Administration of Zinc plus Cyclo-(His-Pro) Increases Hippocampal Neurogenesis in Rats during the Early Phase of Streptozotocin-Induced Diabetes
Abstract
:1. Introduction
2. Results
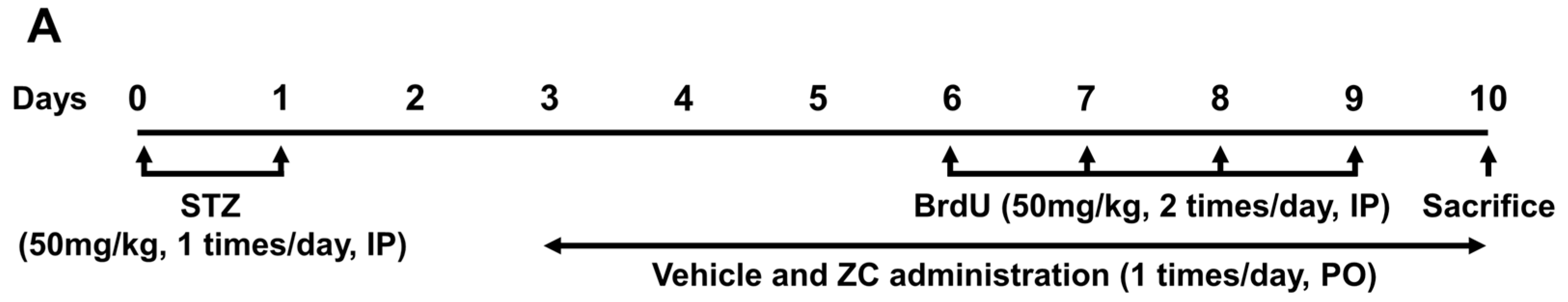
2.1. ZC Treatment Does Not Affect Body Weight or Blood Glucose Level in Diabetic Rats
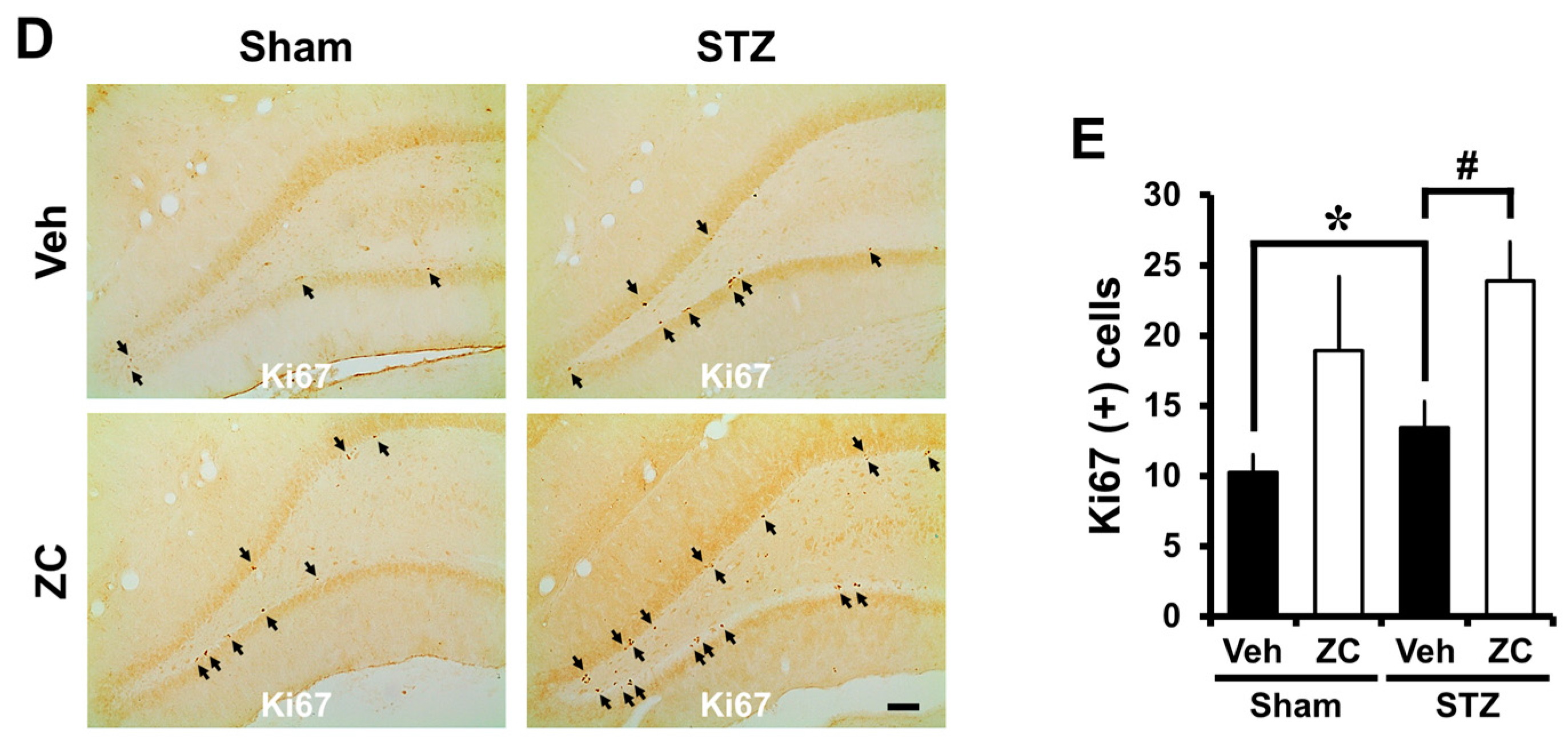
2.2. Short-Term ZC Treatment Increases the NPCs Proliferation in Diabetic Rats
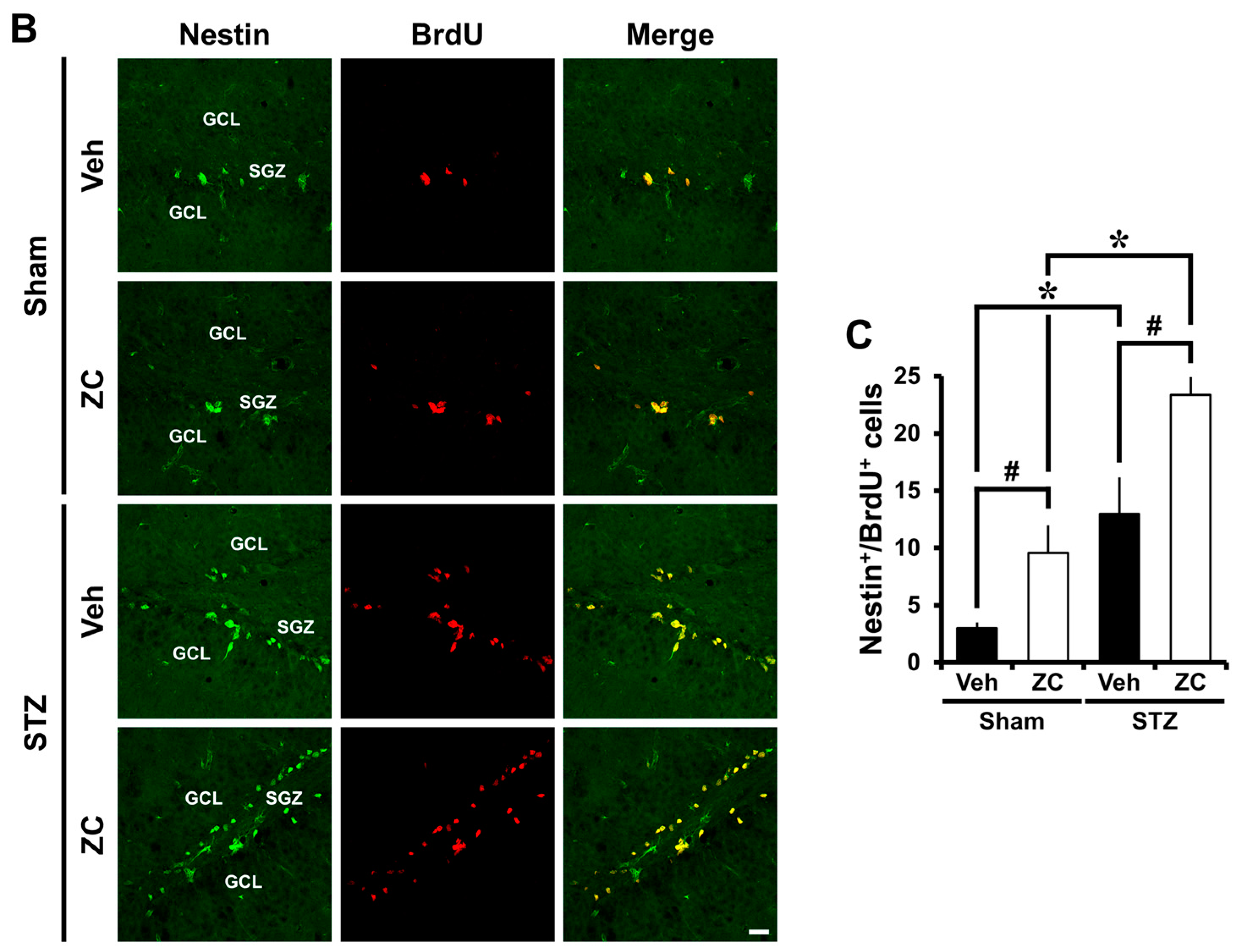
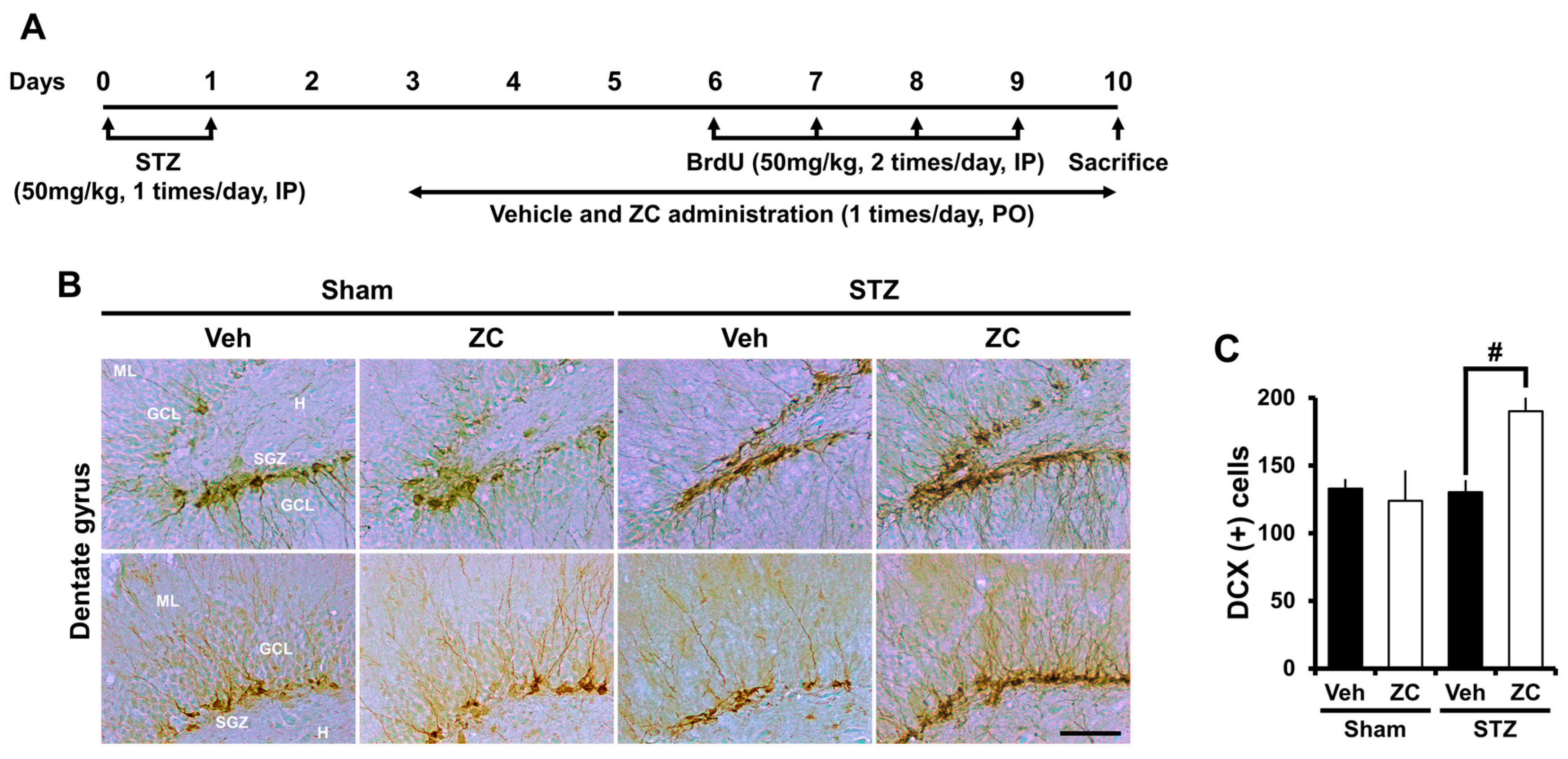
2.3. Short-Term ZC Treatment Increases Neuroblast Production in Diabetic Rats
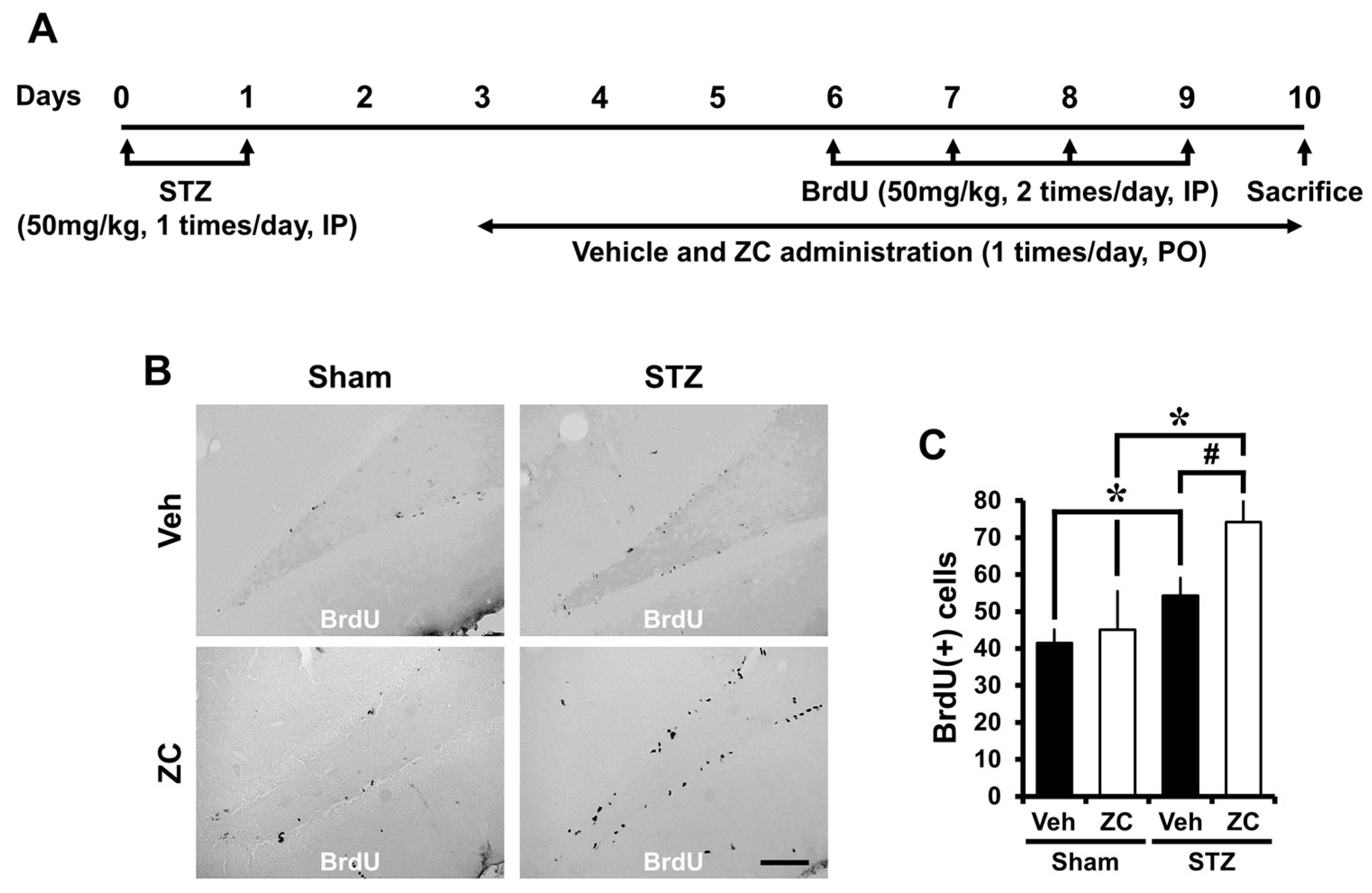
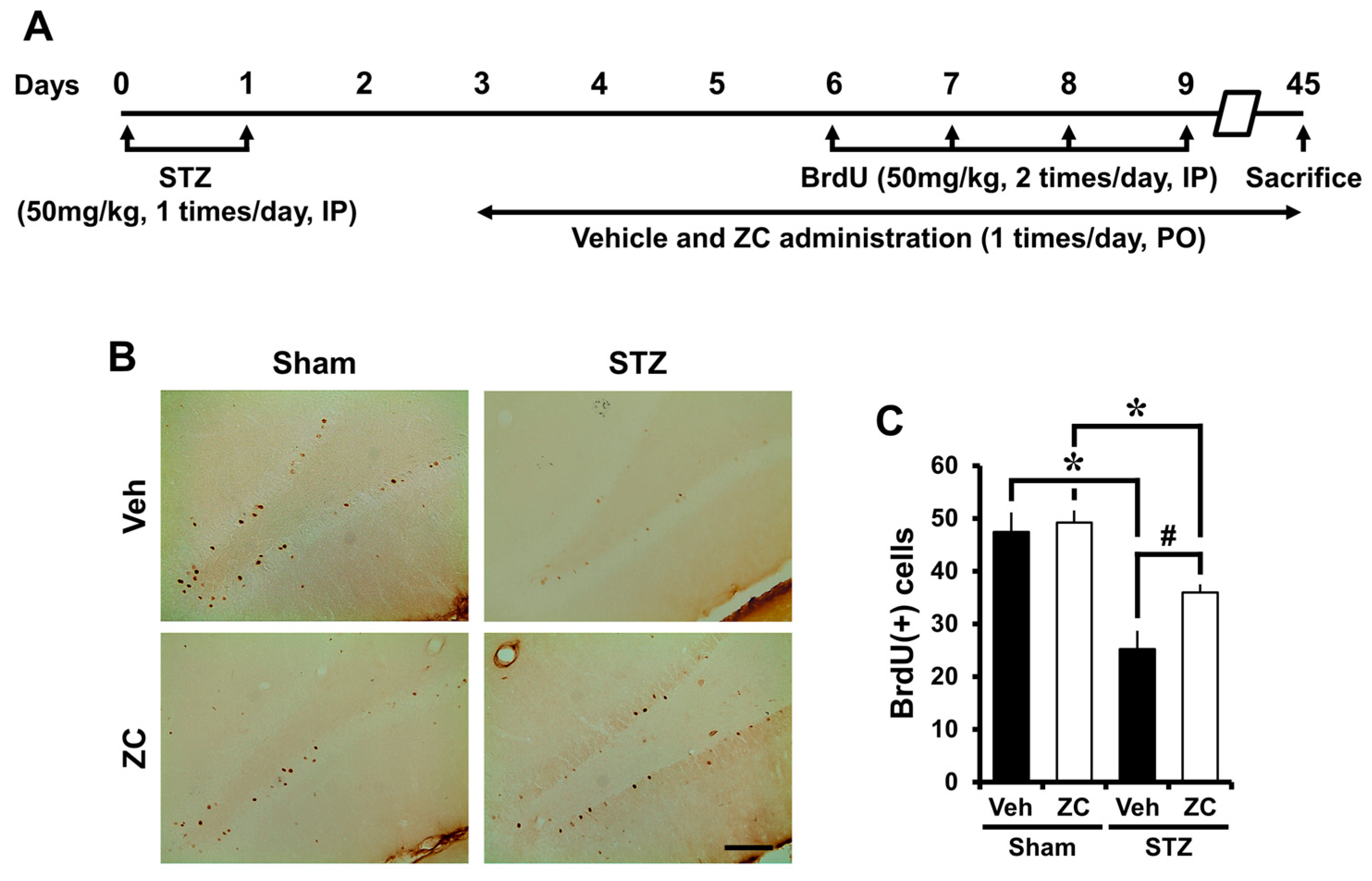
2.4. Long-Term ZC Treatment Increases the Survival of BrdU Positive Cells in Diabetic Rat
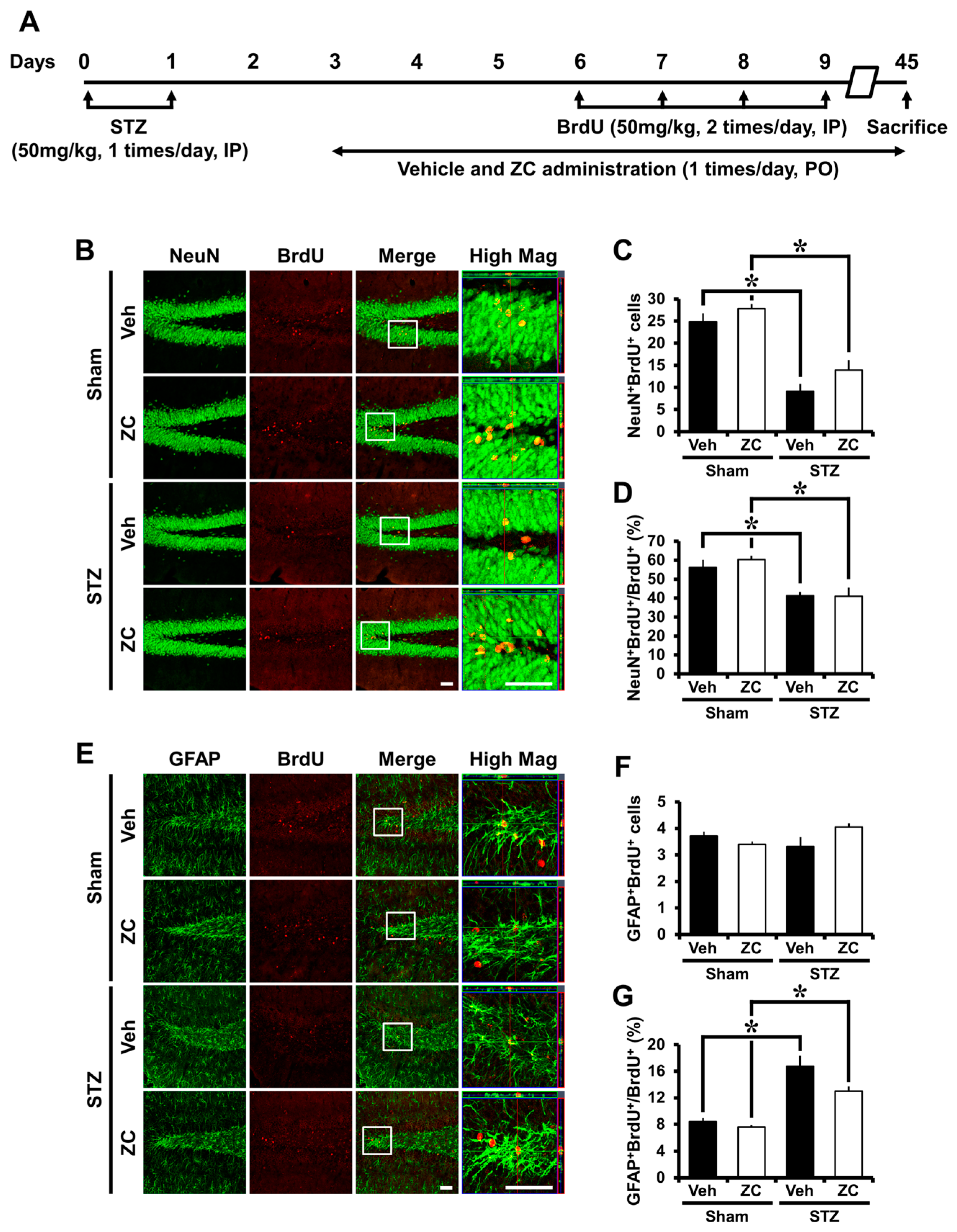
2.5. Long-Term ZC Treatment Does Not Affect the Neurogenesis in Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Animals
4.3. Rat Model of Type 1 Diabetes
4.4. Zinc Supplementation
4.5. BrdU Labeling
4.6. Brain Sections Preparation
4.7. Immunohistochemistry
4.8. Immunofluorescence Staining
4.9. Quantification
4.10. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ZC | Zinc plus cyclo-(His-Pro) |
| DG | Dentate gyrus |
| DCX | Doublecortin |
| STZ | Streptozotocin |
| GCL | Granular cell layer |
| SGZ | Subgranular zone |
| SVZ | Subventricular zone |
| NPCs | Neural progenitor cells |
| BrdU | 5-Bromo-2-Deoxyuridine |
| PFA | Paraformaldehyde |
| PB | Phosphate buffer |
| ABC | Avidin-biotinylated enzyme complex |
| DAB | 3,3′-Diaminobenzidine |
| NeuN | Neuronal nuclei |
| GFAP | Glial fibrillary acidic protein |
| IP | Intraperitoneal |
| PO | Per os |
| PBS | Phosphate-buffered saline |
References
