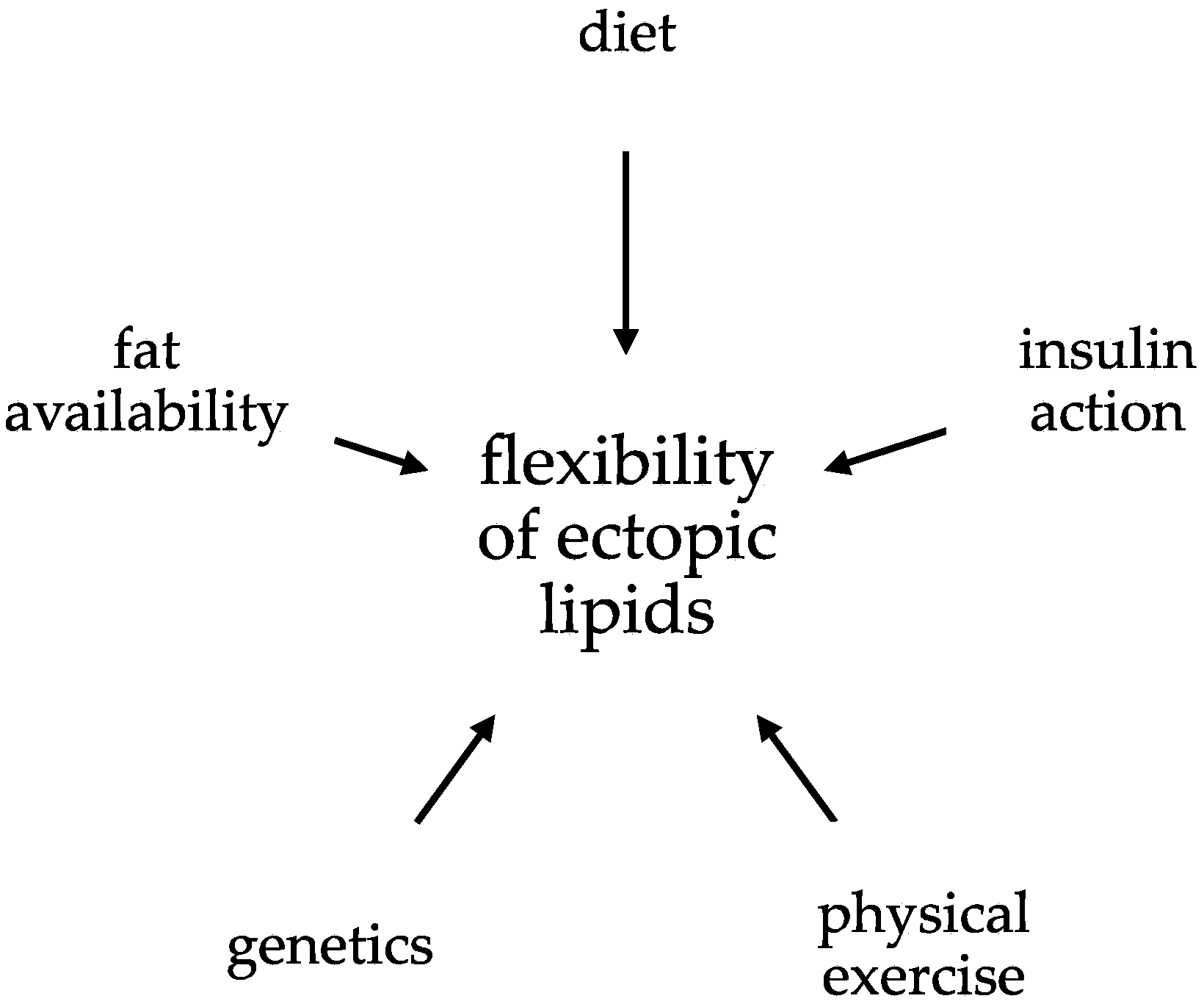
The Flexibility of Ectopic Lipids
Abstract
:1. Introduction
2. Methods to Assess Ectopic Lipids
3. The Effect of Physical Exercise on Ectopic Lipids
3.1. Short-Term Effect: Single Bout of Exercise
3.1.1. IMCL
3.1.2. IHCL
3.1.3. ICCL
3.2. Long-Term Effect: Physical Exercise
3.2.1. IMCL
3.2.2. IHCL
3.2.3. ICCL
4. Nutritional Interventions
4.1. IMCL
4.2. IHCL
4.3. ICCL
4.4. Effect of Bariatric Surgery on Ectopic Lipids
5. Genetics and Drugs
5.1. Genetic Background of Ectopic Lipids
5.2. Medical Therapy for Ectopic Lipids
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Field, A.E.; Coakley, E.H.; Must, A.; Spadano, J.L.; Laird, N.; Dietz, W.H.; Rimm, E.; Colditz, G.A. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 2001, 161, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.G.; Gordon, F.D.; Chopra, S. Nonalcoholic steatohepatitis. Ann. Intern. Med. 1997, 126, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wanless, I.R.; Lentz, J.S. Fatty liver hepatitis (steatohepatitis) and obesity: An autopsy study with analysis of risk factors. Hepatology 1990, 12, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H. Obesity and heart disease: A statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 1997, 96, 3248–3250. [Google Scholar] [CrossRef] [PubMed]
- Engeland, A.; Bjorge, T.; Selmer, R.M.; Tverdal, A. Height and body mass index in relation to total mortality. Epidemiology 2003, 14, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Nonalcoholic Fatty Liver Disease and Risk of Incident Cardiovascular Disease: A Meta-Analysis of Observational Studies. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Katzmarzyk, P.T.; Nichaman, M.Z.; Church, T.S.; Blair, S.N.; Ross, R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity 2006, 14, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Penninx, B.W.; Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Kanaya, A.M.; Pahor, M.; Jingzhong, D.; Harris, T.B. Association of visceral adipose tissue with incident myocardial infarction in older men and women: The Health, Aging and Body Composition Study. Am. J. Epidemiol. 2004, 160, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.A.; Massaro, J.M.; Murabito, J.M.; Kreger, B.E.; Hoffmann, U.; Fox, C.S. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J. Am. Coll. Cardiol. 2013, 62, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Glass, L.; Triplitt, C.; Wajcberg, E.; Mandarino, L.J.; DeFronzo, R.A. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1135–E1143. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Wang, Z.Q.; Werbel, S.; Bell-Farrow, A.; Crouse, J.R., 3rd; Hinson, W.H.; Terry, J.G.; Anderson, R. Contribution of visceral fat mass to the insulin resistance of aging. Metab. Clin. Exp. 1995, 44, 954–959. [Google Scholar] [CrossRef]
- Boyko, E.J.; Fujimoto, W.Y.; Leonetti, D.L.; Newell-Morris, L. Visceral adiposity and risk of type 2 diabetes: A prospective study among Japanese Americans. Diabetes Care 2000, 23, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Richard, D. Thermogenic potential and physiological relevance of human epicardial adipose tissue. Int. J. Obes. Suppl. 2015, 5, S28–S34. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Roden, M. Ectopic lipids and organ function. Curr. Opin. Lipidol. 2009, 20, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Van Herpen, N.A.; Schrauwen-Hinderling, V.B. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol. Behav. 2008, 94, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Taira, S.; Shimabukuro, M.; Higa, M.; Yabiku, K.; Kozuka, C.; Ueda, R.; Sunagawa, S.; Ohshiro, Y.; Doi, M.; Nanba, T.; et al. Lipid deposition in various sites of the skeletal muscles and liver exhibits a positive correlation with visceral fat accumulation in middle-aged Japanese men with metabolic syndrome. Intern. Med. 2013, 52, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Snel, M.; Jonker, J.T.; Schoones, J.; Lamb, H.; de Roos, A.; Pijl, H.; Smit, J.W.; Meinders, A.E.; Jazet, I.M. Ectopic fat and insulin resistance: Pathophysiology and effect of diet and lifestyle interventions. Int. J. Endocrinol. 2012, 2012, 983814. [Google Scholar] [CrossRef] [PubMed]
- Kotronen, A.; Westerbacka, J.; Bergholm, R.; Pietiläinen, K.H.; Yki-Järvinen, H. Liver fat in the metabolic syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 3490–3497. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.I.; Caddy, S.; Ilic, V.; Fielding, B.A.; Frayn, K.N.; Borthwick, A.C.; Taylor, R. Intramuscular triglyceride and muscle insulin sensitivity: Evidence for a relationship in nondiabetic subjects. Metab. Clin. Exp. 1996, 45, 947–950. [Google Scholar] [CrossRef]
- Banerji, M.A.; Buckley, M.C.; Chaiken, R.L.; Gordon, D.; Lebovitz, H.E.; Kral, J.G. Liver fat, serum triglycerides and visceral adipose tissue in insulin-sensitive and insulin-resistant black men with NIDDM. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 846–850. [Google Scholar] [PubMed]
- Browning, J.D.; Baxter, J.; Satapati, S.; Burgess, S.C. The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J. Lipid Res. 2012, 53, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Green, J.G.; Johnson, N.A.; Sachinwalla, T.; Cunningham, C.W.; Thompson, M.W.; Stannard, S.R. Low-carbohydrate diet does not affect intramyocellular lipid concentration or insulin sensitivity in lean, physically fit men when protein intake is elevated. Metab. Clin. Exp. 2010, 59, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Stannard, S.R.; Thompson, M.W.; Fairbairn, K.; Huard, B.; Sachinwalla, T.; Thompson, C.H. Fasting for 72 h increases intramyocellular lipid content in nondiabetic, physically fit men. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1185–E1191. [Google Scholar] [CrossRef] [PubMed]
- Wietek, B.M.; Machann, J.; Mader, I.; Thamer, C.; Häring, H.U.; Claussen, C.D.; Stumvoll, M.; Schick, F. Muscle type dependent increase in intramyocellular lipids during prolonged fasting of human subjects: A proton MRS study. Horm. Metab. Res. 2004, 36, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Machann, J.; Etzel, M.; Thamer, C.; Haring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Morning to evening changes of intramyocellular lipid content in dependence on nutrition and physical activity during one single day: A volume selective 1H-MRS study. MAGMA 2011, 24, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, O.P.; Dahl, D.B.; Brechtel, K.; Machann, J.; Haap, M.; Maier, T.; Loviscach, M.; Stumvoll, M.; Claussen, C.D.; Schick, F.; et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes 2001, 50, 2579–2584. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Tamura, Y.; Takeno, K.; Kumashiro, N.; Sato, F.; Kakehi, S.; Ikeda, S.; Ogura, Y.; Saga, N.; Naito, H.; et al. Determinants of intramyocellular lipid accumulation after dietary fat loading in non-obese men. J. Diabetes Investig. 2011, 2, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Zderic, T.W.; Davidson, C.J.; Schenk, S.; Byerley, L.O.; Coyle, E.F. High-fat diet elevates resting intramuscular triglyceride concentration and whole body lipolysis during exercise. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E217–E225. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Borkhsenious, O.N.; Gullett, J.C.; Russell, R.D.; Devries, M.C.; Smith, S.R.; Ravussin, E. Effect of dietary fat on serum and intramyocellular lipids and running performance. Med. Sci. Sports Exerc. 2008, 40, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Deldicque, L.; van Dyck, R.; Ramaekers, M.; Hespel, P. High-fat diet overrules the effects of training on fiber-specific intramyocellular lipid utilization during exercise. J. Appl. Physiol. 2011, 111, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Lindeboom, L.; Nabuurs, C.I.; Hesselink, M.K.; Wildberger, J.E.