1. Introduction
The transplantation of stem cells has been proposed as a novel approach for the treatment of patients with several diseases, including ischemic heart injury [
1]. Various cells, including bone marrow derived as mononuclear cells, endothelial progenitor cells, mesenchymal stem cells (MSCs), and skeletal myoblasts, have been examined in ischemic heart models [
1,
2,
3,
4]. A major issue with this promising therapy is the proper vascularization and maintenance of an adequate number of surviving cells after cell transplantation [
5,
6,
7,
8,
9,
10] Tissue replacement has been proposed to repair congenital defects or diseased tissue [
1,
2,
3,
4]. Endothelial progenitor cells (EPCs) of bone marrow have been proposed to have potential therapeutic applications for ischemic cardiovascular diseases [
5,
6,
7,
8]. Bone marrow-derived EPCs are known to circulate to neovascularization sites where they differentiate into endothelial cells and contribute to vasculogenesis [
9,
11,
12] and the neovascularization of ischemic injuries [
5,
6,
7,
8]. Stem cells derived from bone marrow have distinct and specific functions: hematopoietic stem cells, EPCs, and non-hematopoietic stem cells [
13]. While hematopoietic stem cells give rise to blood components, EPCs secrete various endothelial growth factors, promoting angiogenesis in pathological conditions [
14]. Non-hematopoietic stem cells as MSCs can differentiate to myogenic, osteogenic, chondrogenic, and adipogenicmesodermal lineages. Moreover, the MSCs are able to differentiate into ectodermal tissues, such as glial cells [
15,
16]. One way to identify hematopoietic, endothelial, and mesenchymal stem cells is through their membrane markers. Hematopoietic stem cells exhibit the membrane markers CD45+ and CD34+. While endothelial cells are phenotypically identified by CD34+ and CD45−, MSC show the CD45− and CD34− phenotypes [
17]. In addition, there are other cells that are also recognized by the CD45− and CD34+ phenotypes, e.g., platelets and cells oferythroid lineage; however, the EPCs can be differentiated by specific markers, such as CD31+.
Thyroid and ovarian hormones regulate cellular homeostasis, proliferation, apoptosis, and differentiation [
18]. Investigating the hormonal regulation of stem cells allows for an understanding of stem/progenitor cell physiological and biological activities that are required for important therapeutic applications. The role of thyroid and ovarian hormones in stem cell function is still unclear. Thyroid hormones have been shown to inhibit the proliferation of some cells, including cultured embryonic neural stem cells, and have been suggested to regulate the activity of insulin-like growth factor type 1 (IGF-1) [
19,
20]; however, their roles in the proliferation of bone marrow stem cells have not been clarified [
21]. Previous investigators have reported that ovariectomy upregulates B lymphopoiesis in rat bone marrow and that decreased estrogen is involved in the expansion of B cell precursor and mature B cell populations; however, the role of B lineage cells in ovariectomy-induced bone loss in vivo is unclear [
22,
23]. It was previously reported that there are bidirectional interactions between the hypothalamic-pituitary-thyroid (HPT) axis and the immune system [
24]. While hypothyroidism leads to an inhibition of humoral and cell-mediated immune responses, hyperthyroidism was found to upregulate immunity.
The formation of new blood vessels is essential for successful bone marrow stem cell transplantation and tissue regeneration [
25]. On the other hand, the proliferation, differentiation, and survival of progenitor populations depends on various factors secreted by other cell types. Specifically, a wide range of secreted factors regulates the proliferation and fate of stem cells; however, the mechanisms have not been identified. Hormonal action is regulated by multiple signaling molecules that control vital biological processes of various body cell types, such as growth, development, proliferation, and differentiation. The end result of hormonal action is a general increase in functional activity throughout mammalian cells, including cell proliferation [
26]. Thyroid hormones can act indirectly to regulate the transcription of genes that influence metabolism, cell growth, and the development of all organ systems. Therefore, the clinical manifestation of thyroid disturbances are typically insidious, multiple, and diverse. The main form of thyroid hormone secreted by the thyroid gland is 3,5,3′,5′-tetraiodo-L-thyronine (T4 or thyroxine). The thyroid gland also secretes a smaller quantity of 3,5,3′-triiodothyronine (T3), the active form of the hormone. In addition, the concentration of T4 is about 45 times higher than that of T3 (90 nm versus 2 nm), and the primary production source of T3 occurs by the conversion of T4 to T3 in peripheral tissues [
27]. Besides, the actions of these hormones require receptors in both the cell membrane and the nucleus [
28]. In the presence of these hormones, thyroid hormone receptors bind with co-activator protein and allow for the organization and activation of transcription machinery [
29]. Hypothyroidism is the most common pathology of the thyroid gland. In most cases, it is caused by abnormalities of the thyroid gland itself (primary hypothyroidism), such as, not producing enough of the hormone. Thyroid hormone level has been suggested to modulate the level of critical cell cycle proteins, specifically cyclin A, resulting in an alteration of S-phase progression [
30]. Moreover, the action of pituitary leptin as a local inhibitor of Thyroid Stimulating Hormone (TSH) release also disappears in hypothyroidism [
31].
