Bioinformatics Identification of Drug Resistance-Associated Gene Pairs in Mycobacterium tuberculosis
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
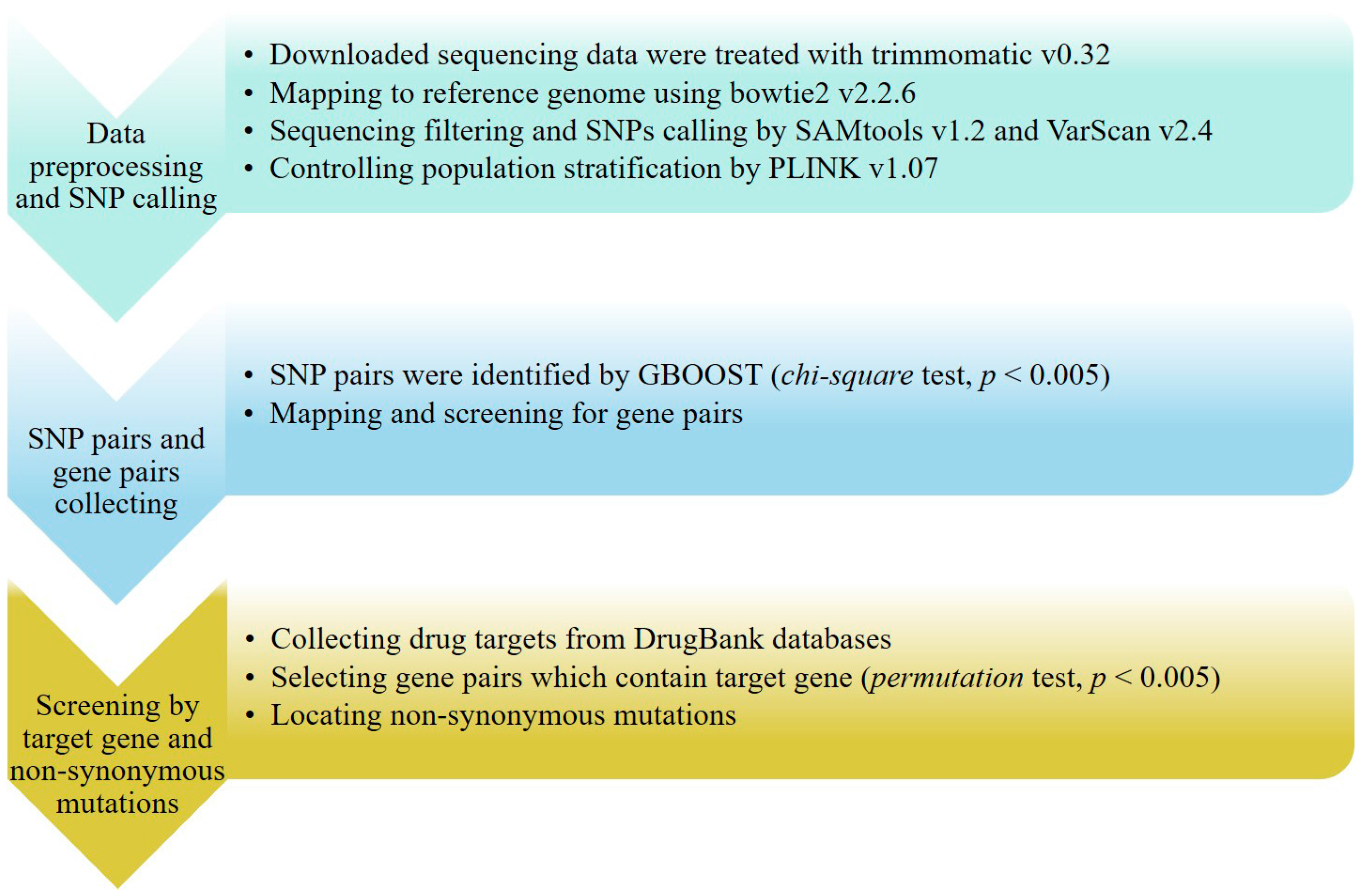
3.1. Data Source and SNP Calling
3.2. SNP Pair and Gene Pair Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooper, A.M. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009, 27, 393–422. [Google Scholar] [CrossRef] [PubMed]
- 3rd Global GLC meeting World Health Organization, Geneva, Switzerland, 17–19 October 2012 Meeting Report. Available online: http://apps.who.int/iris/bitstream/10665/77948/1/WHO_HTM_TB_2012.13_eng.pdf (accessed on 11 January 2013).
