RETRACTED: Advanced Glycation End-Products Enhance Lung Cancer Cell Invasion and Migration
Abstract
:1. Introduction
2. Results
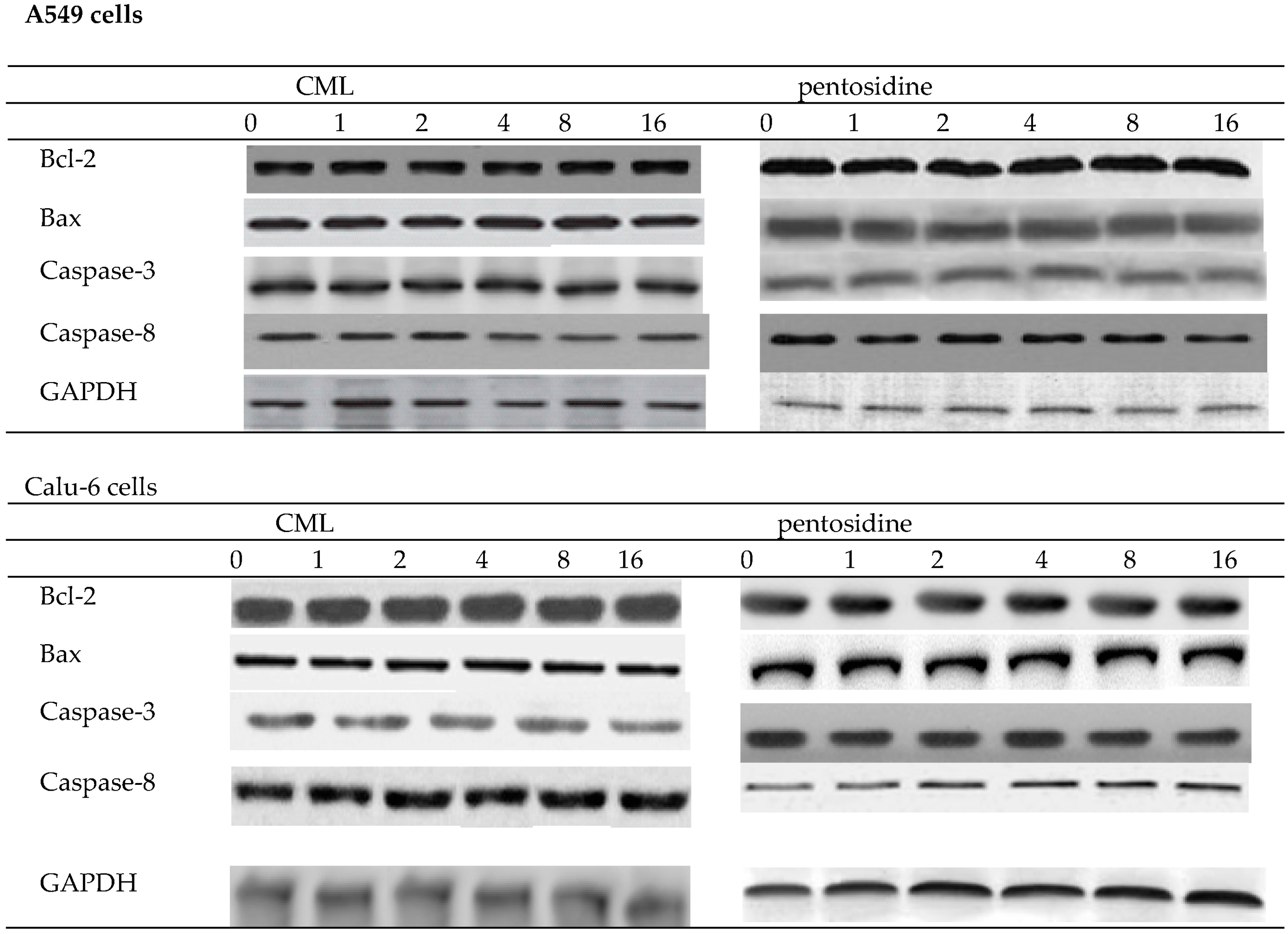
2.1. Effects of CML and Pentosidine upon Invasion and Migration of Lung Cancer Cells
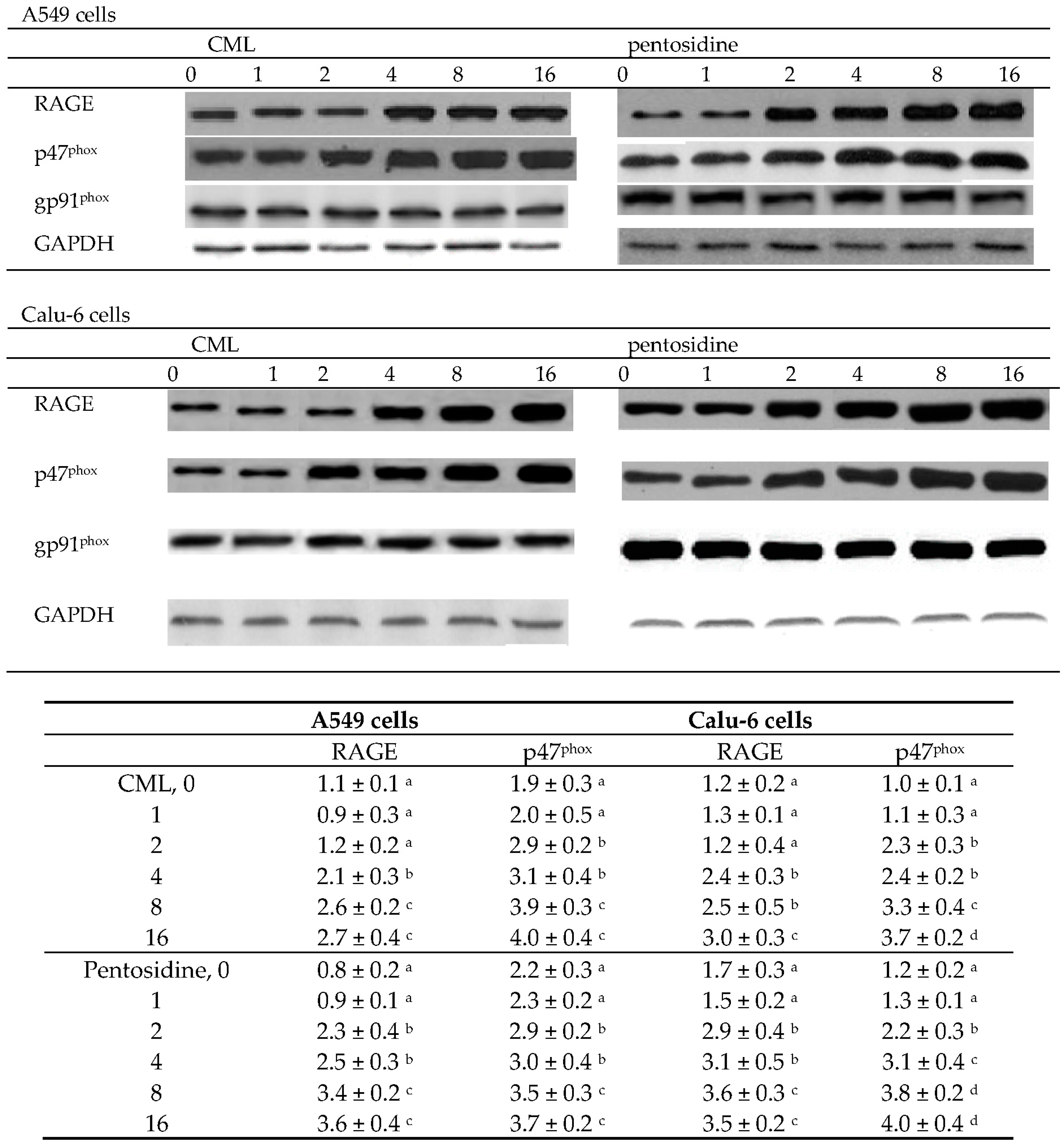
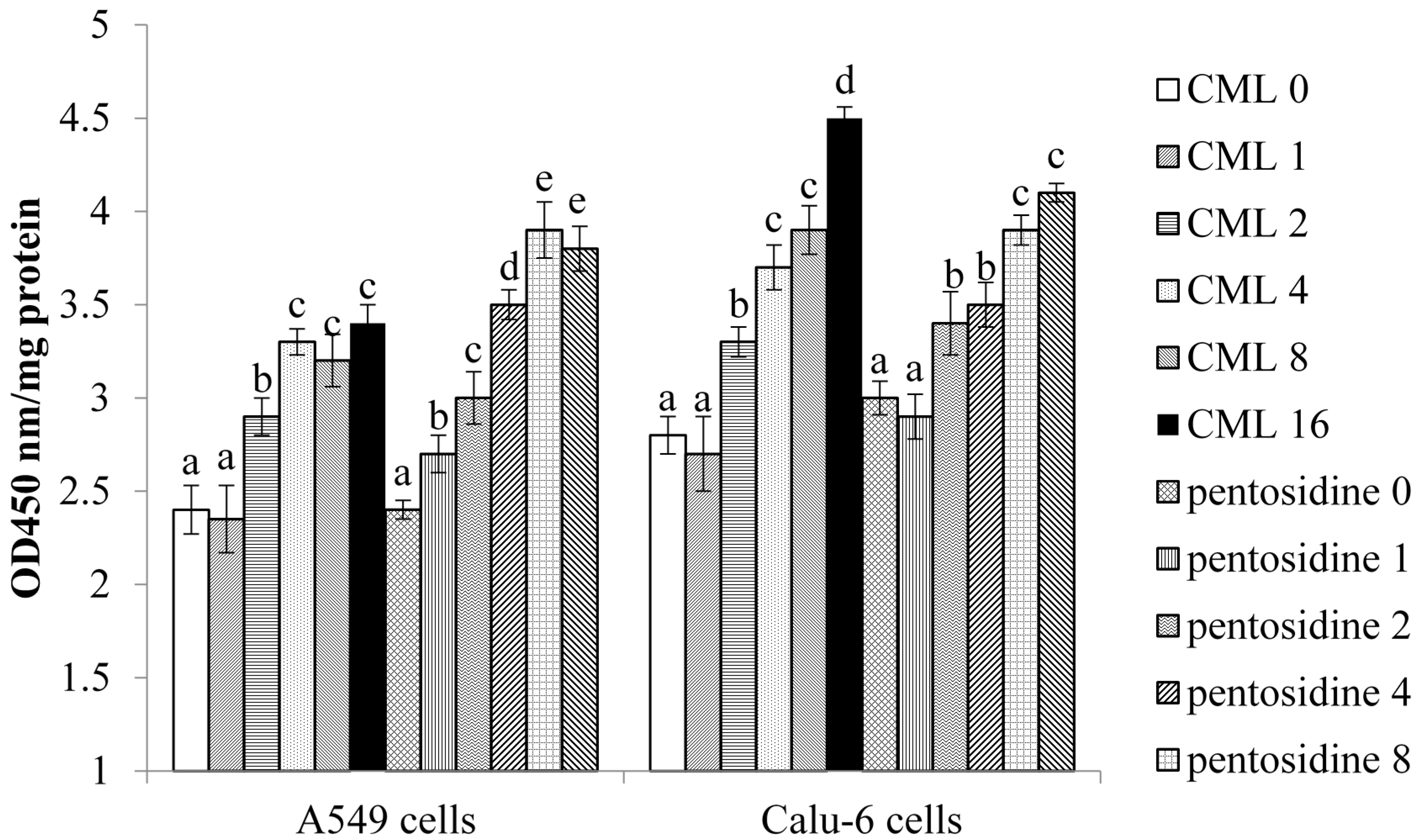
2.2. Effects of CML and Pentosidine upon Oxidative and Inflammatory Factors
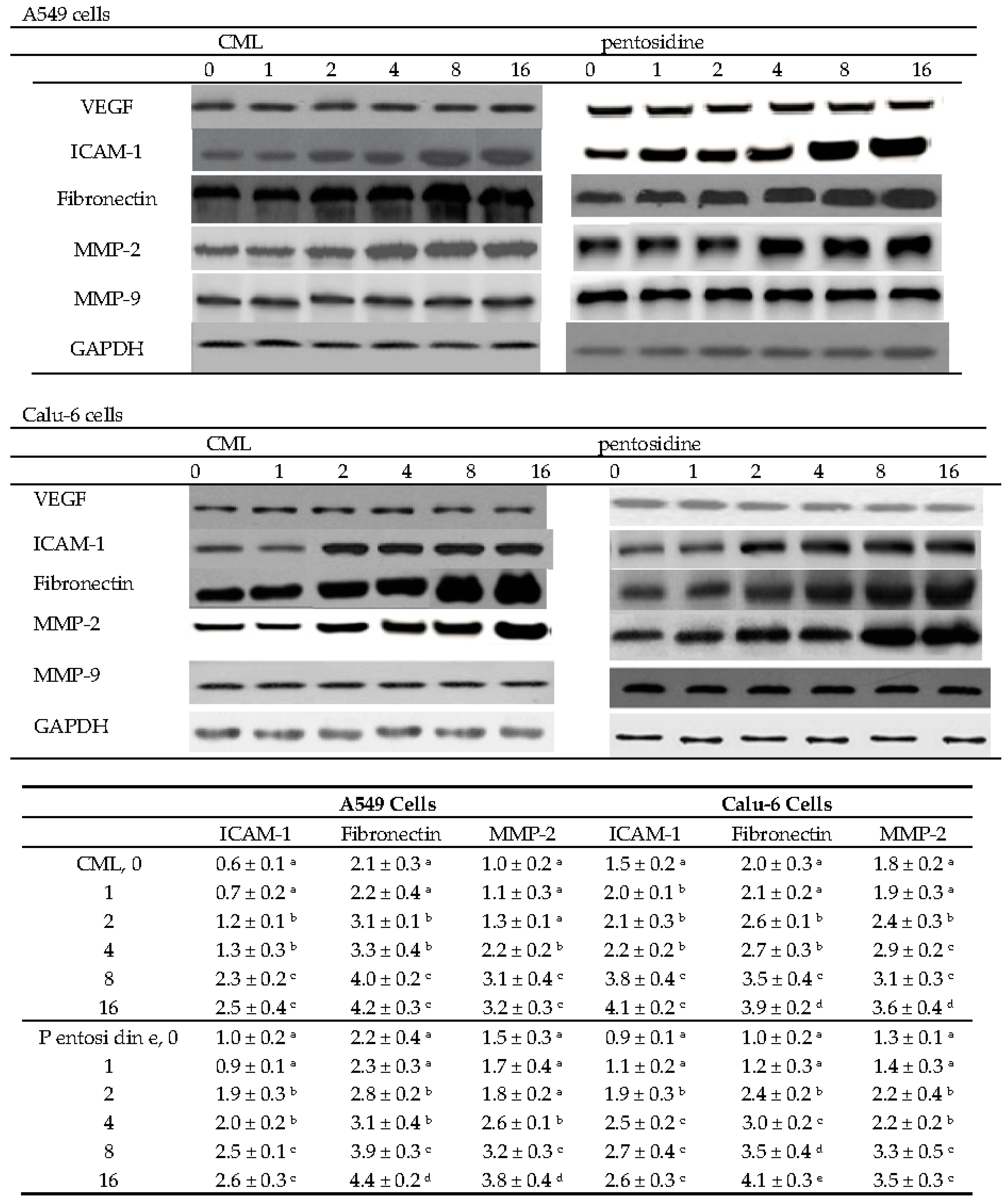