Ovarian estrogens regulate the proliferation and growth of sex organs and other tissues related to reproduction, but their role is not limited to reproductive functions. Estrogens also play an important role in the balance between bone formation and resorption, and their loss causes critical physiological and pathological problems [
32].
Given the above-mentioned facts, there is a growing interest to study hormonal influence on the proliferation of stem cells, especially the role of the thyroid and ovarian hormones in females. Many patients are already being subjected to bone marrow samples, with the purpose of isolating hematopoietic stem cells from bone marrow, without prior knowledge of the proliferative state of the cells to be transplanted. Increased or decreased stem cell proliferation maybe a significant factor in determining an efficient therapeutic result. To ensure greater success in transplantation, the integration of transplanted stem cells in vivo or ex vivo is favored by the increased proliferative capacity of the stem cells. In this study, we analyzed proliferative activity by detecting bromodeoxyuridine (BRDU), which is incorporated by cells during the S-phase of mitosis. This marker does not require immunohistochemistry and can be quantified by flow cytometry [
33].
In the present study, we used bone marrow-derived mesenchymal stem cells, which have been reported to be very efficient in regenerative medicine due to their multi-lineage differentiation character and high potential to repair tissues. We compared the proliferative activity of these cells with other bone marrow-derived stem cells (BMDSCs) of rats subjected to the same conditions (ovariectomy and/or thyroidectomy). This enabled us to analyze the role of hormonal interactions (in vivo) in regulating BMDSCs expansion. We used specific markers and flow cytometry to assess the response of affected cell populations. Our results revealed diverse effects of thyroid and ovarian hormonal loss on BMDSCs proliferation.
3. Discussion
The use of BMDSCs has made a number of contributions to the study of regenerative medicine; however, the hormonal regulation of the biological activities of these cells is not fully understood. Experimental thyroidectomy and ovariectomy in animal models have led to many advances in the fields of cell biology, endocrinology, and metabolism, particularly with regard to studying the roles of thyroid and ovarian hormones in regulating mechanisms of cell survival, proliferation, differentiation, and programmed cell death. In the present study, we investigated endocrine-dependent events of bone marrow stem cell survival and proliferation upon the removal of the thyroid gland or/and ovaries. Some previous studies have studied the hormonal influence on cell biology by using thyroid inhibitors at the receptor and histone acetylation levels. Other studies have focused on disturbing