- Brands, A.M.; Kessels, R.P.; Hoogma, R.P.; Henselmans, J.M.; van der Beek Boter, J.W.; Kappelle, L.J.; de Haan, E.H.; Biessels, G.J. Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes. Diabetes 2006, 55, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.M.; Williams, T.M.; Finegold, D.N.; Orchard, T.J. Cognitive dysfunction in adults with type 1 (insulin-dependent) diabetes mellitus of long duration: Effects of recurrent hypoglycaemia and other chronic complications. Diabetologia 1993, 36, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Weinger, K.; Jacobson, A.M.; Musen, G.; Lyoo, I.K.; Ryan, C.M.; Jimerson, D.C.; Renshaw, P.F. The effects of type 1 diabetes on cerebral white matter. Diabetologia 2008, 51, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Reeves, A.J.; Fallah, M.; Tanapat, P.; Gross, C.G.; Fuchs, E. Hippocampal neurogenesis in adult old world primates. Proc. Natl. Acad. Sci. USA 1999, 96, 5263–5267. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P.; Gage, F.H. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J. Neurosci. Res. 2002, 69, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Abrous, D.N.; Koehl, M.; Le Moal, M. Adult neurogenesis: From precursors to network and physiology. Physiol. Rev. 2005, 85, 523–569. [Google Scholar] [CrossRef] [PubMed]
- Shors, T.J.; Miesegaes, G.; Beylin, A.; Zhao, M.; Rydel, T.; Gould, E. Neurogenesis in the adult is involved in the formation of trace memories. Nature 2001, 410, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Rampon, C.; Tang, Y.P.; Shrom, D.; Jin, J.; Kyin, M.; Sopher, B.; Miller, M.W.; Ware, C.B.; Martin, G.M.; et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron 2001, 32, 911–926. [Google Scholar] [CrossRef]