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Proton magnetic resonance spectroscopy reveals increased hepatic lipid content after a single high-fat meal with no additional modulation by added protein. Am. J. Clin. Nutr. 2015, 101, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, R.W.; Hammer, S.; Smit, J.W.; Frölich, M.; Bax, J.J.; Diamant, M.; Rijzewijk, L.J.; de Roos, A.; Romijn, J.A.; Lamb, H.J. Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes 2007, 56, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, R.W.; Hammer, S.; Lamb, H.J.; Frölich, M.; Diamant, M.; Rijzewijk, L.J.; de Roos, A.; Romijn, J.A.; Smit, J.W. Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J. Clin. Endocrinol. Metab. 2008, 93, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Kreis, R.; Debard, C.; Cariou, B.; Faeh, D.; Chetiveaux, M.; Ith, M.; Vermathen, P.; Stefanoni, N.; Lê, K.A.; et al. High protein intake reduces intrahepatocellular lipid deposition in humans. Am. J. Clin. Nutr. 2009, 90, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M.; Christ, E.R.; Ith, M.; Acheson, K.J.; Pouteau, E.; Kreis, R.; Trepp, R.; Diem, P.; Boesch, C.; Décombaz, J. Intramyocellular lipid stores increase markedly in athletes after 1.5 days lipid supplementation and are utilized during exercise in proportion to their content. Eur. J. Appl. Physiol. 2006, 98, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Newcomer, B.R.; Hunter, G.R. Influence of endurance running and recovery diet on intramyocellular lipid content in women: A 1H NMR study. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E95–E106. [Google Scholar] [PubMed]
- Décombaz, J.; Schmitt, B.; Ith, M.; Decarli, B.; Diem, P.; Kreis, R.; Hoppeler, H.; Boesch, C. Postexercise fat intake repletes intramyocellular lipids but no faster in trained than in sedentary subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R760–R769. [Google Scholar] [PubMed]
- Brehm, A.; Krššák, M.; Schmid, A.I.; Nowotny, P.; Waldhäusl, W.; Roden, M. Acute elevation of plasma lipids does not affect ATP synthesis in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E33–E38. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Stannard, S.R.; Rowlands, D.S.; Chapman, P.G.; Thompson, C.H.; O’Connor, H.; Sachinwalla, T.; Thompson, M.W. Effect of short-term starvation versus high-fat diet on intramyocellular triglyceride accumulation and insulin resistance in physically fit men. Exp. Physiol. 2006, 91, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J.; Fleith, M.; Hoppeler, H.; Kreis, R.; Boesch, C. Effect of diet on the replenishment of intramyocellular lipids after exercise. Eur. J. Nutr. 2000, 39, 244–247. [Google Scholar] [CrossRef]
- Bucher, J.; Krüsi, M.; Zueger, T.; Ith, M.; Stettler, C.; Diem, P.; Boesch, C.; Kreis, R.; Christ, E. The effect of a single 2 h bout of aerobic exercise on ectopic lipids in skeletal muscle, liver and the myocardium. Diabetologia 2014, 57, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Egger, A.; Kreis, R.; Allemann, S.; Stettler, C.; Diem, P.; Buehler, T.; Boesch, C.; Christ, E.R. The effect of aerobic exercise on intrahepatocellular and intramyocellular lipids in healthy subjects. PLoS ONE 2013, 8, e70865. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Ferguson, M.A.; McCoy, S.C.; Kim, H. Intramyocellular lipid changes in men and women during aerobic exercise: A 1H-magnetic resonance spectroscopy study. J. Clin. Endocrinol. Metab. 2003, 88, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- White, L.J.; Robergs, R.A.; Sibbitt, W.L., Jr.; Ferguson, M.A.; McCoy, S.; Brooks, W.M. Effects of intermittent cycle exercise on intramyocellular lipid use and recovery. Lipids 2003, 38, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Vermathen, P.; Saillen, P.; Boss, A.; Zehnder, M.; Boesch, C. Skeletal muscle 1H-MRSI before and after prolonged exercise. I. muscle specific depletion of intramyocellular lipids. Magn. Reson. Med. 2012, 68, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Dresselaers, T.; Kiens, B.; Richter, E.A.; van Hecke, P.; Hespel, P. Evaluation of intramyocellular lipid breakdown during exercise by biochemical assay, NMR spectroscopy, and Oil Red O staining. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E428–E434. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M.; Ith, M.; Kreis, R.; Saris, W.; Boutellier, U.; Boesch, C. Gender-specific usage of intramyocellular lipids and glycogen during exercise. Med. Sci. Sports Exerc. 2005, 37, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen-Hinderling, V.B.; van Loon, L.J.; Koopman, R.; Nicolay, K.; Saris, W.H.; Kooi, M.E. Intramyocellular lipid content is increased after exercise in nonexercising human skeletal muscle. J. Appl. Physiol. 2003, 95, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Schrauwen-Hinderling, V.B.; Koopman, R.; Wagenmakers, A.J.; Hesselink, M.K.; Schaart, G.; Kooi, M.E.; Saris, W.H. Influence of prolonged endurance cycling and recovery diet on intramuscular triglyceride content in trained males. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E804–E811. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Stannard, S.R.; Mehalski, K.; Trenell, M.I.; Sachinwalla, T.; Thompson, C.H.; Thompson, M.W. Intramyocellular triacylglycerol in prolonged cycling with high- and low-carbohydrate availability. J. Appl. Physiol. 2003, 94, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Brechtel, K.; Niess, A.M.; Machann, J.; Rett, K.; Schick, F.; Claussen, C.D.; Dickhuth, H.H.; Haering, H.U.; Jacob, S. Utilisation of intramyocellular lipids (IMCLs) during exercise as assessed by proton magnetic resonance spectroscopy (1H-MRS). Horm. Metab. Res. 2001, 33, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Petersen, K.F.; Bergeron, R.; Price, T.; Laurent, D.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramuscular glycogen and intramyocellular lipid utilization during prolonged exercise and recovery in man: A 13C and 1H nuclear magnetic resonance spectroscopy study. J. Clin. Endocrinol. Metab. 2000, 85, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sanz, J.; Moosavi, M.; Thomas, E.L.; McCarthy, J.; Coutts, G.A.; Saeed, N.; Bell, J.D. In vivo evaluation of the effects of continuous exercise on skeletal muscle triglycerides in trained humans. Lipids 2000, 35, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Ith, M.; Huber, P.M.; Egger, A.; Schmid, J.P.; Kreis, R.; Christ, E.; Boesch, C. Standardized protocol for a depletion of intramyocellular lipids (IMCL). NMR Biomed. 2010, 23, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.; Slotboom, J.; Hoppeler, H.; Kreis, R. In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn. Reson. Med. 1997, 37, 484–493. [Google Scholar] [CrossRef] [PubMed]
- De Bock, K.; Richter, E.A.; Russell, A.P.; Eijnde, B.O.; Derave, W.; Ramaekers, M.; Koninckx, E.; Léger, B.; Verhaeghe, J.; Hespel, P. Exercise in the fasted state facilitates fibre type-specific intramyocellular lipid breakdown and stimulates glycogen resynthesis in humans. J. Physiol. 2005, 564, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Hurley, B.F.; Nemeth, P.M.; Martin, W.H., 3rd; Hagberg, J.M.; Dalsky, G.P.; Holloszy, J.O. Muscle triglyceride utilization during exercise: Effect of training. J. Appl. Physiol. 1986, 60, 562–567. [Google Scholar] [PubMed]