mechanisms involved in thyroid hormone biosynthesis, cellular uptake, cellular transport, or nuclear action. These strategies are known to cause many problems, such as a disruption of oxygen consumption and peripheral metabolic abnormalities via unknown mechanisms [
34,
35,
36]. Importantly, these methods were not efficient enough to analyze the effects of a complete loss of hormonal action. Therefore, to understand the role of a complete lack of ovarian or/and thyroid hormones in BMDSCs proliferation, we performed ovariectomies or/and thyroidectomies, respectively, in female Wistar rats.
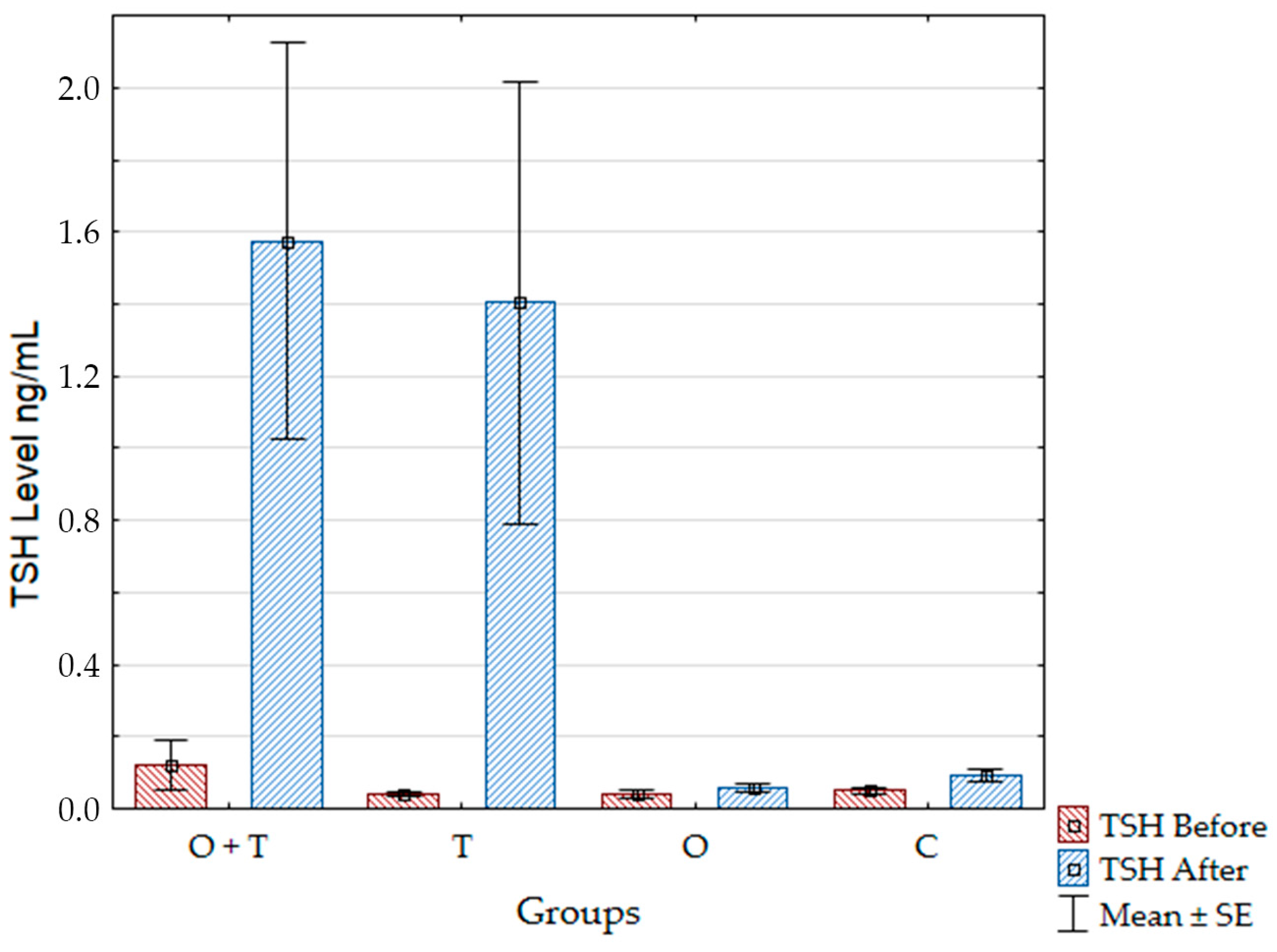
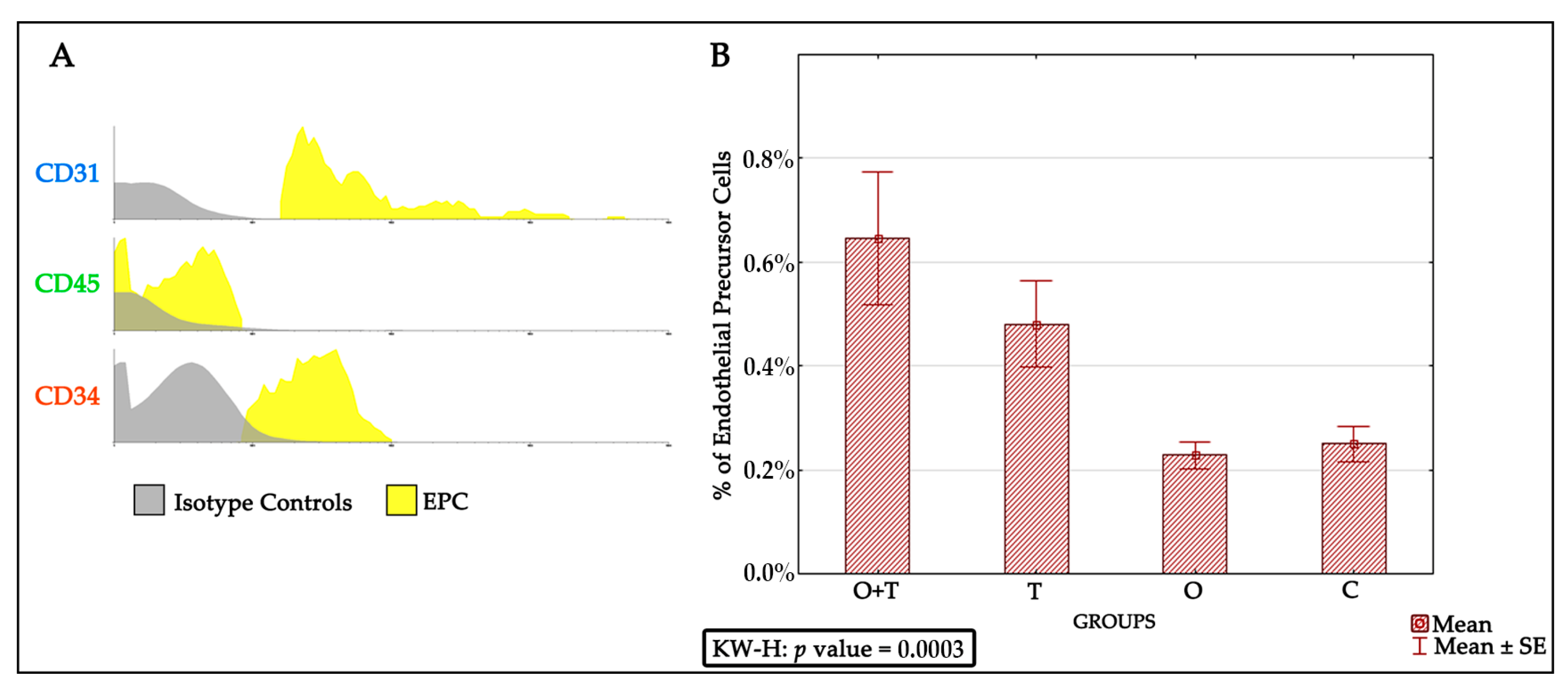
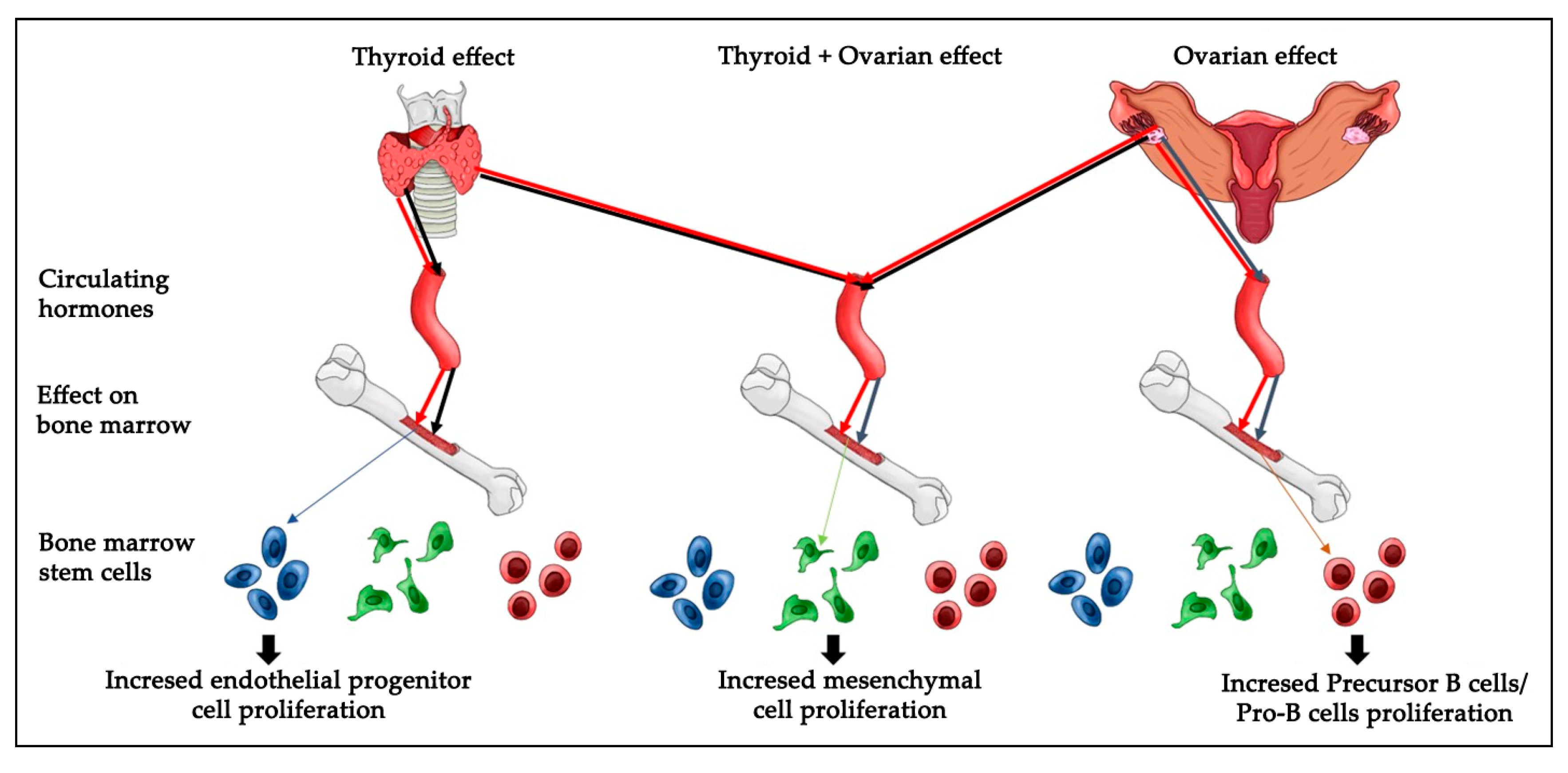
The endothelial progenitor cells were identified via detecting for positivity of the CD31+, CD45−, and CD34+ phenotypes. We found an increased endothelial progenitor cell number in the thyroidectomized group. However, our analysis did not detect any change in the endothelial progenitor cell proliferation of the ovariectomized or the control groups. Because decreased estrogen affects total bone marrow mass, we asked if a decrease in ovarian hormones could block the stimulatory effect of thyroid removal on endothelial progenitor cells proliferation. Interestingly, our results indicate that the absence of ovarian hormone does not change the increased endothelial progenitor cell number caused by thyroid hormone removal (
Figure 3).