- Suh, S.W.; Fan, Y.; Hong, S.M.; Liu, Z.; Matsumori, Y.; Weinstein, P.R.; Swanson, R.A.; Liu, J. Hypoglycemia induces transient neurogenesis and subsequent progenitor cell loss in the rat hippocampus. Diabetes 2005, 54, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, B.G.; Choi, B.Y.; Kwon, L.M.; Sohn, M.; Song, H.K.; Suh, S.W. Zinc chelation reduces hippocampal neurogenesis after pilocarpine-induced seizure. PLoS ONE 2012, 7, e48543. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Kim, J.H.; Kim, H.J.; Lee, B.E.; Kim, I.Y.; Sohn, M.; Suh, S.W. Zinc chelation reduces traumatic brain injury-induced neurogenesis in the subgranular zone of the hippocampal dentate gyrus. J. Trace Elem. Med. Biol. 2014, 28, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Kim, I.Y.; Kim, J.H.; Lee, B.E.; Lee, S.H.; Kho, A.R.; Sohn, M.; Suh, S.W. Zinc plus cyclo-(His-Pro) promotes hippocampal neurogenesis in rats. Neuroscience 2016, 339, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Rosenthal, M.J.; Song, A.M.; Uyemura, K.; Yang, H.; Ament, M.E.; Yamaguchi, D.T.; Cornford, E.M. Body weight reduction in rats by oral treatment with zinc plus cyclo-(His-Pro). Br. J. Pharmacol. 2009, 158, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Rosenthal, M.J.; Hong, S.; Harris, D.M.; Hwang, I.; Yip, I.; Golub, M.S.; Ament, M.E.; Go, V.L. Synergistic antidiabetic activities of zinc, cyclo (His-Pro), and arachidonic acid. Metabolism 2001, 50, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, K.; McKay, R.D. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J. Neurosci. 1988, 8, 1144–1151. [Google Scholar] [PubMed]
- Kodl, C.T.; Seaquist, E.R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008, 29, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Gispen, W.H.; Biessels, G.J. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000, 23, 542–549. [Google Scholar] [CrossRef]