- van Loon, L.J.; Koopman, R.; Stegen, J.H.; Wagenmakers, A.J.; Keizer, H.A.; Saris, W.H. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J. Physiol. 2003, 553, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.O.; Cocks, M.; Tipton, K.D.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.; Shaw, C.S. Preferential utilization of perilipin 2-associated intramuscular triglycerides during 1 h of moderate-intensity endurance-type exercise. Exp. Physiol. 2012, 97, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.O.; Cocks, M.; Tipton, K.D.; Ranasinghe, A.M.; Barker, T.A.; Burniston, J.G.; Wagenmakers, A.J.; Shaw, C.S. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. J. Physiol. 2013, 591, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Boon, H.; Gijsen, A.P.; Stegen, J.H.; Kuipers, H.; van Loon, L.J. Carbohydrate supplementation during prolonged cycling exercise spares muscle glycogen but does not affect intramyocellular lipid use. Pflug. Arch. Eur. J. Physiol. 2007, 454, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Heigenhauser, G.J.; Dyck, D.J.; Spriet, L.L. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J. Physiol. 2002, 541, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Jenni, S.; Oetliker, C.; Allemann, S.; Ith, M.; Tappy, L.; Wuerth, S.; Egger, A.; Boesch, C.; Schneiter, P.; Diem, P.; et al. Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus—A prospective single-blinded randomised crossover trial. Diabetologia 2008, 51, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Trepp, R.; Flück, M.; Stettler, C.; Boesch, C.; Ith, M.; Kreis, R.; Hoppeler, H.; Howald, H.; Schmid, J.P.; Diem, P.; et al. Effect of GH on human skeletal muscle lipid metabolism in GH deficiency. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E1127–E1134. [Google Scholar] [CrossRef] [PubMed]
- Christ, E.R.; Egger, A.; Allemann, S.; Buehler, T.; Kreis, R.; Boesch, C. Effects of aerobic exercise on ectopic lipids in patients with growth hormone deficiency before and after growth hormone replacement therapy. Sci. Rep. 2016, 6, 19310. [Google Scholar] [CrossRef] [PubMed]
- Rico-Sanz, J.; Hajnal, J.V.; Thomas, E.L.; Mierisová, S.; Ala-Korpela, M.; Bell, J.D. Intracellular and extracellular skeletal muscle triglyceride metabolism during alternating intensity exercise in humans. J. Physiol. 1998, 510, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Bilet, L.; Brouwers, B.; van Ewijk, P.A.; Hesselink, M.K.; Kooi, M.E.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Acute exercise does not decrease liver fat in men with overweight or NAFLD. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; van Overbeek, D.; Chapman, P.G.; Thompson, M.W.; Sachinwalla, T.; George, J. Effect of prolonged exercise and pre-exercise dietary manipulation on hepatic triglycerides in trained men. Eur. J. Appl. Physiol. 2012, 112, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.S.; Shepherd, S.O.; Wagenmakers, A.J.; Hansen, D.; Dendale, P.; van Loon, L.J. Prolonged exercise training increases intramuscular lipid content and perilipin 2 expression in type I muscle fibers of patients with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1158–E1165. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Mogensen, M.; Vind, B.F.; Sahlin, K.; Højlund, K.; Schrøder, H.D.; Ortenblad, N. Increased subsarcolemmal lipids in type 2 diabetes: Effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E706–E713. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.T.; de Mol, P.; de Vries, S.T.; Widya, R.L.; Hammer, S.; van Schinkel, L.D.; van der Meer, R.W.; Gans, R.O.; Webb, A.G.; Kan, H.E.; et al. Exercise and type 2 diabetes mellitus: Changes in tissue-specific fat distribution and cardiac function. Radiology 2013, 269, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Kriketos, A.D.; Cooney, G.J.; Hawley, J.A. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with Type 2 diabetes. Diabetologia 2004, 47, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.S.; Kim, C.K. Effect of exercise training on muscle glucose transporter 4 protein and intramuscular lipid content in elderly men with impaired glucose tolerance. Eur. J. Appl. Physiol. 2004, 93, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Green, H.J.; Tarnopolsky, M.A.; Heigenhauser, G.J.; Grant, S.M. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am. J. Physiol. 1996, 270, E265–E272. [Google Scholar] [PubMed]
- Stellingwerff, T.; Boon, H.; Jonkers, R.A.; Senden, J.M.; Spriet, L.L.; Koopman, R.; van Loon, L.J. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1715–E1723. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Manders, R.J.; Jonkers, R.A.; Hul, G.B.; Kuipers, H.; van Loon, L.J. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur. J. Appl. Physiol. 2006, 96, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, M.; Saltin, B.; Osada, T.; van Hall, G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J. Physiol. 2002, 540, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Wendling, P.S.; Peters, S.J.; Heigenhauser, G.J.; Spriet, L.L. Variability of triacylglycerol content in human skeletal muscle biopsy samples. J. Appl. Physiol. 1996, 81, 1150–1155. [Google Scholar] [PubMed]
- Starling, R.D.; Trappe, T.A.; Parcell, A.C.; Kerr, C.G.; Fink, W.J.; Costill, D.L. Effects of diet on muscle triglyceride and endurance performance. J. Appl. Physiol. 1997, 82, 1185–1189. [Google Scholar] [PubMed]
- Kiens, B.; Richter, E.A. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am. J. Physiol. 1998, 275, E332–E337. [Google Scholar] [PubMed]
- Essén, B.; Hagenfeldt, L.; Kaijser, L. Utilization of blood-borne and intramuscular substrates during continuous and intermittent exercise in man. J. Physiol. 1977, 265, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Kiens, B.; Essen-Gustavsson, B.; Christensen, N.J.; Saltin, B. Skeletal muscle substrate utilization during submaximal exercise in man: Effect of endurance training. J. Physiol. 1993, 469, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Howald, H.; Boesch, C.; Kreis, R.; Matter, S.; Billeter, R.; Essen-Gustavsson, B.; Hoppeler, H. Content of intramyocellular lipids derived by electron microscopy, biochemical assays, and 1H-MR spectroscopy. J. Appl. Physiol. 2002, 92, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Theriault, R.; Watkins, S.C.; Kelley, D.E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metab. Clin. Exp. 2000, 49, 467–472. [Google Scholar] [CrossRef]
- Schick, F.; Eismann, B.; Jung, W.I.; Bongers, H.; Bunse, M.; Lutz, O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: Two lipid compartments in muscle tissue. Magn. Reson. Med. 1993, 29, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.; Décombaz, J.; Slotboom, J.; Kreis, R. Observation of intramyocellular lipids by means of 1H magnetic resonance spectroscopy. Proc. Nutr. Soc. 1999, 58, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Boesch, C.; Machann, J.; Vermathen, P.; Schick, F. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2006, 19, 968–988. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Babcock, E.E.; Schick, F.; Dobbins, R.L.; Garg, A.; Burns, D.K.; McGarry, J.D.; Stein, D.T. Measurement of intracellular triglyceride stores by H spectroscopy: Validation in vivo. Am. J. Physiol. 1999, 276, E977–E989. [Google Scholar] [PubMed]
- Ahmed, A.; Wong, R.J.; Harrison, S.A. Nonalcoholic Fatty Liver Disease Review: Diagnosis, Treatment, and Outcomes. Clin. Gastroenterol. Hepatol. 2015, 13, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Ricci, C.; Masutti, F.; Vidimari, R.; Crocé, L.S.; Bercich, L.; Tiribelli, C.; Dalla Palma, L. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Investig. Radiol. 1993, 28, 297–302. [Google Scholar] [CrossRef]
- Thomsen, C.; Becker, U.; Winkler, K.; Christoffersen, P.; Jensen, M.; Henriksen, O. Quantification of liver fat using magnetic resonance spectroscopy. Magn. Reson. Imaging 1994, 12, 487–495. [Google Scholar] [CrossRef]
- Parente, D.B.; Rodrigues, R.S.; Paiva, F.F.; Oliveira Neto, J.A.; Machado-Silva, L.; Lanzoni, V.; Campos, C.F.; Eiras-Araujo, A.L.; do Brasil, P.E.; Garteiser, P.; et al. Is MR spectroscopy really the best MR-based method for the evaluation of fatty liver in diabetic patients in clinical practice? PLoS ONE 2014, 9, e112574. [Google Scholar] [CrossRef] [PubMed]