- World Health Organisation Global Tuberculosis Report. Available online: http://www.who.int/tb/Global_TB_Facts.pdf (assessed on 28 October 2015).
- Narendran, G.; Swaminathan, S. TB–HIV co-infection: A catastrophic comradeship. Oral Dis. 2016, 22, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Bates, J.H. Tuberculosis chemotherapy: The need for new antituberculosis drugs is urgent. Am. J. Respir. Crit. Care Med. 1995, 151, 942–943. [Google Scholar] [PubMed]
- Pontali, E.; Matteelli, A.; Migliori, G.B. Drug-resistant tuberculosis. Curr. Opin. Pulm. Med. 2013, 19, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Falzon, D.; Jaramillo, E.; Schünemann, H.J.; Arentz, M.; Bauer, M.; Bayona, J.; Blanc, L.; Caminero, J.A.; Daley, C.L.; Duncombe, C.; et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur. Respir. J. 2011, 38, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yew, W.W. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int. J. Tuberc. Lung. Dis. 2015, 19, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, B.; Carpena, X.; Feliz, M.; Donald, L.J.; Pons, M.; Fita, I.; Loewen, P.C. Isonicotinic acid hydrazide conversion to isonicotinyl-NAD by catalase-peroxidases. J. Biol. Chem. 2010, 285, 26662–26673. [Google Scholar] [CrossRef] [PubMed]
- Sreevatsan, S.; Stockbauer, K.E.; Pan, X. Ethambutol resistance in Mycobacterium tuberculosis: Critical role of embB mutations. Antimicrob. Agents Chemother. 1997, 41, 1677–1681. [Google Scholar] [PubMed]
- Caminero, J.A.; Sotgiu, G.; Zumla, A.; Migliori, G.B. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect. Dis. 2010, 10, 621–629. [Google Scholar] [CrossRef]
- Morlock, G.P.; Metchock, B.; Sikes, D.; Crawford, J.T.; Cooksey, R.C. ethA, inhA, and katG Loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 2003, 47, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Borrell, S.; Rose, G.; Gagneux, S. The heterogeneous evolution of multidrug-resistant Mycobacterium tuberculosis. Trends Genet. 2013, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.R.; Moll, A.; Sturm, A.W.; Pawinski, R.; Govender, T.; Lalloo, U.; Zeller, K.; Andrews, J.; Friedland, G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006, 368, 1575–1580. [Google Scholar] [CrossRef]