- Flood, J.F.; Mooradian, A.D.; Morley, J.E. Characteristics of learning and memory in streptozocin-induced diabetic mice. Diabetes 1990, 39, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Yasuda, H.; Kikkawa, R.; Koyama, N.; Yokota, T.; Shigeta, Y. Electrophysiological study of dorsal column function in streptozocin-induced diabetic rats: Comparison with 2,5-hexanedione intoxication. J. Neurol. Sci. 1993, 115, 58–66. [Google Scholar] [CrossRef]
- Biessels, G.J.; Cristino, N.A.; Rutten, G.J.; Hamers, F.P.; Erkelens, D.W.; Gispen, W.H. Neurophysiological changes in the central and peripheral nervous system of streptozotocin-diabetic rats—Course of development and effects of insulin treatment. Brain 1999, 122, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Morano, S.; Sensi, M.; di Gregorio, S.; Pozzessere, G.; Petrucci, A.F.; Valle, E.; Pugliese, G.; Caltabiano, V.; Vetri, M.; di Mario, U.; et al. Peripheral, but not central, nervous system abnormalities are reversed by pancreatic islet transplantation in diabetic lewis rats. Eur. J. Neurosci. 1996, 8, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Stevens, C.F.; Gage, F.H. Neural stem cells from adult hippocampus develop essential properties of functional cns neurons. Nat. Neurosci. 2002, 5, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Solway, K.; Messing, R.O.; Sharp, F.R. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J. Neurosci. 1998, 18, 7768–7778. [Google Scholar] [PubMed]
- Raber, J.; Fan, Y.; Matsumori, Y.; Liu, Z.; Weinstein, P.R.; Fike, J.R.; Liu, J. Irradiation attenuates neurogenesis and exacerbates ischemia-induced deficits. Ann. Neurol. 2004, 55, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Perez-Clausell, J.; Danscher, G. Intravesicular localization of zinc in rat telencephalic boutons. A histochemical study. Brain Res. 1985, 337, 91–98. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [PubMed]