- Bannas, P.; Kramer, H.; Hernando, D.; Agni, R.; Cunningham, A.M.; Mandal, R.; Motosugi, U.; Sharma, S.D.; Munoz del Rio, A.; Fernandez, L.; et al. Quantitative MR Imaging of Hepatic Steatosis: Validation in Ex Vivo Human Livers. Hepatology 2015, 62, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Cowin, G.J.; Jonsson, J.R.; Bauer, J.D.; Ash, S.; Ali, A.; Osland, E.J.; Purdie, D.M.; Clouston, A.D.; Powell, E.E.; Galloway, G.J. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J. Magn. Reson. Imaging 2008, 28, 937–945. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Jonsson, J.R.; Cowin, G.J.; O’Rourke, P.; Clouston, A.D.; Volp, A.; Horsfall, L.; Jothimani, D.; Fawcett, J.; Galloway, G.J.; et al. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J. Hepatol. 2009, 51, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, L.S.; Nurenberg, P.; Leonard, D.; Browning, J.D.; Reingold, J.S.; Grundy, S.; Hobbs, H.H.; Dobbins, R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E462–E468. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Kan, H.E. Quantitative proton MR techniques for measuring fat. NMR Biomed. 2013, 26, 1609–1629. [Google Scholar] [CrossRef] [PubMed]
- Ith, M.; Stettler, C.; Xu, J.; Boesch, C.; Kreis, R. Cardiac lipid levels show diurnal changes and long-term variations in healthy human subjects. NMR Biomed. 2014, 27, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Reingold, J.S.; McGavock, J.M.; Kaka, S.; Tillery, T.; Victor, R.G.; Szczepaniak, L.S. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: Reproducibility and sensitivity of the method. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E935–E939. [Google Scholar] [CrossRef] [PubMed]
- Faller, K.M.; Lygate, C.A.; Neubauer, S.; Schneider, J.E. 1H-MR spectroscopy for analysis of cardiac lipid and creatine metabolism. Heart Fail. Rev. 2013, 18, 657–668. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.D.; Xu, J.; Ewald, G.A.; Ackerman, J.J.; Peterson, L.R.; Gropler, R.J.; Bashir, A. Intramyocardial triglyceride quantification by magnetic resonance spectroscopy: In vivo and ex vivo correlation in human subjects. Magn. Reson. Med. 2011, 65, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, R.W.; Doornbos, J.; Kozerke, S.; Schär, M.; Bax, J.J.; Hammer, S.; Smit, J.W.; Romijn, J.A.; Diamant, M.; Rijzewijk, L.J.; et al. Metabolic imaging of myocardial triglyceride content: Reproducibility of 1H-MR spectroscopy with respiratory navigator gating in volunteers. Radiology 2007, 245, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Vermathen, P.; Kreis, R.; Boesch, C. Distribution of intramyocellular lipids in human calf muscles as determined by MR spectroscopic imaging. Magn. Reson. Med. 2004, 51, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Reeder, S.B.; Cruite, I.; Hamilton, G.; Sirlin, C.B. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J. Magn. Reson. Imaging 2011, 34, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.; Martini, N.; Boesiger, P.; Kozerke, S. Metabolic MR imaging of regional triglyceride and creatine content in the human heart. Magn. Reson. Med. 2012, 68, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Jansson, E.; Kaijser, L. Substrate utilization and enzymes in skeletal muscle of extremely endurance-trained men. J. Appl. Physiol. 1987, 62, 999–1005. [Google Scholar] [PubMed]
- Jansson, E.; Kaijser, L. Effect of diet on the utilization of blood-borne and intramuscular substrates during exercise in man. Acta Physiol. Scand. 1982, 115, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Bergman, B.C.; Hunerdosse, D.M.; Playdon, M.C.; Eckel, R.H. Inflexibility in intramuscular triglyceride fractional synthesis distinguishes prediabetes from obesity in humans. Obesity 2010, 18, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Kiens, B. Gender differences in skeletal muscle substrate metabolism—Molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen-Hinderling, V.B.; Schrauwen, P.; Hesselink, M.K.; van Engelshoven, J.M.; Nicolay, K.; Saris, W.H.; Kessels, A.G.; Kooi, M.E. The increase in intramyocellular lipid content is a very early response to training. J. Clin. Endocrinol. Metab. 2003, 88, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Koopman, R.; Manders, R.; van der Weegen, W.; van Kranenburg, G.P.; Keizer, H.A. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E558–E565. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.; Sauers, S.E.; Goodpaster, B.H. Exercise-induced alterations in intramyocellular lipids and insulin resistance: The athlete’s paradox revisited. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E882–E888. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Amati, F.; Toledo, F.G.; Stefanovic-Racic, M.; Rossi, A.; Coen, P.; Goodpaster, B.H. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011, 54, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; He, J.; Watkins, S.; Kelley, D.E. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J. Clin. Endocrinol. Metab. 2001, 86, 5755–5761. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.E.; Short, F.A.; Cobb, L.A. Effect of long-term exercise on skeletal muscle lipid composition. Am. J. Physiol. 1969, 216, 82–86. [Google Scholar] [PubMed]
- Pruchnic, R.; Katsiaras, A.; He, J.; Kelley, D.E.; Winters, C.; Goodpaster, B.H. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E857–E862. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Goodpaster, B.H. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflug. Arch. Eur. J. Physiol. 2006, 451, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A.; Rennie, C.D.; Robertshaw, H.A.; Fedak-Tarnopolsky, S.N.; Devries, M.C.; Hamadeh, M.J. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1271–R1278. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Kirk, E.P.; Mittendorfer, B.; Patterson, B.W.; Klein, S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 2012, 55, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Stufflebam, A.; Hilton, T.N.; Sinacore, D.R.; Klein, S.; Villareal, D.T. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity 2009, 17, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Sachinwalla, T.; Walton, D.W.; Smith, K.; Armstrong, A.; Thompson, M.W.; George, J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009, 50, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Pugh, C.J.; Spring, V.S.; Kemp, G.J.; Richardson, P.; Shojaee-Moradie, F.; Umpleby, A.M.; Green, D.J.; Cable, N.T.; Jones, H.; Cuthbertson, D.J. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1298–H1306. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Meex, R.; van der Made, S.; Schär, M.; Lamb, H.; Wildberger, J.E.; Glatz, J.; Snoep, G.; Kooi, M.E.; et al. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J. Clin. Endocrinol. Metab. 2010, 95, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Essén-Gustavsson, B.; Tesch, P.A. Glycogen and triglyceride utilization in relation to muscle metabolic characteristics in men performing heavy-resistance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Nurjhan, N.; Campbell, P.J.; Kennedy, F.P.; Miles, J.M.; Gerich, J.E. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes 1986, 35, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Møller, N.; Jørgensen, J.O.; Schmitz, O.; Møller, J.; Christiansen, J.; Alberti, K.G.; Orskov, H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am. J. Physiol. 1990, 258, E86–E91. [Google Scholar] [PubMed]
- Hansen, T.K.; Gravholt, C.H.; Ørskov, H.; Rasmussen, M.H.; Christiansen, J.S.; Jørgensen, J.O. Dose dependency of the pharmacokinetics and acute lipolytic actions of growth hormone. J. Clin. Endocrinol. Metab. 2002, 87, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Møller, N.; Jørgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [PubMed]