- Phelan, J.; Coll, F.; McNerney, R.; Ascher, D.B.; Pires, D.E.; Furnham, N.; Coeck, N.; Hill-Cawthorne, G.A.; Nair, M.B.; Mallard, K.; et al. Mycobacterium tuberculosis whole genome sequencing and protein structure modelling provides insights into anti-tuberculosis drug resistance. BMC Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, D.; Zhao, L.; Fleming, J.; Lin, N.; Wang, T.; Liu, Z.; Li, C.; Galwey, N.; Deng, J.; et al. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat. Genet. 2013, 45, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Trauner, A.; Borrell, S.; Reither, K.; Gagneux, S. Evolution of drug resistance in tuberculosis: Recent progress and implications for diagnosis and therapy. Drugs 2014, 74, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhang, X. Protein targets for structure-based anti-Mycobacterium tuberculosis drug discovery. Protein Cell 2010, 1, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Jessica, N.T.; Lynthia, V.P.; Timothy, C.R.; Victor, T.C.; Amallraja, A.M.; Elghraoui, A.; Goodmanson, A.P.; Ramirez-Busby, S.M.; Chawla, A.; Zadorozhny, V.; et al. Novel katG mutations causing isoniazid resistance in clinical M. tuberculosis isolates. Emerg. Microbes Infect. 2015. [Google Scholar] [CrossRef]
- Banerjee, A.; Dubnau, E.; Quemard, A.; Balasubramanian, V.; Um, K.S.; Wilson, T.; Collins, D.; Delisle, G.; Jacobs, W.R. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994, 263, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.V.; Amin, A.G.; Goksel, S.; Stager, C.E.; Dou, S.J.; El Sahly, H.; Moghazeh, S.L.; Kreiswirth, B.N.; Musser, J.M. Molecular genetic analysis of nucleotide polymorphisms associated with ethambutol resistance in human isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2000, 44, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, H.; Yu, S.; Wang, F.; Sacchettini, J.C.; Magliozzo, R.S. Hydrogen peroxide-mediated isoniazid activation catalyzed by Mycobacterium tuberculosis catalase-peroxidase (KatG) and its S315T mutant. Biochemistry 2006, 45, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Sampson, S.L. Mycobacterial PE/PPE proteins at the host-pathogen interface. Clin. Dev. Immunol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Das, A.; Mukhopadhyay, S. Immunoregulatory functions and expression patterns of PE/PPE family members: Roles in pathogenicity and impact on anti-tuberculosis vaccine and drug design. IUBMB Life 2015, 67, 414–427. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.; Pellegrini, M.; Eisenberg, D. Identifying cognate binding pairs among a large set of paralogs: The case of PE/PPE proteins of Mycobacterium tuberculosis. PLoS Comput. Biol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rao, R.N.; Reddy, J.R.; Prasad, R.; Kotturu, S.K.; Ghosh, S.; Mukhopadhyay, S. PE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodeling and virulence. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Balaji, K.N. The PE and PPE proteins of Mycobacterium tuberculosis. Tuberculosis 2011, 91, 441–447. [Google Scholar] [CrossRef]
- Duan, Z.L.; Li, Q.; Wang, S.; Chen, X.Y.; Liu, H.F.; Chen, B.K.; Li, D.Z.; Huang, X.; Wen, J.S. Identification of Mycobacterium tuberculosis PPE68-specific HLA-A*0201-restricted epitopes for tuberculosis diagnosis. Curr. Microbiol. 2015, 70, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Demangel, C.; Brodin, P.; Cockle, P.J.; Brosch, R.; Majlessi, L.; Leclerc, C.; Cole, S.T. Cell envelope protein PPE68 contributes to Mycobacterium tuberculosis RD1 immunogenicity independently of a 10-kilodalton culture filtrate protein and ESAT-6. Infect. Immun. 2004, 72, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miltner, E.; Wu, M.; Petrofsky, M.; Bermudez, L.E. A Mycobacterium avium PPE gene is associated with the ability of the bacterium to grow in macrophages and virulence in mice. Cell. Microbiol. 2005, 7, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and samtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Pavy, N.; Deschenes, A.; Blais, S.; Lavigne, P.; Beaulieu, J.; Isabel, N.; Mackay, J.; Bousquet, J. The landscape of nucleotide polymorphism among 13,500 genes of the conifer picea glauca, relationships with functions, and comparison with Medicago truncatula. Genome Biol. Evol. 2013, 5, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.; Inouye, M. Fast principal component analysis of large-scale genome-wide data. PLoS ONE 2014, 9, e93766. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.S.; Yang, C.; Wan, X.; Yu, W. GBOOST: A GPU-based tool for detecting gene-gene interactions in genome-wide case control studies. Bioinformatics 2011, 27, 1309–1310. [Google Scholar] [CrossRef] [PubMed]
| Group | Drug |
|---|---|
| Group 1: First-line oral agents | isoniazid; rifampicin; ethambutol; pyrazinamide |
| Group 2: Injectable agents | kanamycin; amikacin; capreomycin; streptomycin |
| Group 3: Fluoroquinolones | ofloxacin; levofloxacin; moxifloxacin; gatifloxacin |
| Group 4: Oral bacteriostatic second-line agents | p-aminosalicylic acid; cycloserine; terizidone; ethionamide; protionamide |
| Group 5: Agents with unclear efficacy | clofazimine; linezolid; amoxicillin; imipenem; clarithromycin; thioacetazone |
| Drug | Genes Involved in Resistance |
|---|---|
| Capreomycin (CPM) | rrs, tlyA |
| Ethambutol (EMB) | embA, embB, embC |
| Ethionamide (ETH) | inhA, katG |
| Isoniazid (INH) | katG, inhA, ahpC, fabG, fadE24 |
| Kanamycin (KAN) | rpsL rrs |
| Ofloxacin (OFX) | gyrA, gyrB |
| Rifampicin (RMP) | rpoA, rpoB, rpoC |
| Streptomycin (STR) | gidB, rpsL rrs |
| Gene ID (Target Name) | Gene ID (Gene Name) | SNP1 | SNP2 | Chi-Square Test | Permutation Test |
|---|---|---|---|---|---|
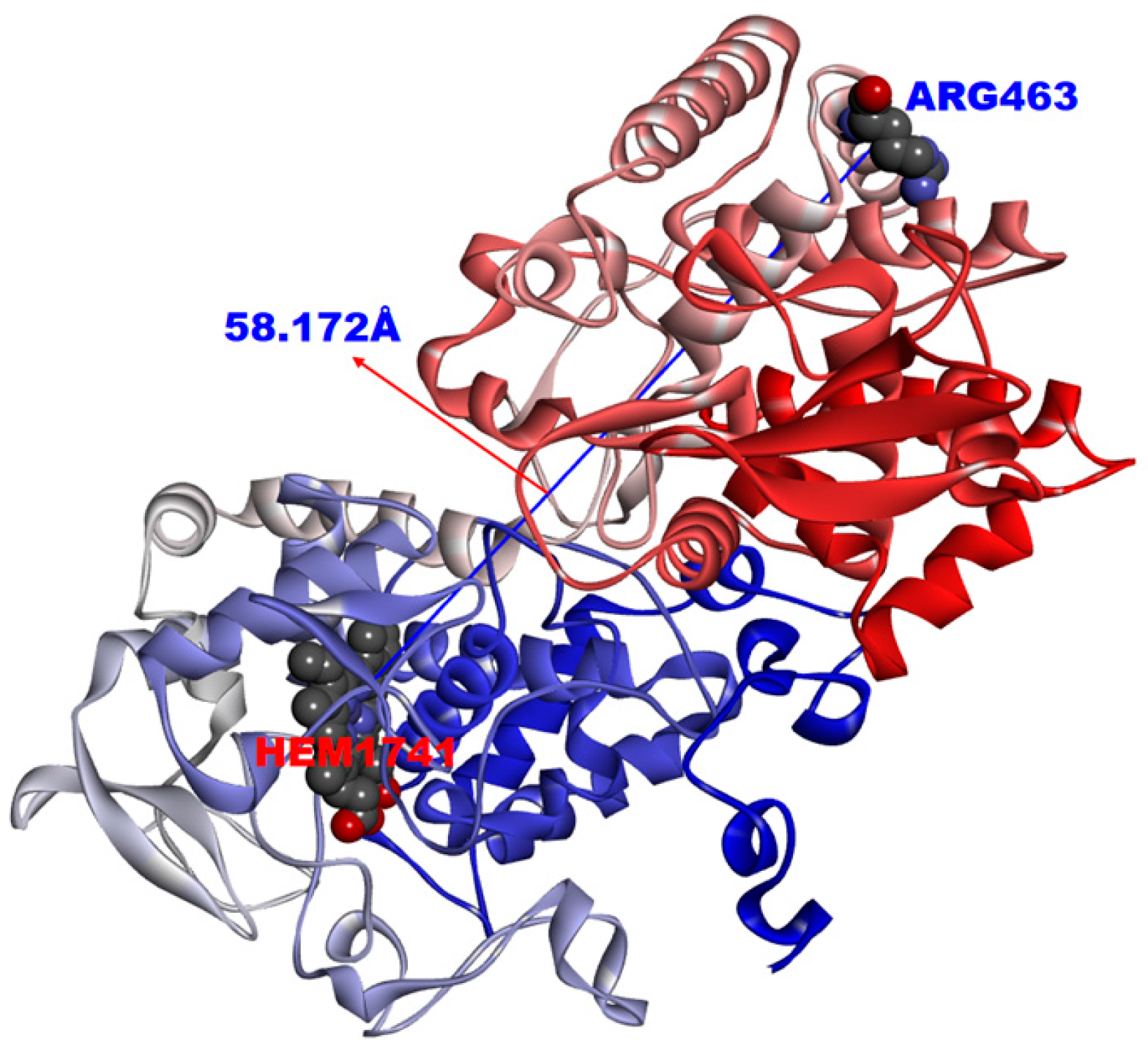
| Rv1908c (katG) a | Rv3343c (PPE54) a | rs_5245 | rs_8894 | 1.69 × 10−3 | <0.0001 |
| Rv0667 (rpoB) b | Rv3343c (PPE54) b | rs_2019 | rs_8894 | 1.28 × 10−4 | <0.0001 |
| Gene ID (Target Name) | Gene ID (Gene Name) | SNP1 | SNP2 | Chi-Square Test | Permutation Test |
|---|---|---|---|---|---|
| Rv3794 (embA) a | Rv3873 (PPE68) a | rss_17400 | rss_17926 | 1.98 × 10−3 | 0.0016 |
| Rv3795 (embB) a | Rv3343c (PPE54) a | rss_17410 | rss_15182 | 2.29 × 10−3 | <0.0001 |
| Rv3795 (embB) a | Rv3343c (PPE54) a | rss_17411 | rss_15181 | 8.55 × 10−4 | 0.0004 |
| Rv3795 (embB) a | Rv3343c (PPE54) a | rss_17411 | rss_15182 | 2.19 × 10−3 | <0.0001 |