- Stewart, G.R.; Frederickson, C.J.; Howell, G.A.; Gage, F.H. Cholinergic denervation-induced increase of chelatable zinc in mossy-fiber region of the hippocampal formation. Brain Res. 1984, 290, 43–51. [Google Scholar] [CrossRef]
- Dvergsten, C.L.; Fosmire, G.J.; Ollerich, D.A.; Sandstead, H.H. Alterations in the postnatal development of the cerebellar cortex due to zinc deficiency. I. Impaired acquisition of granule cells. Brain Res. 1983, 271, 217–226. [Google Scholar] [CrossRef]
- Sandstead, H.H.; Frederickson, C.J.; Penland, J.G. History of zinc as related to brain function. J. Nutr. 2000, 130, 496S–502S. [Google Scholar] [PubMed]
- Golub, M.S.; Takeuchi, P.T.; Keen, C.L.; Gershwin, M.E.; Hendrickx, A.G.; Lonnerdal, B. Modulation of behavioral performance of prepubertal monkeys by moderate dietary zinc deprivation. Am. J. Clin. Nutr. 1994, 60, 238–243. [Google Scholar] [PubMed]
- Keller, K.A.; Chu, Y.; Grider, A.; Coffield, J.A. Supplementation with l-histidine during dietary zinc repletion improves short-term memory in zinc-restricted young adult male rats. J. Nutr. 2000, 130, 1633–1640. [Google Scholar] [PubMed]
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 2009, 29, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- King, A.J. The use of animal models in diabetes research. Br. J. Pharmacol. 2012, 166, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Beltramini, M.; Zambenedetti, P.; Raso, M.; IbnlKayat, M.I.; Zatta, P. The effect of zn(II) and streptozotocin administration in the mouse brain. Brain Res. 2006, 1109, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.J.; Lee, D.D.; Tao, J.; Wilson, R.A.; Park, S.Y.; Bell, G.I.; Chong, A.S. Glycemic control promotes pancreatic β-cell regeneration in streptozotocin-induced diabetic mice. PLoS ONE 2010, 5, e8749. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, O. Iib group metal ions (Zn2+, Cd2+, Hg2+) stimulate glucose transport activity by post-insulin receptor kinase mechanism in rat adipocytes. J. Biol. Chem. 1989, 264, 16118–16122. [Google Scholar] [PubMed]
- Kimball, S.R.; Vary, T.C.; Jefferson, L.S. Regulation of protein synthesis by insulin. Annu. Rev. Physiol. 1994, 56, 321–348. [Google Scholar] [CrossRef] [PubMed]
- Simons, T.J. Calcium-dependent zinc efflux in human red blood cells. J. Membr. Biol. 1991, 123, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.P.; Balbis, A.; Bartke, A.; Turyn, D. Role of hyperinsulinemia on hepatic insulin receptor concentration and autophosphorylation in the presence of high growth hormone levels in transgenic mice overexpressing growth hormone gene. J. Endocrinol. 1998, 159, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in b cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Rosenthal, M.J.; Hwang, I.K.; Song, M.K. Effects of arachidonic acid and cyclo (His-Pro) on zinc transport across small intestine and muscle tissues. Life Sci. 2001, 70, 337–348. [Google Scholar] [CrossRef]
- Kagabu, Y.; Mishiba, T.; Okino, T.; Yanagisawa, T. Effects of thyrotropin-releasing hormone and its metabolites, cyclo(his-pro) and trh-oh, on growth hormone and prolactin synthesis in primary cultured pituitary cells of the common carp, cyprinus carpio. Gen. Comp. Endocrinol. 1998, 111, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Morgan, W.T. Human serum histidine-rich glycoprotein. I. Interactions with heme, metal ions and organic ligands. Biochim. Biophys. Acta. 1978, 535, 319–333. [Google Scholar] [CrossRef]