2.3. Effects of CML and Pentosidine upon VEGF, ICAM-1, Fibronectin, MMP-2 and MMP-9 Expression
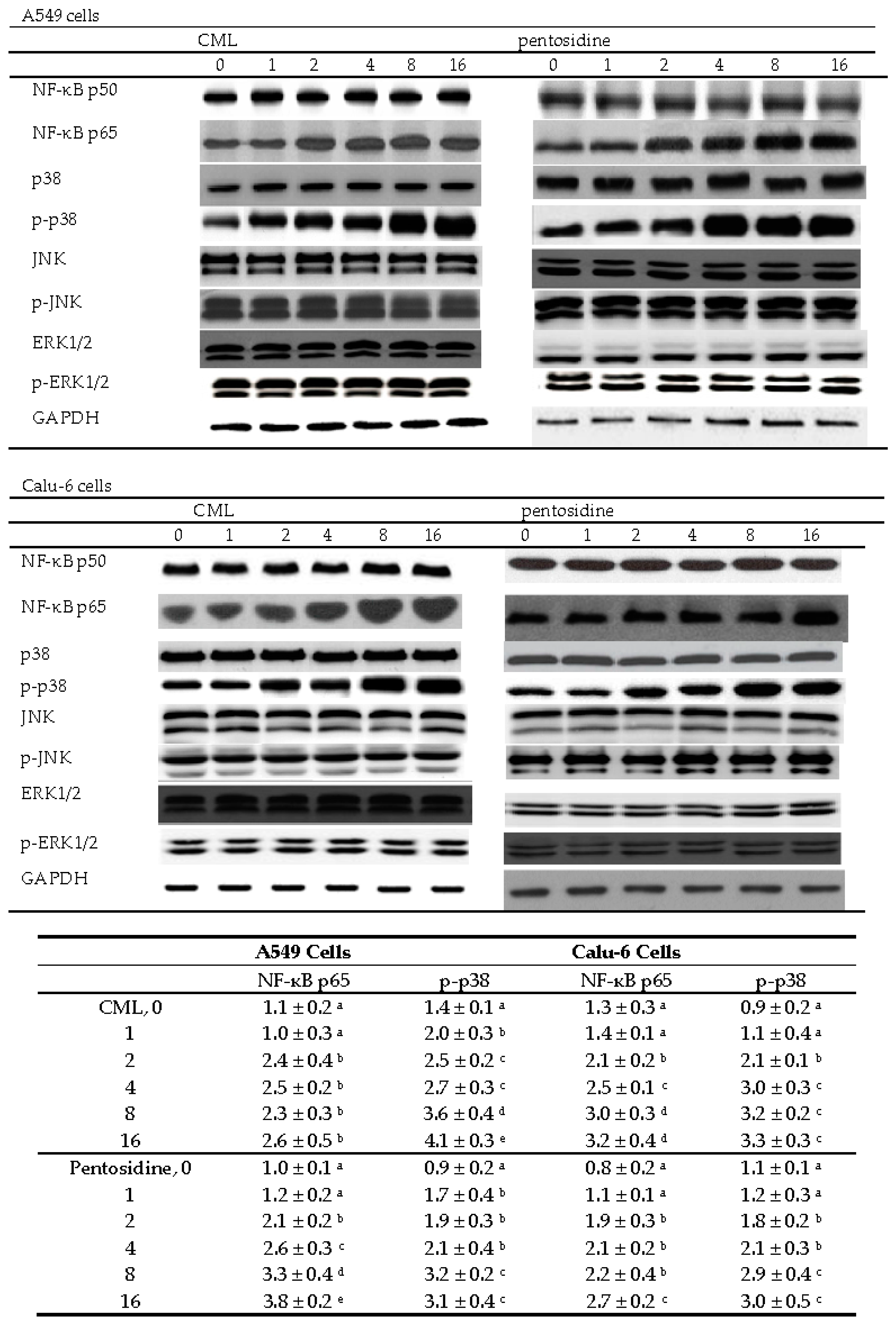
2.4. Effects of CML and Pentosidine upon NF-κB and MAPK Pathways
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Proliferation
4.4. Cell Invasion and Migration
4.5. Measurement of ROS, Interleukin (IL)-6, TNF-α and TGF-β1
4.6. Assay for NF-κB p50/65 DNA Binding Activity
4.7. Western Blot Analyses
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CML | carboxymethyllysine |
