; Banet, N.; Muallem, H.; Woodward, K.; et al. Epstein-Barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect. Agent. Cancer 2012, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yao, K. Molecular characterization and clinical implications of spindle cells in nasopharyngeal carcinoma: A novel molecule-morphology model of tumor progression proposed. PLoS ONE 2013, 8, e83135. [Google Scholar] [CrossRef] [PubMed]
- Aneja, K.K.; Yuan, Y. Reactivation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus: An Update. Front. Microbiol. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, D.P.; Damania, B. Kaposi’s sarcoma-associated herpesvirus: Immunobiology, oncogenesis, and therapy. J. Clin. Investig. 2016, 126, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Myoung, J. Primary lymphocyte infection models for KSHV and its putative tumorigenesis mechanisms in B cell lymphomas. J. Microbiol. 2017, 55, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kaaya, E.E.; Castaños-Velez, E.; Amir, H.; Lema, L.; Luande, J.; Kitinya, J.; Patarroyo, M.; Biberfeld, P. Expression of adhesion molecules in endemic and epidemic Kaposi’s sarcoma. Histopathology 1996, 29, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; Benelli, R.; Borsotti, P.; Rusnati, M.; Presta, M.; Giavazzi, R.; Ruco, L.; Albini, A. Thrombospondin-1 inhibits Kaposi’s sarcoma (KS) cell and HIV-1 Tat-induced angiogenesis and is poorly expressed in KS lesions. J. Pathol. 1999, 188, 76–81. [Google Scholar] [CrossRef]
- Samols, M.A.; Skalsky, R.L.; Maldonado, A.M.; Riva, A.; Lopez, M.C.; Baker, H.V.; Renee, R. Identification of cellular genes targeted by KSHV-encoded microRNAs. PLoS Pathog. 2007, 3, e65. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qiao, J.; Lin, Z.; Zabaleta, J.; Dai, L.; Qin, Z. Up-regulation of tumor suppressor genes by exogenous dhC16-Cer contributes to its anti-cancer activity in primary effusion lymphoma. Oncotarget 2017, 8, 15220–15229. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; MacDonald, M.; You, J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr. Opin. Virol. 2016, 20, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Koljonen, V.; Jahkola, T.; Tukiainen, E.; Granroth, G.; Haglund, C.; Böhling, T. Tenascin-C in primary Merkel cell carcinoma. J. Clin. Pathol. 2005, 58, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Koljonen, V.; Böhling, T.; Tukiainen, E.; Haglund, C.; Jahkola, T. Tenascin-C expression in Merkel cell carcinoma lymph node metastasis. APMIS 2006, 114, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, R.; Payne, A.S.; Schowalter, R.M.; Spurgeon, M.E.; Lambert, P.F.; Xu, X.; Buck, C.B.; You, J. Identifying the Target Cells and Mechanisms of Merkel Cell Polyomavirus Infection. Cell Host Microbe 2016, 19, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Kwun, H.J.; Feng, H.; Chang, Y.; Moore, P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Investig. 2011, 121, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Matsuda, A.; Maenaka, K. Antibody-mediated molecular-targeted therapy for adult T-cell leukemia: Recent progress and future challenges in the treatment of cancers. Cancer Cell Microenviron. 2016, 3, e1201. [Google Scholar] [CrossRef]

| Viruses | Viral Genes/Oncogenes | Matricellular Proteins | Phenomena/Mechanisms | References |
|---|---|---|---|---|
| HTLV-I | Tax | OPN | Transcriptional upregulation of OPN resulted in the activation of PI3K/Akt pathway | [63] |
| ? | OPN | Stromal cell-derived OPN involved in the tumorigenesis and metastasis | [64,65] | |
| ? | SPARC | SPARC inhibition resulted in caspase 3-dependent apoptosis by bortezomib | [71] | |
| HBV | HBx | OPN | 5-LOX-dependent upregulation of OPN promoted cell migration | [74,75] |
| ? | OPN | Elevated production in cirrhosis and HCC | [76,77,78] | |
| ? | TSP-1 | Elevated production in fibrosis | [80] | |
| HCV | NS3/4A, NS5A | TSP-1 | Proteolytic activation of TGF-β by intracellular TSP-1 | [83] |
| Core | TSP-1 | Increased secretion of TSP-1 activated TGF-β | [84] | |
| ? | OPN | Antisense ODNs suppressed lung metastasis via downregulating MMP-2 and uPA | [92] | |
| HPV | E6, E7 | TSP-1 | Decreased expression in keratinocytes | [96,97,98] |
| ? | OPN | Induction of furin via p38 and NF-kB resulted in cancer progression | [99] | |
| ? | Tenascin * | Expressed during the premalignant stage | [103] | |
| EBV | ? | OPN | Elevated expression | [107] |
| LMP1, EBER | SPARC POSTN | Elevated expression | [107,108] | |
| KSHV | ? | Tenascin * | Expressed in the vessel walls | [112] |
| ? | TSP-1 | Viral miRNA-dependent TSP-1 reduction decreased TGF-β activity | [113,114] | |
| ? | OPN | Viral miRNA-dependent reduction | [114] | |
| MCPyV | sT antigen? | TNC | Tumorigenesis and metastasis | [118,119] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, N.; Maenaka, K. The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 2198. https://doi.org/10.3390/ijms18102198
Maeda N, Maenaka K. The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. International Journal of Molecular Sciences. 2017; 18(10):2198. https://doi.org/10.3390/ijms18102198
Chicago/Turabian StyleMaeda, Naoyoshi, and Katsumi Maenaka. 2017. "The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets" International Journal of Molecular Sciences 18, no. 10: 2198. https://doi.org/10.3390/ijms18102198
APA StyleMaeda, N., & Maenaka, K. (2017). The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. International Journal of Molecular Sciences, 18(10), 2198. https://doi.org/10.3390/ijms18102198