- Kambe, T.; Yamaguchi-Iwai, Y.; Sasaki, R.; Nagao, M. Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 2004, 61, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Wilber, J.F. The distribution of histidyl-proline diketopiperazine (cyclo(His-Pro)) in discrete rat hypothalamic nuclei. Neuropeptides 1989, 13, 221–223. [Google Scholar] [CrossRef]
- Choi, B.Y.; Kim, J.H.; Kim, H.J.; Yoo, J.H.; Song, H.K.; Sohn, M.; Won, S.J.; Suh, S.W. Pyruvate administration reduces recurrent/moderate hypoglycemia-induced cortical neuron death in diabetic rats. PLoS ONE 2013, 8, e81523. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, A.; Zizi, S.; Israili, Z.H.; Lyoussi, B. Hypoglycemic and hypolipidemic effects of coriandrum sativum l. In meriones shawi rats. J. Ethnopharmacol. 2011, 137, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Rosenthal, M.J.; Naliboff, B.D.; Phanumas, L.; Kang, K.W. Effects of bovine prostate powder on zinc, glucose, and insulin metabolism in old patients with non-insulin-dependent diabetes mellitus. Metabolism 1998, 47, 39–43. [Google Scholar] [CrossRef]
| Groups | Body Weight (g) | |
| 10 Days | ||
| Initial | Final | |
| Sham + Vehicle (n = 12) | 177.17 ± 1.89 | 224.75 ± 3.39 |
| Sham + ZC (n = 7) | 167.57 ± 5.36 | 227.29 ± 6.89 |
| STZ + Vehicle (n = 12) | 172.75 ± 3.08 | 165.92 ± 5.09 * |
| STZ + ZC (n = 10) | 166.40 ± 1.56 | 162.00 ± 2.71 * |
| Groups | Body Weight (g) | |
| 45 Days | ||
| Initial | Final | |
| Sham + Vehicle (n = 7) | 133.14 ± 4.34 | 405.00 ± 7.51 |
| Sham + ZC (n = 8) | 127.00 ± 2.42 | 387.75 ± 9.66 |
| STZ + Vehicle (n = 7) | 133.20 ± 5.34 | 213.60 ± 24.41 * |
| STZ + ZC (n = 6) | 132.60 ± 2.52 | 184.40 ± 13.08 * |
| Groups | Blood Glucose Level (mg/dL) | |
| 10 Days | ||
| Initial | Final | |
| Sham + Vehicle (n = 12) | 116.92 ± 4.39 | 115.83 ± 4.04 |
| Sham + ZC (n = 7) | 117.29 ± 5.85 | 114.00 ± 4.48 |