Bone marrow EPCs are derived from CD34+ stem cells and undergo continuous differentiation into erythrocytes, thrombocytes, leukocytes, and mature endothelial cells [
9,
37]; however, the regulatory mechanisms are incompletely elucidated. EPCs are involved in neovascularization to improve cardiac function after cardiac ischemia [
38]. In humans with ischemic hearts, the outcome highly correlates to the number of EPCs [
39]. Thus, the modulation of EPC proliferation might be a useful therapeutic approach in the cardiovascular field.
Our results indicate significant thyroid hormonal action on EPCs number; however, other bone marrow signals that stimulate the proliferation of this lineage cannot be excluded. Further studies should investigate molecular pathways that govern the effect of thyroid hormones on endothelial progenitor cell proliferation. Thyroid hormone stimulates expression of its target genes through the thyroid receptor’s activation [
40]. Transcription downstream of the thyroid hormones can be activated by the ligand-bound form of thyroid receptors. Previous reports have shown that the absence of thyroid hormone affects the proliferation of cancer cells via regulating the cell cycle gene [
41]. Therefore, changes of EPCs number upon thyroid removal may involve the expression of thyroid hormone targets that possibly include genes governing cell division, such as cyclins and/or cyclin-dependent kinases. Our results provide a good basis for future studies investigating mechanisms that regulate thyroid hormone-dependent EPCs proliferation.
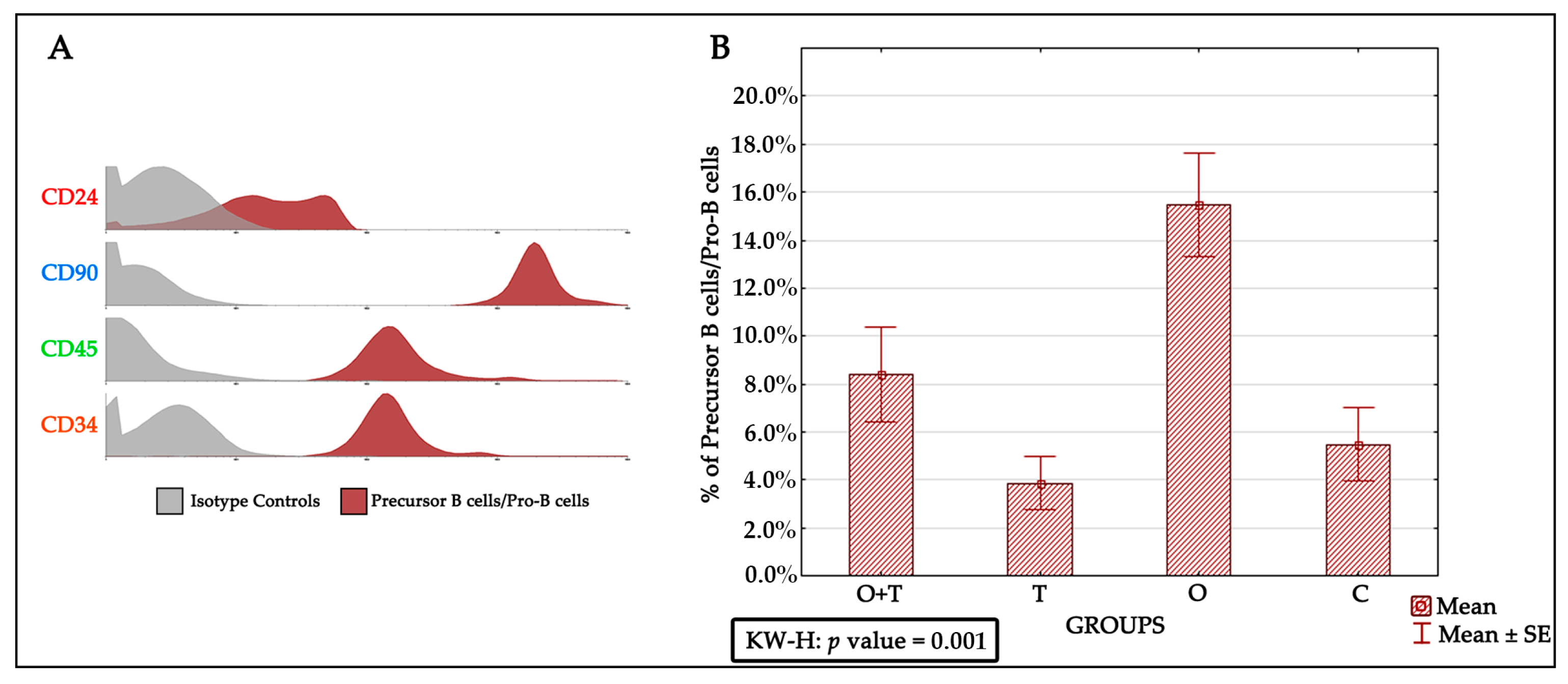
Previous studies have proposed various mechanisms that affect precursor B cells/Pro-B cells in aged animals. These include survival factors, altered differentiation, and impaired production of precursor B cells/Pro-B cells. However, our understanding of how cell proliferation and its mechanisms are regulated by hormones remains fragmentary. One of the major goals of this study was to understand the regulation of precursor B cells/Pro-B cells number in aged female Wistar rats. Earlier works have shown that the precursor B cells/Pro-B cells pool is decreased by ~fourfold in aged adults [
36]; however, this decrease was postulated to be an effect of decreased function caused by the advanced age of many different body systems that impair the physiology of the aged animals. In addition, precursor B cells/Pro-B cells number in old mice has been proposed to be regulated by neighboring bone marrow stromal cells and soluble molecules [
42,
43,