- Standl, E.; Lotz, N.; Dexel, T.; Janka, H.U.; Kolb, H.J. Muscle triglycerides in diabetic subjects. Effect of insulin deficiency and exercise. Diabetologia 1980, 18, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Boon, H.; Blaak, E.E.; Saris, W.H.; Keizer, H.A.; Wagenmakers, A.J.; van Loon, L.J. Substrate source utilisation in long-term diagnosed type 2 diabetes patients at rest, and during exercise and subsequent recovery. Diabetologia 2007, 50, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, C.H.; Roepstorff, C.; Madsen, M.; Kiens, B. Myocellular triacylglycerol breakdown in females but not in males during exercise. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E634–E642. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, C.; Donsmark, M.; Thiele, M.; Vistisen, B.; Stewart, G.; Vissing, K.; Schjerling, P.; Hardie, D.G.; Galbo, H.; Kiens, B. Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1106–E1114. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, C.; Steffensen, C.H.; Madsen, M.; Stallknecht, B.; Kanstrup, I.L.; Richter, E.A.; Kiens, B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E435–E447. [Google Scholar] [CrossRef] [PubMed]
- Høeg, L.; Roepstorff, C.; Thiele, M.; Richter, E.A.; Wojtaszewski, J.F.; Kiens, B. Higher intramuscular triacylglycerol in women does not impair insulin sensitivity and proximal insulin signaling. J. Appl. Physiol. 2009, 107, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Dufour, S.; Petersen, K.F.; LeBon, V.; Enoksson, S.; Ma, Y.Z.; Savoye, M.; Rothman, D.L.; Shulman, G.I.; Caprio, S. Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: Relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 2002, 51, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Moro, C.; Galgani, J.E.; Luu, L.; Pasarica, M.; Mairal, A.; Bajpeyi, S.; Schmitz, G.; Langin, D.; Liebisch, G.; Smith, S.R. Influence of gender, obesity, and muscle lipase activity on intramyocellular lipids in sedentary individuals. J. Clin. Endocrinol. Metab. 2009, 94, 3440–3447. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Lowther, S.A.; Glover, A.W.; Hamadeh, M.J.; Tarnopolsky, M.A. IMCL area density, but not IMCL utilization, is higher in women during moderate-intensity endurance exercise, compared with men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2336–R2342. [Google Scholar] [CrossRef] [PubMed]
- Santosa, S.; Jensen, M.D. The Sexual Dimorphism of Lipid Kinetics in Humans. Front. Endocrinol. 2015, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.J.; Merkel, M. Lipoprotein lipase: Physiology, biochemistry, and molecular biology. Front. Biosci. 2001, 6, D388–D405. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta 2014, 1841, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Ehehalt, R.; Füllekrug, J.; Pohl, J.; Ring, A.; Herrmann, T.; Stremmel, W. Translocation of long chain fatty acids across the plasma membrane—Lipid rafts and fatty acid transport proteins. Mol. Cell. Biochem. 2006, 284, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.S.; Luiken, J.J.; Snook, L.A.; Han, X.X.; Holloway, G.P.; Glatz, J.F.; Bonen, A. Fatty acid transport and transporters in muscle are critically regulated by Akt2. FEBS Lett. 2015, 589, 2769–2775. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. Fatty acid transport: Difficult or easy? J. Lipid Res. 1998, 39, 467–481. [Google Scholar] [PubMed]
- Alsted, T.J.; Nybo, L.; Schweiger, M.; Fledelius, C.; Jacobsen, P.; Zimmermann, R.; Zechner, R.; Kiens, B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E445–E453. [Google Scholar] [CrossRef] [PubMed]
- Oscai, L.B.; Essig, D.A.; Palmer, W.K. Lipase regulation of muscle triglyceride hydrolysis. J. Appl. Physiol. 1990, 69, 1571–1577. [Google Scholar] [PubMed]
- Roepstorff, C.; Vistisen, B.; Donsmark, M.; Nielsen, J.N.; Galbo, H.; Green, K.A.; Hardie, D.G.; Wojtaszewski, J.F.; Richter, E.A.; Kiens, B. Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J. Physiol. 2004, 560, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Spriet, L.L. Regulation and role of hormone-sensitive lipase activity in human skeletal muscle. Proc. Nutr. Soc. 2004, 63, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Jocken, J.W.; Blaak, E.E. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol. Behav. 2008, 94, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Meex, R.C.; Schrauwen, P.; Hesselink, M.K. Modulation of myocellular fat stores: Lipid droplet dynamics in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R913–R924. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Falk Petersen, K.; Dresner, A.; DiPietro, L.; Vogel, S.M.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Dubé, J.J.; Amati, F.; Stefanovic-Racic, M.; Ferrell, R.E.; Toledo, F.G.; Goodpaster, B.H. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 2010, 59, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Dubé, J.J.; Alvarez-Carnero, E.; Chomentowski, P.; Coen, P.M.; Switzer, G.E.; Bickel, P.E.; Stefanovic-Racic, M.; Toledo, F.G.; Goodpaster, B.H. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: Another paradox in endurance-trained athletes? Diabetes 2011, 60, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Badin, P.M.; Langin, D.; Moro, C. Dynamics of skeletal muscle lipid pools. Trends Endocrinol. Metab. 2013, 24, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010, 103, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Va, P.; Bray, F.; Gao, S.; Gao, J.; Li, H.L.; Xiang, Y.B. The role of pre-existing diabetes mellitus on hepatocellular carcinoma occurrence and prognosis: A meta-analysis of prospective cohort studies. PLoS ONE 2011, 6, e27326. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M. Nonalcoholic fatty liver disease: A review of current understanding and future impact. Clin. Gastroenterol. Hepatol. 2004, 2, 1048–1058. [Google Scholar] [CrossRef]
- Petersen, K.F.; Dufour, S.; Savage, D.B.; Bilz, S.; Solomon, G.; Yonemitsu, S.; Cline, G.W.; Befroy, D.; Zemany, L.; Kahn, B.B.; et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 12587–12594. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Stein, D.T.; Barzilai, N.; Cui, M.H.; Tonelli, J.; Kishore, P.; Hawkins, M. Increased intrahepatic triglyceride is associated with peripheral insulin resistance: In vivo MR imaging and spectroscopy studies. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1663–E1669. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, J.; Kiens, B. Regulation and limitations to fatty acid oxidation during exercise. J. Physiol. 2012, 590, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002, 45, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Pernigo, M.; Bergamini, C.; Bonapace, S.; Lipari, P.; Pichiri, I.; Bertolini, L.; Valbusa, F.; Barbieri, E.; Zoppini, G.; et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0135329. [Google Scholar] [CrossRef] [PubMed]
- Granér, M.; Nyman, K.; Siren, R.; Pentikäinen, M.O.; Lundbom, J.; Hakkarainen, A.; Lauerma, K.; Lundbom, N.; Nieminen, M.S.; Taskinen, M.R. Ectopic fat depots and left ventricular function in nondiabetic men with nonalcoholic fatty liver disease. Circ. Cardiovasc. Imaging 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Winhofer, Y.; Smajis, S.; Jankovic, D.; Anderwald, C.H.; Trattnig, S.; Luger, A.; Krebs, M.; Krššák, M. Pericardial- Rather than Intramyocardial Fat Is Independently Associated with Left Ventricular Systolic Heart Function in Metabolically Healthy Humans. PLoS ONE 2016, 11, e0151301. [Google Scholar] [CrossRef] [PubMed]