| STZ + Vehicle (n = 12) | 112.75 ± 3.67 | 518.08 ± 17.24 * |
| STZ + ZC (n = 10) | 115.90 ± 3.64 | 488.50 ± 34.20 * |
| Groups | Blood Glucose Level (mg/dL) | |
| 45 Days | ||
| Initial | Final | |
| Sham + Vehicle (n = 7) | 110.29 ± 3.63 | 90.57 ± 3.73 |
| Sham + ZC (n = 8) | 109.63 ± 4.74 | 87.00 ± 3.35 |
| STZ + Vehicle (n = 7) | 105.00 ± 3.78 | 551.60 ± 20.99 * |
| STZ + ZC (n = 6) | 105.40 ± 5.10 | 562.80 ± 16.92 * |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.Y.; Kim, I.Y.; Kim, J.H.; Lee, B.E.; Lee, S.H.; Kho, A.R.; Sohn, M.; Suh, S.W. Administration of Zinc plus Cyclo-(His-Pro) Increases Hippocampal Neurogenesis in Rats during the Early Phase of Streptozotocin-Induced Diabetes. Int. J. Mol. Sci. 2017, 18, 73. https://doi.org/10.3390/ijms18010073
Choi BY, Kim IY, Kim JH, Lee BE, Lee SH, Kho AR, Sohn M, Suh SW. Administration of Zinc plus Cyclo-(His-Pro) Increases Hippocampal Neurogenesis in Rats during the Early Phase of Streptozotocin-Induced Diabetes. International Journal of Molecular Sciences. 2017; 18(1):73. https://doi.org/10.3390/ijms18010073
Chicago/Turabian StyleChoi, Bo Young, In Yeol Kim, Jin Hee Kim, Bo Eun Lee, Song Hee Lee, A Ra Kho, Min Sohn, and Sang Won Suh. 2017. "Administration of Zinc plus Cyclo-(His-Pro) Increases Hippocampal Neurogenesis in Rats during the Early Phase of Streptozotocin-Induced Diabetes" International Journal of Molecular Sciences 18, no. 1: 73. https://doi.org/10.3390/ijms18010073
APA StyleChoi, B. Y., Kim, I. Y., Kim, J. H., Lee, B. E., Lee, S. H., Kho, A. R., Sohn, M., & Suh, S. W. (2017). Administration of Zinc plus Cyclo-(His-Pro) Increases Hippocampal Neurogenesis in Rats during the Early Phase of Streptozotocin-Induced Diabetes. International Journal of Molecular Sciences, 18(1), 73. https://doi.org/10.3390/ijms18010073