44]. We therefore sought to investigate the specific roles of ovarian or/and thyroid hormones in the regulation of precursor B cells’/Pro-B cells’ viability and multiplication. To study the proliferation of precursor B cells/Pro-B cells after the removal of ovarian and/or thyroid hormones by ovariectomy or/thyroidectomy, we first characterized these cells by the CD24+, CD90+, CD45+, and CD34+ phenotypes. Interestingly, our analysis revealed an increase in precursor B cells/Pro-B cells number upon the removal of ovarian hormones by ovariectomy. This increase was not seen in the thyroidectomy group, which showed no significant difference from the control group. We were then interested to know if the removal of thyroid hormone would affect the increase in precursor B cells/Pro-B cells proliferation caused by a loss of ovarian hormones. When we removed both the thyroid and ovaries, we detected a slight (non-significant) increase in precursor B cells/Pro-B cells number (
Figure 4). This suggests that a loss of thyroid hormone does not affect the decrease in precursor B cells/Pro-B cells number caused by a lack of ovarian hormone.
Previous results have shown an association between certain regulatory genes and the developmental sequence of precursor B cells/Pro-B cells [
43,
44]. For instance, precursor B cells/Pro-B cells production is greatly reduced when there is a decreased amount of mRNA of the recombination activating genes 1 and 2 in aged bone marrow [
42,
45]. Moreover, signals from stromal cells have been shown to potentiate the effect of other gene groups, such as the interleukin 7 receptor, that modulate the proliferation and differentiation of developing B cells [
46,
47,
48,
49,
50]. These events may require direct cell communication with stromal cells and biological responses to signaling growth factors [
51,
52]. Future studies may investigate if ovarian hormones control precursor B cells/Pro-B cells number through these molecular mechanisms. Identification of pathways downstream of ovarian hormones may shed new light towards dissecting the genetic programs controlling cell proliferation of the developing B cell lineage. In addition, determination of the pro-B cells that receive hormonal signals will improve our understanding of the physiology of the bone marrow of aging mammals.
The majority of immature bone marrow precursor B cells/Pro-B cells undergo significant proliferation to reach the optimum number [
53,
54,
55]. It is important to note that some B lineage precursors may die to balance cell proliferation in the bone marrow. Therefore, the production of this lineage is accompanied by growth, differentiation, and apoptosis [
55,
56,
57,
58,
59]. A disturbance of these processes might be affected by ovarian hormone action. Since we do not see a difference in the number of dying precursor B cell/Pro-B cells, we suggest that the ovarian control of Pro-B cells mainly affects cell proliferation.