| Rv1908c (katG) b | Rv3343c (PPE54) b | rss_8886 | rss_15184 | 2.16 × 10−3 | 0.0023 |
| Rv1908c (katG) b | Rv0576 (transcriptional regulator) b | rss_8886 | rss_3040 | 1.86 × 10−3 | <0.0001 |
| Rv1908c (katG) b | Rv0529 (ccsA) b | rss_8886 | rss_2852 | 1.78 × 10−3 | 0.0038 |
| Rv1908c (katG) b | Rv3554 (fdxB) b | rss_8886 | rss_16297 | 1.20 × 10−3 | 0.0009 |
| Rv1908c (katG) b | Rv0575c (oxidoreductase) b | rss_8900 | rss_3037 | 1.80 × 10−3 | 0.0009 |
| Drug | SNP | SNP Loci | Gene ID | Target | Base Mutation | Amino Acid Mutation | Previously Reported |
|---|---|---|---|---|---|---|---|
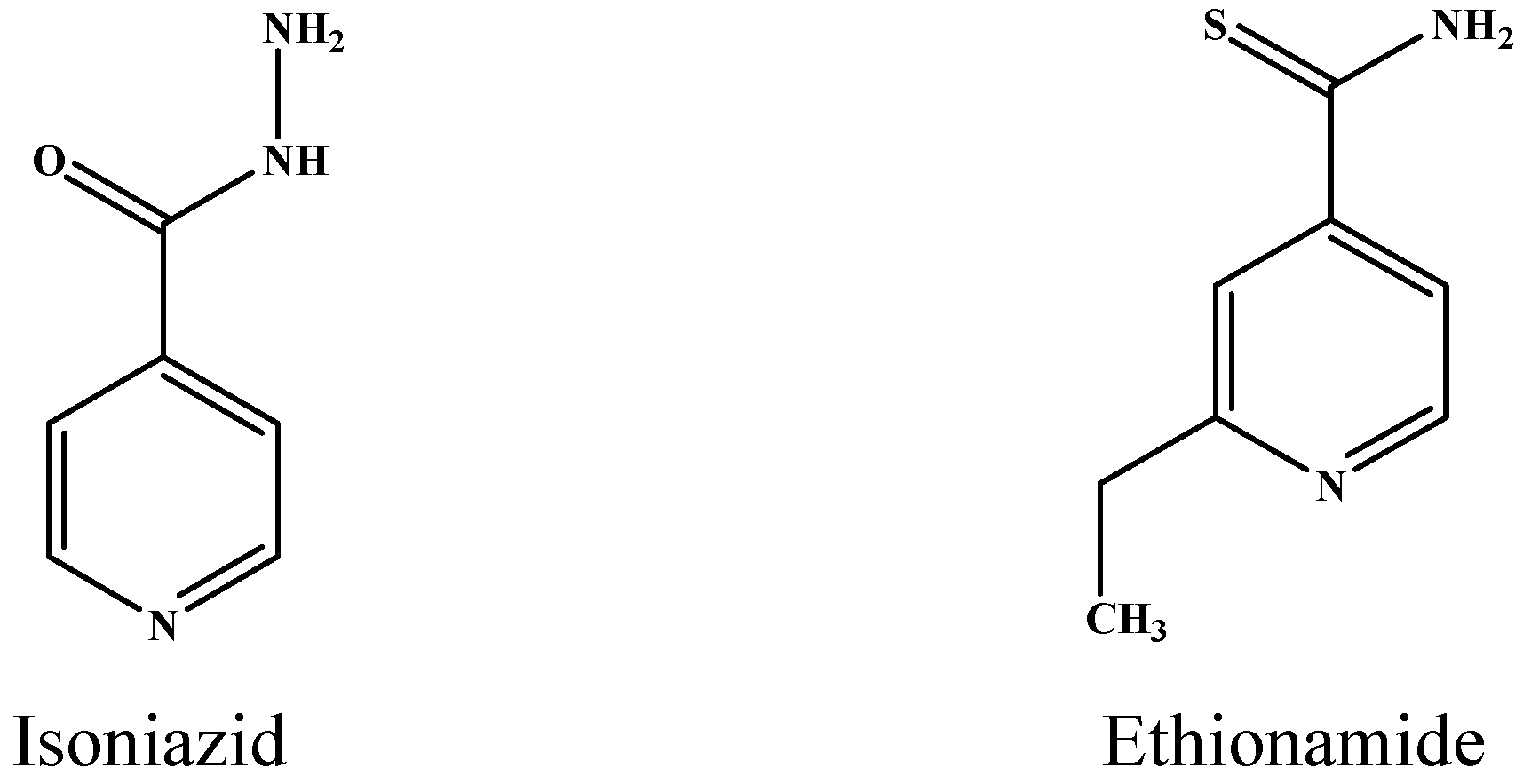
| INH a | rs_5245 | 2154724 | Rv1908c | KatG | CGG > CTG | R463L | [19] |
| EMB b | rss_17410 | 4247709 | Rv3795 | embB | AAC > ACC | N399T | – |
| EMB b | rss_17411 | 4247729 | Rv3795 | embB | GGC > AGC | G406S | [16,21] |
| ETH c | rss_8886 | 2154724 | Rv1908c | KatG | CGG > CTG | R463L | – |
| ETH c | rss_8900 | 2155648 | Rv1908c | KatG | TAC > TGC | Y155C | [16] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Z.-J.; Yang, Q.-Y.; Zhang, H.-Y.; Zhu, Q.; Zhang, Q.-Y. Bioinformatics Identification of Drug Resistance-Associated Gene Pairs in Mycobacterium tuberculosis. Int. J. Mol. Sci. 2016, 17, 1417. https://doi.org/10.3390/ijms17091417
Cui Z-J, Yang Q-Y, Zhang H-Y, Zhu Q, Zhang Q-Y. Bioinformatics Identification of Drug Resistance-Associated Gene Pairs in Mycobacterium tuberculosis. International Journal of Molecular Sciences. 2016; 17(9):1417. https://doi.org/10.3390/ijms17091417
Chicago/Turabian StyleCui, Ze-Jia, Qing-Yong Yang, Hong-Yu Zhang, Qiang Zhu, and Qing-Ye Zhang. 2016. "Bioinformatics Identification of Drug Resistance-Associated Gene Pairs in Mycobacterium tuberculosis" International Journal of Molecular Sciences 17, no. 9: 1417. https://doi.org/10.3390/ijms17091417
APA StyleCui, Z.-J., Yang, Q.-Y., Zhang, H.-Y., Zhu, Q., & Zhang, Q.-Y. (2016). Bioinformatics Identification of Drug Resistance-Associated Gene Pairs in Mycobacterium tuberculosis. International Journal of Molecular Sciences, 17(9), 1417. https://doi.org/10.3390/ijms17091417