- Bilet, L.; van de Weijer, T.; Hesselink, M.K.; Glatz, J.F.; Lamb, H.J.; Wildberger, J.; Kooi, M.E.; Schrauwen, P.; Schrauwen-Hinderling, V.B. Exercise-induced modulation of cardiac lipid content in healthy lean young men. Basic Res. Cardiol. 2011, 106, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Thamer, C.; Machann, J.; Bachmann, O.; Haap, M.; Dahl, D.; Wietek, B.; Tschritter, O.; Niess, A.; Brechtel, K.; Fritsche, A.; et al. Intramyocellular lipids: Anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J. Clin. Endocrinol. Metab. 2003, 88, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen-Hinderling, V.B.; Meex, R.C.; Hesselink, M.K.; van de Weijer, T.; Leiner, T.; Schär, M.; Lamb, H.J.; Wildberger, J.E.; Glatz, J.F.; Schrauwen, P.; et al. Cardiac lipid content is unresponsive to a physical activity training intervention in type 2 diabetic patients, despite improved ejection fraction. Cardiovasc. Diabetol. 2011, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Finucane, F.M.; Sharp, S.J.; Purslow, L.R.; Horton, K.; Horton, J.; Savage, D.B.; Brage, S.; Besson, H.; de Lucia Rolfe, E.; Sleigh, A.; et al. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: A randomised controlled trial. Diabetologia 2010, 53, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Higuchi, M. Endurance Exercise Reduces Hepatic Fat Content and Serum Fibroblast Growth Factor 21 Levels in Elderly Men. J. Clin. Endocrinol. Metab. 2016, 101, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bacha, F.; Hannon, T.; Kuk, J.L.; Boesch, C.; Arslanian, S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: A randomized, controlled trial. Diabetes 2012, 61, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Shojaee-Moradie, F.; Sprung, V.S.; Jones, H.; Pugh, C.J.; Richardson, P.; Kemp, G.J.; Barrett, M.; Jackson, N.C.; Thomas, E.L.; et al. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin. Sci. 2016, 130, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, K.; Thoma, C.; Hollingsworth, K.G.; Cassidy, S.; Anstee, Q.M.; Day, C.P.; Trenell, M.I. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin. Sci. 2015, 129, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, K.; Fattakhova, G.; Hollingsworth, K.G.; Thoma, C.; Moore, S.; Taylor, R.; Day, C.P.; Trenell, M.I. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011, 60, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Kienesberger, P.C.; Pulinilkunnil, T.; Nagendran, J.; Dyck, J.R. Myocardial triacylglycerol metabolism. J. Mol. Cell. Cardiol. 2013, 55, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- Brechtel, K.; Dahl, D.B.; Machann, J.; Bachmann, O.P.; Wenzel, I.; Maier, T.; Claussen, C.D.; Häring, H.U.; Jacob, S.; Schick, F. Fast elevation of the intramyocellular lipid content in the presence of circulating free fatty acids and hyperinsulinemia: A dynamic 1H-MRS study. Magn. Reson. Med. 2001, 45, 179–183. [Google Scholar] [CrossRef]
- Hoeks, J.; Mensink, M.; Hesselink, M.K.; Ekroos, K.; Schrauwen, P. Long- and medium-chain fatty acids induce insulin resistance to a similar extent in humans despite marked differences in muscle fat accumulation. J. Clin. Endocrinol. Metab. 2012, 97, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Boesch, C.; Kuk, J.L.; Arslanian, S. Effects of an overnight intravenous lipid infusion on intramyocellular lipid content and insulin sensitivity in African-American versus Caucasian adolescents. Metab. Clin. Exp. 2013, 62, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Fielding, B.A.; Callow, J.; Owen, R.M.; Samra, J.S.; Matthews, D.R.; Frayn, K.N. Postprandial lipemia: The origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am. J. Clin. Nutr. 1996, 63, 36–41. [Google Scholar] [PubMed]
- Boden, G.; Lebed, B.; Schatz, M.; Homko, C.; Lemieux, S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 2001, 50, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Deldicque, L.; Van Proeyen, K.; Francaux, M.; Hespel, P. The unfolded protein response in human skeletal muscle is not involved in the onset of glucose tolerance impairment induced by a fat-rich diet. Eur. J. Appl. Physiol. 2011, 111, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Frøsig, C.; Roepstorff, C.; Brandt, N.; Maarbjerg, S.J.; Birk, J.B.; Wojtaszewski, J.F.; Richter, E.A.; Kiens, B. Reduced malonyl-CoA content in recovery from exercise correlates with improved insulin-stimulated glucose uptake in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E787–E795. [Google Scholar] [CrossRef] [PubMed]
- Jazet, I.M.; Schaart, G.; Gastaldelli, A.; Ferrannini, E.; Hesselink, M.K.; Schrauwen, P.; Romijn, J.A.; Maassen, J.A.; Pijl, H.; Ouwens, D.M.; et al. Loss of 50% of excess weight using a very low energy diet improves insulin-stimulated glucose disposal and skeletal muscle insulin signalling in obese insulin-treated type 2 diabetic patients. Diabetologia 2008, 51, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Morino, K.; Yoo, P.S.; Cline, G.W.; Shulman, G.I. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc. Natl. Acad. Sci. USA 2012, 109, 8236–8240. [Google Scholar] [CrossRef] [PubMed]
- Lara-Castro, C.; Newcomer, B.R.; Rowell, J.; Wallace, P.; Shaughnessy, S.M.; Munoz, A.J.; Shiflett, A.M.; Rigsby, D.Y.; Lawrence, J.C.; Bohning, D.E.; et al. Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metab. Clin. Exp. 2008, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Jeukendrup, A.E.; Oseto, M.C.; Hodgkinson, B.J.; Zderic, T.W. Low-fat diet alters intramuscular substrates and reduces lipolysis and fat oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E391–E398. [Google Scholar] [PubMed]
- Van Herpen, N.A.; Schrauwen-Hinderling, V.B.; Schaart, G.; Mensink, R.P.; Schrauwen, P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J. Clin. Endocrinol. Metab. 2011, 96, E691–E695. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen-Hinderling, V.B.; Kooi, M.E.; Hesselink, M.K.; Moonen-Kornips, E.; Schaart, G.; Mustard, K.J.; Hardie, D.G.; Saris, W.H.; Nicolay, K.; Schrauwen, P. Intramyocellular lipid content and molecular adaptations in response to a 1-week high-fat diet. Obes. Res. 2005, 13, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Helge, J.W.; Watt, P.W.; Richter, E.A.; Rennie, M.J.; Kiens, B. Fat utilization during exercise: Adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J. Physiol. 2001, 537, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Helge, J.W.; Wulff, B.; Kiens, B. Impact of a fat-rich diet on endurance in man: Role of the dietary period. Med. Sci. Sports Exerc. 1998, 30, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Puntschart, A.; Howald, H.; Mueller, B.; Mannhart, C.; Gfeller-Tuescher, L.; Mullis, P.; Hoppeler, H. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Med. Sci. Sports Exerc. 2003, 35, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, E.; Kumahara, H.; Tobina, T.; Matsuda, T.; Ayabe, M.; Kiyonaga, A.; Anzai, K.; Higaki, Y.; Tanaka, H. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J. Obes. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Chan, R.S.; Wong, G.L.; Cheung, B.H.; Chu, W.C.; Yeung, D.K.; Chim, A.M.; Lai, J.W.; Li, L.S.; Sea, M.M.; et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: A randomized controlled trial. J. Hepatol. 2013, 59, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Heilbronn, L.K.; Redman, L.M.; Newcomer, B.R.; Frisard, M.I.; Anton, S.; Smith, S.R.; Alfonso, A.; Ravussin, E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006, 29, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; Snel, M.; Lamb, H.J.; Jazet, I.M.; van der Meer, R.W.; Pijl, H.; Meinders, E.A.; Romijn, J.A.; de Roos, A.; Smit, J.W. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J. Am. Coll. Cardiol. 2008, 52, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of β cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Sobrecases, H.; Lê, K.A.; Bortolotti, M.; Schneiter, P.