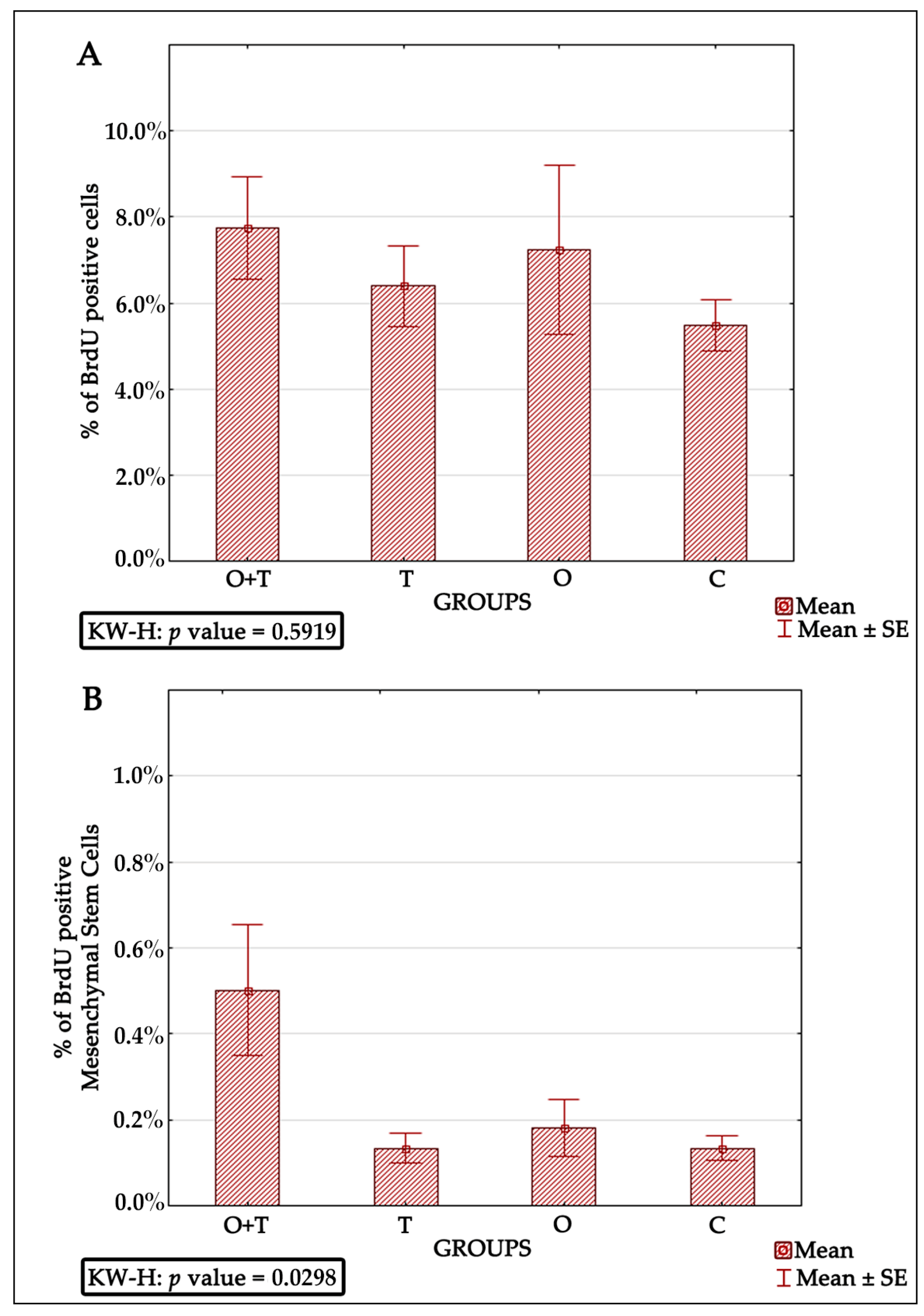
Bone marrow-derived mesenchymal stem cells have been reported to be very efficient in regenerative medicine due to their multi-lineage differentiation character [
60,
61] and high potential to repair tissues [
62,
63,
64]. However, a critical obstacle is how their cell number is controlled [
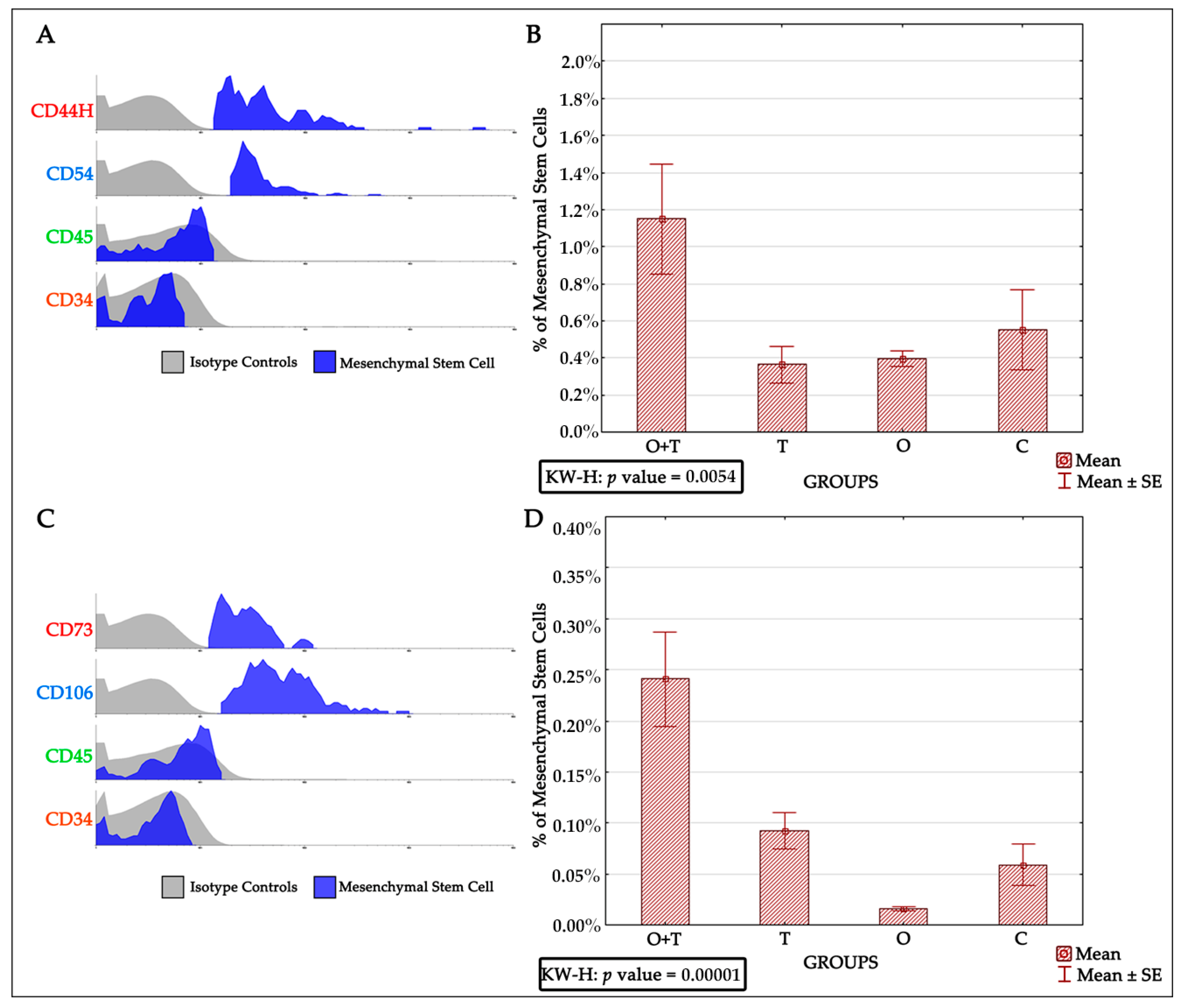
65]. Thus, successful mesenchymal cell therapy requires better elucidation of the regulatory mechanisms controlling the proliferation of these cells. Our analysis also evaluated the roles of thyroid or/and ovarian hormones in the proliferation of mesenchymal bone marrow stem cells. Adult female rats from the thyroidectomy and/or ovariectomy groups were analyzed for changes in mesenchymal cell number and compared with control animals. MSCs were identified by the presence of the CD44H+, CD54+, CD73+, CD106+, CD45−, and CD34− phenotypes (
Figure 5). Our BRDU analysis revealed no effect of thyroidectomy on the number of mesenchymal bone marrow cells when compared to the control group. Interestingly, the removal of both ovaries and thyroid glands caused a significant increase in mesenchymal stem cell number in the bone marrow in comparison to the control group (
Figure 6). Earlier works have shown that MSCs can maintain their multipotency ability and proliferate rapidly at considerably low densities, but this expansion may not generate adequate numbers of mesenchymal cells for therapeutic applications [
63,
64,
66,
67]. Our results of increased MSCs upon the removal of both thyroid and ovarian hormones indicate a necessity to generate highly proliferating mesenchymal cells, and may thus enhance efficient therapeutic regeneration.
Cell viability and proliferation are major concerns in the field of stem cell biology as well as in therapeutic applications. It is critical to understand the in vivoresponse of BMDSCs, as successful models widely used in translational research, to physiologic and pathologic endocrine alterations. Future studies could investigate if monitoring the conditions of patients receiving stem cell therapy is related to thyroid and ovarian hormones homeostasis. Overall, the strength of our findings is that they were performed in an in vivo model. In summary, the present results reveal that hypothyroidism causes a significant increase in endothelial progenitor cells, whereas a loss of ovarian hormones significantly increased the numbers of precursor B cells/Pro-B cells and decreased the bone marrow mesenchymal cell number. The removal of both ovaries and thyroid glands caused a significant increase in mesenchymal stem cells number (
Figure 7). However, it is not clear which molecular pathways regulate the cellular response to hormonal loss in each condition and which amount of each hormone is sufficient to induce specific changes in cell number. Thus, further research is important to elucidate the molecular and cellular biological mechanisms regulating thyroid or/and ovarian hormonal effects on BMDSCs number.