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab. 2010, 36, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Westerbacka, J.; Lammi, K.; Häkkinen, A.M.; Rissanen, A.; Salminen, I.; Aro, A.; Yki-Järvinen, H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J. Clin. Endocrinol. Metab. 2005, 90, 2804–2809. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.; Reeds, D.N.; Finck, B.N.; Mayurranjan, S.M.; Patterson, B.W.; Klein, S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009, 136, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Baker, J.A.; Rogers, T.; Davis, J.; Satapati, S.; Burgess, S.C. Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 2011, 93, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Haufe, S.; Engeli, S.; Kast, P.; Böhnke, J.; Utz, W.; Haas, V.; Hermsdorf, M.; Mähler, A.; Wiesner, S.; Birkenfeld, A.L.; et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011, 53, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Brøns, C.; Jensen, C.B.; Storgaard, H.; Hiscock, N.J.; White, A.; Appel, J.S.; Jacobsen, S.; Nilsson, E.; Larsen, C.M.; Astrup, A.; et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J. Physiol. 2009, 587, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Assy, N.; Nasser, G.; Kamayse, I.; Nseir, W.; Beniashvili, Z.; Djibre, A.; Grosovski, M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can. J. Gastroenterol. 2008, 22, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Ngo Sock, E.T.; Lê, K.A.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br. J. Nutr. 2010, 103, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Lê, K.A.; Ith, M.; Kreis, R.; Faeh, D.; Bortolotti, M.; Tran, C.; Boesch, C.; Tappy, L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am. J. Clin. Nutr. 2009, 89, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Lecoultre, V.; Egli, L.; Carrel, G.; Theytaz, F.; Kreis, R.; Schneiter, P.; Boss, A.; Zwygart, K.; Lê, K.A.; Bortolotti, M.; et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity 2013, 21, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Theytaz, F.; Noguchi, Y.; Egli, L.; Campos, V.; Buehler, T.; Hodson, L.; Patterson, B.W.; Nishikata, N.; Kreis, R.; Mittendorfer, B.; et al. Effects of supplementation with essential amino acids on intrahepatic lipid concentrations during fructose overfeeding in humans. Am. J. Clin. Nutr. 2012, 96, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Campos, V.; Despland, C.; Brandejsky, V.; Kreis, R.; Schneiter, P.; Chiolero, A.; Boesch, C.; Tappy, L. Sugar- and artificially sweetened beverages and intrahepatic fat: A randomized controlled trial. Obesity 2015, 23, 2335–2239. [Google Scholar] [CrossRef] [PubMed]
- Faeh, D.; Minehira, K.; Schwarz, J.M.; Periasamy, R.; Park, S.; Tappy, L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005, 54, 1907–1913. [Google Scholar] [CrossRef] [PubMed]
- Nseir, W.; Nassar, F.; Assy, N. Soft drinks consumption and nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 2579–2588. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, A.C.; Adeli, K. Fructose and the metabolic syndrome: Pathophysiology and molecular mechanisms. Nutr. Rev. 2007, 65, S13–S23. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, R.M.; Bott, S.J.; Harding, M.; Coward, W.A.; Bluck, L.J.; Prentice, A.M. De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. Am. J. Clin. Nutr. 2001, 74, 737–746. [Google Scholar] [PubMed]
- Lê, K.A.; Faeh, D.; Stettler, R.; Ith, M.; Kreis, R.; Vermathen, P.; Boesch, C.; Ravussin, E.; Tappy, L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am. J. Clin. Nutr. 2006, 84, 1374–1379. [Google Scholar] [PubMed]
- Johnston, R.D.; Stephenson, M.C.; Crossland, H.; Cordon, S.M.; Palcidi, E.; Cox, E.F.; Taylor, M.A.; Aithal, G.P.; Macdonald, I.A. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013, 145, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Maiolo, E.; Corazza, M.; Van Dijke, E.; Schneiter, P.; Boss, A.; Carrel, G.; Giusti, V.; Lê, K.A.; Quo Chong, D.G.; et al. Effects of a whey protein supplementation on intrahepatocellular lipids in obese female patients. Clin. Nutr. 2011, 30, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.; van der Meer, R.W.; Lamb, H.J.; de Boer, H.H.; Bax, J.J.; de Roos, A.; Romijn, J.A.; Smit, J.W. Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E714–E718. [Google Scholar] [CrossRef] [PubMed]
- Abdesselam, I.; Dutour, A.; Kober, F.; Ancel, P.; Bege, T.; Darmon, P.; Lesavre, N.; Bernard, M.; Gaborit, B. Time Course of Change in Ectopic Fat Stores after Bariatric Surgery. J. Am. Coll. Cardiol. 2016, 67, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Garg, A. Lipodystrophies: Genetic and acquired body fat disorders. J. Clin. Endocrinol. Metab. 2011, 96, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012, 142, 1592–1609. [Google Scholar] [CrossRef] [PubMed]
- Lanktree, M.B.; Johansen, C.T.; Joy, T.R.; Hegele, R.A. A translational view of the genetics of lipodystrophy and ectopic fat deposition. Prog. Mol. Biol. Trans. Sci. 2010, 94, 159–196. [Google Scholar]
- Tsoukas, M.A.M.C. Lipodystrophy syndromes. In Endocrinology: Adult and Pediatric, 7th ed.; Jameson, J.L., De Groot, L.J., Eds.; Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Haque, W.A.; Shimomura, I.; Matsuzawa, Y.; Garg, A. Serum adiponectin and leptin levels in patients with lipodystrophies. J. Clin. Endocrinol. Metab. 2002, 87, 2395. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Oral, E.A.; Dufour, S.; Befroy, D.; Ariyan, C.; Yu, C.; Cline, G.W.; DePaoli, A.M.; Taylor, S.I.; Gorden, P.; et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Investig. 2002, 109, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Simha, V.; Szczepaniak, L.S.; Wagner, A.J.; DePaoli, A.M.; Garg, A. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care 2003, 26, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Safar Zadeh, E.; Lungu, A.O.; Cochran, E.K.; Brown, R.J.; Ghany, M.G.; Heller, T.; Kleiner, D.E.; Gorden, P. The liver diseases of lipodystrophy: The long-term effect of leptin treatment. J. Hepatol. 2013, 59, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E.; or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN trial team; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Blackett, P.R.; Sanghera, D.K. Genetic determinants of cardiometabolic risk: A proposed model for phenotype association and interaction. J. Clin. Lipidol. 2013, 7, 65–81. [Google Scholar] [CrossRef] [PubMed]
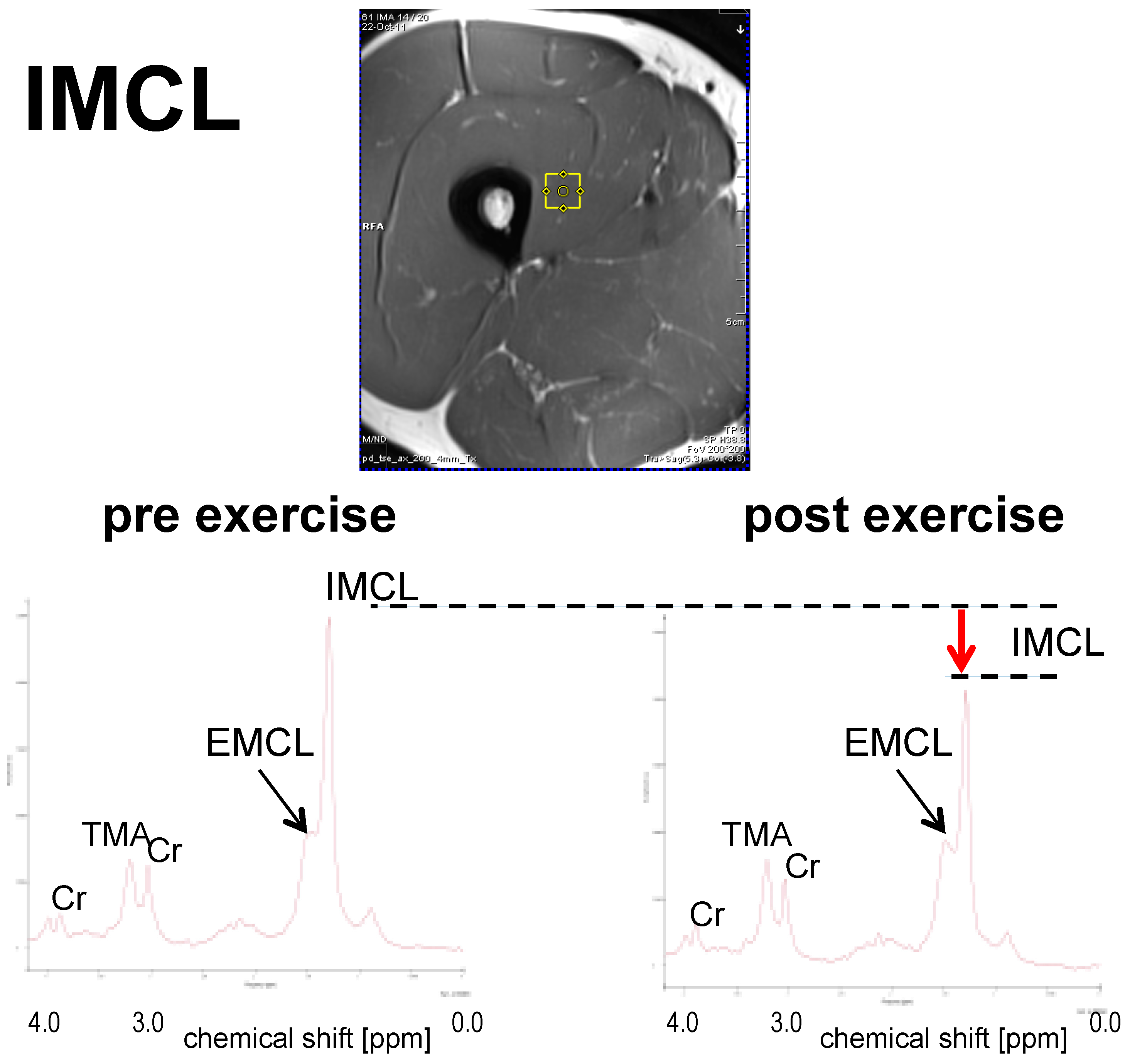
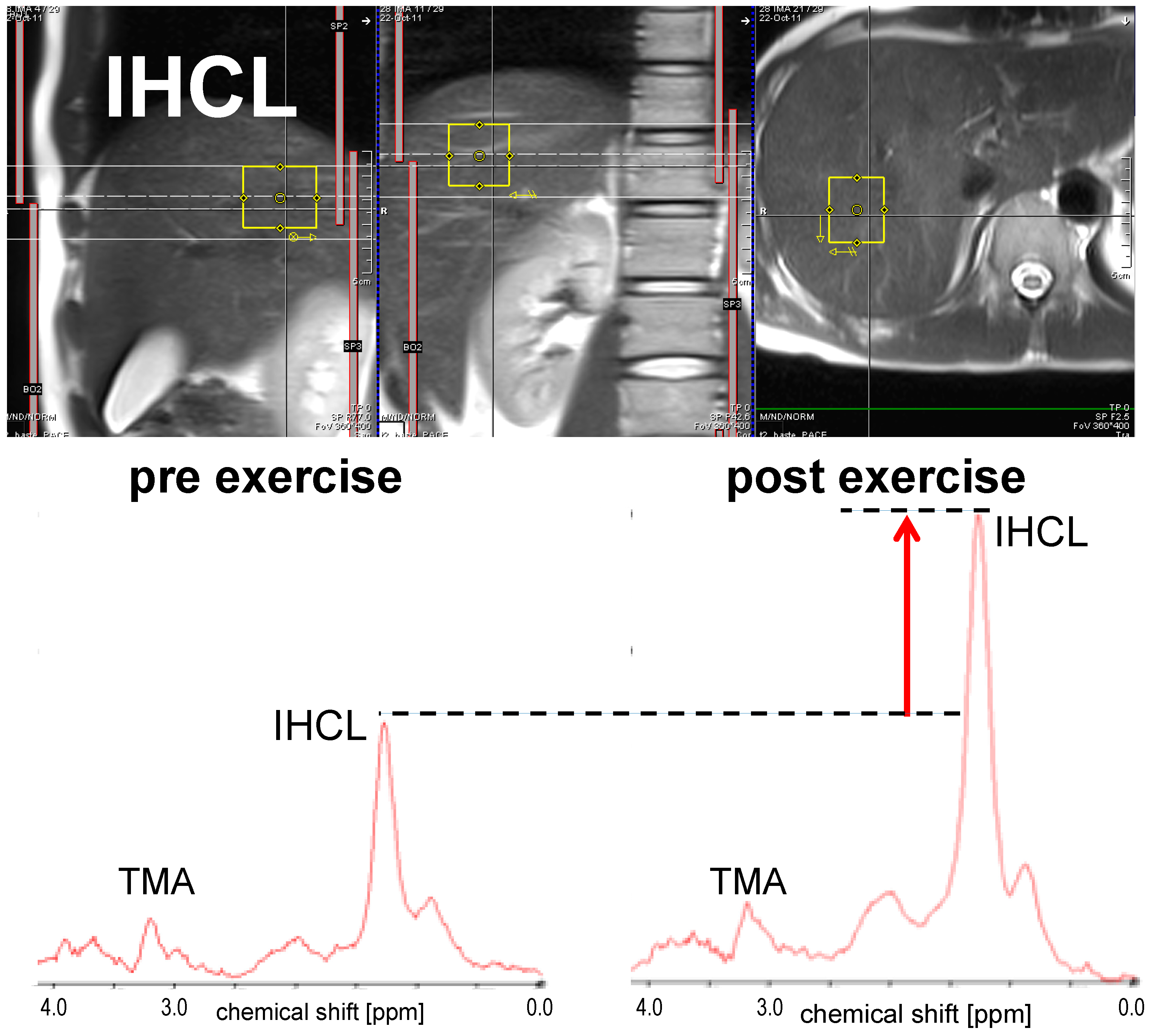
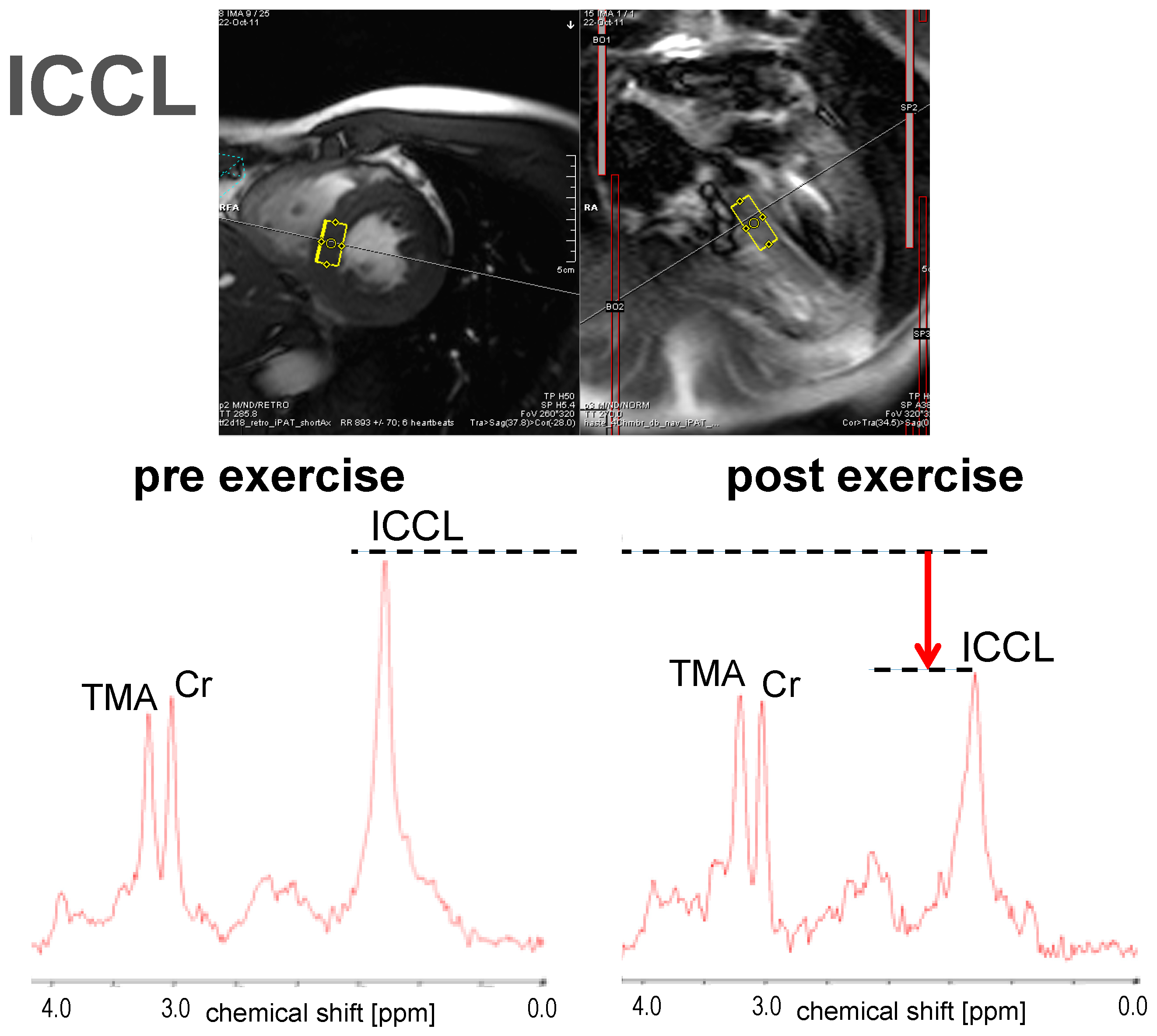
| Author (Year) | n | Subjects | Gender | Intervention | IMCL | % Change | Muscle Investigated | Comments |
|---|---|---|---|---|---|---|---|---|
| Christ (2016) [67] | 10 | Volunteers with adult-onset GHD | m, f | 2 h exercise at 50%–60% VO2 max on a treadmill | ↓ * | −9.3 to −13.5 | M. tibialis anterior | No significant effect of growth hormone replacement therapy on IMCL and IHCL, IHCL ↑ * |
| Bucher (2014) [43] | 10 | Healthy volunteers | m | 2 h exercise on bicycle ergometer at 50%–60% VO2 max | ↓ * | −16.8 | M. vastus intermedius | IHCL ↑ *, ICCL ↓ * |
| Egger (2013) [44] | 18 | Healthy volunteers | m, f | 2 h exercise on treadmill at 50%–60% VO2 max | ↓ * | −22.6 | M. tibialis anterior | IHCL ↑ * |
| Vermathen (2012) [47] | 8 | Trained cyclists or runners | m | 3 h exercise on bicycle ergometer or treadmill at 50% Wmax | ↓ * | −3 to −50 | Thigh (M. vastus intermedius, vastus lateralis, vastus lateralis, adductor magnus, biceps femoris; rectus femoris) or lower leg muscle (tibialis anterior, soleus lateralis, soleus medialis, gastrocnemius lateralis, gastrocnemius medialis, extensor digitorum) | In M. biceps femoris and rectus femoris no significant decrease |
| Jenni (2008) [65] | 7 | Physically active men with T1DM | m | 2 h cycling at 55%–60% VO2 max | ↓ * | −11.5 to −16.2 | M. vastus intermedius | |
| Trepp (2008) [66] | 15 | Volunteers with adult-onset GHD | m, f | 1 h walking at heart rate corresponding to 50% VO2 max, on three days and low fat diet | ↓ * | −35 to −47.5 ** | M. tibialis anterior | No significant effect of growth hormone replacement therapy on IMCL |
| De Bock (2007) [48] | 9 | Physically active men | m | 2 h cycling at 75% VO2 peak | ↓ * | −47 | M. vastus lateralis | |
| Zehnder (2006) [37] | 11 | Endurance trained cyclists | m | 3 h cycling at 50% Wmax | ↓ * | −21 to −41 | M. vastus intermedius | |
| Zehnder (2005) [49] | 18 | Cyclists or triathletes | m, f | 3 h cycling at 50% Wmax | ↓ * | −42 to −59 | M. vastus intermedius | Larger reduction in males |
| Schrauwen-Hinderling (2003) [50] | 8 | Highly trained cyclists | m | 3 h cycling at 55% Wmax | ↓ * | −20.4 | M. vastus lateralis | M. biceps brachii ↑ * |
| Van Loon (2003) [51] | 9 | Endurance-trained cyclists | m | 3 h cycling at 55% Wmax | ↓ * | −21 | M. vastus lateralis | No difference between normal and low-fat diet |
| White (2003) [46] | 9 | Moderately active | m | 45 min cycling, intervals at 50% and 110% of ventilator threshold | ↓ * | −38 | M. vastus lateralis | |
| White (2003) [45] | 18 | Moderately active | m, f | 1 h cycling at 65% VO2 max | ↓ * | −11.5 to −17.1 | M. vastus lateralis | |
| Johnson (2003) [52] | 6 | Highly trained cyclists | m | 3 h cycling at 70% VO2 max | ↓ * | −57 to −64 | M. vastus lateralis | Higher IMCL degradation in low carbohydrate condition |
| Larson-Meyer (2002) [38] | 7 | Well-trained endurance runners | f | 2 h running at 65% VO2 max | ↓ * | −25 | M. soleus | |
| Brechtel (2001) [53] | 12 | Well-trained subjects | m | Running: parallel design 60%–70% VO2 max, 80%–90% VO2 max 21/42 km | ↓ | −10 to −42 | M. tibialis anterior, M. soleus | |
| Krssak (2000) [54] | 9 | Trained subjects | m, f | 3–4 bouts of 45 min of running at 65%–70% peak oxygen until exhaustion | ↓ * | −33.5 ** | M. soleus | |
| Rico-Sanz (2000) [55] | 5 | Trained subjects | m | 90 min running at 64% VO2 max | ↓ * | −15.7 to −32.2 ** | M. soleus, tibialis, gastrocnemius | in M. gastrocnemius no sign decrease |
| Rico-Sanz (1998) [68] | 8 | Trained subjects | m | 13.2 km running, jogging, sprinting | → | +9 to −2.4 ** | M. soleus, gastrocnemius, tibialis |
| Author (Year) | n | Subjects | Gender | Intervention | IHCL | Comments |
|---|---|---|---|---|---|---|
| Christ (2016) [67] | 10 | Volunteers with adult-onset GHD | m, f | 2 h exercise at 50%–60% VO2 max on a treadmill | ↑ * | No significant effect of growth hormone replacement therapy on IMCL and IHCL, IMCL ↓ * |
| Bilet (2015) [69] | 21 | Overweight subjects | m | 2 h cycling at 50% Wmax | → | |
| Bucher (2014) [43] | 10 | Healthy volunteers | m | 2 h cycling at 50%–60% VO2 max | ↑ * | ICCL ↓ *, IMCL ↓ * |
| Egger (2013) [44] | 18 | Healthy volunteers | m, f | 2 h aerobic exercise on treadmill at 50%–60% VO2 max | ↑ * | |
| Johnson (2012) [70] | 6 | Healthy trained volunteers | m | 90 min cycling at 65% VO2 peak | ↑ * | At 4.5 h post-exercise |
| Author (Year) | n | Subjects | Gender | Intervention | IMCL | Comments: Method, Muscle Investigated |
|---|---|---|---|---|---|---|
| Browning (2012) [23] | 18 | Healthy individuals | m, f | Fasting for 48 h | ↑ * | 1H-MRS M. soleus, only in women, not in men |
| Green (2010) [24] | 6 | Healthy physically fit men | m | Fasting for 67 h | ↑ * | 1H-MRS M. vastus lateralis |
| Stannard (2002) [25] | 6 | Nondiabetic, physically fit men | m | Fasting for 72 h | ↑ * | 1H-MRS M. vastus lateralis |
| Wietek (2004) [26] | 4 | Healthy volunteers | m, f | Fasting for 120 h | ↑ * | 1H-MRS M. tibialis anterior, soleus |
| Machann (2011) [27] | 12 | Healthy volunteers | m | Fasting for 12 h | ↓ * | 1H-MRS M. tibialis anterior, soleus |
| Bachmann (2001) [28] | 12 | Healthy volunteers | m | High-fat diet for 3 days | ↑ * | 1H-MRS M. tibialis anterior, soleus (increase in M. tibialis, not in M. soleus) |
| Sakurai (2011) [29] | 37 | Healthy volunteers | m | Isocaloric, high-fat diet for 3 days | ↑ * | 1H-MRS M. tibialis anterior, M. soleus |
| Zderic (2004) [30] | 6 | Endurance-trained cyclists | m | Isocaloric, high-fat diet for 2 days | ↑ * | Biopsy M. vastus lateralis |
| Larson-Meyer (2008) [31] | 21 | Endurance-trained runners | m, f | Isoenergetic, high-fat diet for 3 days | ↑ * | Biopsy M. vastus lateralis Sign higher |
| Lindeboom (2015) [33] | 9 | Lean healthy subjects | m, f | Single high-energy, high-fat meal | → | 1H-MRS M. tibialis anterior, ↑ * IHCL |
| Brechtel (2001) [186] | 5 | Healthy male subjects | m | 5 h hyperinsulinemic euglycemic clamp and intralipid infusion | ↑ * | 1H-MRS M. tibialis anterior, M. soleus |
| Bachmann (2001) [28] | 12 | Healthy volunteers | m | 6 h lipid infusion during hyperinsulinemic euglycemic clamp | ↑ * | 1H-MRS M. tibialis anterior, M.soleus; only in presence of insulin infusion |
| Hoeks (2012) [187] | 9 | Healthy lean males | m | 6 h euglycemic hyperinsulinemic clamp and lipid or glycerol infusion | ↑ * | Only in long-chain triacylglycerols emulsion, not in medium chain glycerols emulsion Biopsy M. vastus lateralis |
| Lee (2013) [188] | 28 | Normal-weight adolescents | m,f | 12 h lipid infusion and 3 h hyperinsulinemic euglycemic clamp | ↑ * | 1H-MRS M. tibialis anterior |
| Brehm (2010) [40] | 8 | Glucose-tolerant volunteers | m | 3 h Euglycemic pancreatic clamp, and intralipid infusion | → | 1H-MRS M. soleus |
| Author (Year) | n | Subjects | Gender | Intervention | IHCL | Comments |
|---|---|---|---|---|---|---|
| Van der Meer (2007) [34] | 14 | Healthy, non-obese men | m | 3 days very low calorie diet | ↓ * | ICCL increased |
| Browning (2012) [23] | 18 | Healthy individuals | m, f | 48 h fasting | ↑ * | in males, no sign increase in women |
| Lindeboom (2015) [33] | 9 | Lean healthy subjects | m, f | Single high-energy, high-fat meal | ↑ * | |
| Van der Meer (2008) [35] | 15 | Healthy men | m | 3 days high-fat, high-energy diet | ↑ * | No effect on ICCL |
| Bortolotti (2009) [36] | 10 | Healthy young men | m | 4 days hypercaloric high-fat diet | ↑ * | Protein co-ingestion blunts effect of high fat diet |
| Johnson (2012) [70] | 6 | Healthy trained males | m | High-fat diet | → | compared to Isocaloric control diet |
| Kirk (2009) [211] | 22 | Obese subjects | m, f | 48 h energy-deficient, high-fat diet | ↓ * | |
| Ngo Sock (2010) [217] | 11 | Healthy men | m | 7 days hypercaloric, high-fructose diet | ↑ * | |
| Lê (2009) [218] | 24 | Healthy offspring of T2DM patients and control subjects | m | 7 days high-fructose diet | ↑ * | also significant increase in IMCL |
| Lecoultre (2013) [219] | 55 | Healthy young males | m | 6–7 days high-fructose diet | ↑ * | Only if at least 3 g fructose/kg/day |
| Theytaz (2012) [220] | 9 | Healthy male volunteers | m | 6 days high-fructose diet | ↑ * | Supplementation with amino acids blunts increase |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loher, H.; Kreis, R.; Boesch, C.; Christ, E. The Flexibility of Ectopic Lipids. Int. J. Mol. Sci. 2016, 17, 1554. https://doi.org/10.3390/ijms17091554
Loher H, Kreis R, Boesch C, Christ E. The Flexibility of Ectopic Lipids. International Journal of Molecular Sciences. 2016; 17(9):1554. https://doi.org/10.3390/ijms17091554
Chicago/Turabian StyleLoher, Hannah, Roland Kreis, Chris Boesch, and Emanuel Christ. 2016. "The Flexibility of Ectopic Lipids" International Journal of Molecular Sciences 17, no. 9: 1554. https://doi.org/10.3390/ijms17091554
APA StyleLoher, H., Kreis, R., Boesch, C., & Christ, E. (2016). The Flexibility of Ectopic Lipids. International Journal of Molecular Sciences, 17(9), 1554. https://doi.org/10.3390/ijms17091554