| AGE | advanced glycation end-product |
| NSCLC | non-small cell lung cancer |
| MMP | matrix metalloproteinase |
| RAGE | receptor for advanced glycation end-product |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor κ-B |
| ROS | reactive oxygen species |
| TNF | tumor necrosis factor |
| ICAM | intercellular adhesion molecule |
| TGF | transforming growth factor |
| VEGF | vascular endothelial growth factor |
References
- Chao, P.C.; Hsu, C.C.; Yin, M.C. Analysis of glycative products in sauces and sauce-treated foods. Food Chem. 2009, 113, 262–266. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.C.; Huang, C.N.; Hsu, C.C.; Yin, M.C.; Guo, Y.R. Association of dietary AGEs with circulating AGEs, glycated LDL, IL-1α and MCP-1 levels in type 2 diabetic patients. Eur. J. Nutr. 2010, 49, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Piroddi, M.; Palazzetti, I.; Quintaliani, G.; Pilolli, F.; Montaldi, M.; Valentina, V.; Libetta, C.; Galli, F. Circulating levels and dietary intake of the advanced glycation end-product marker carboxymethyl lysine in chronic kidney disease patients on conservative predialysis therapy: A pilot study. J. Ren. Nutr. 2011, 21, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.P. Advanced glycation end-products: A biological consequence of lifestyle contributing to cancer disparity. Cancer Res. 2015, 75, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, H.K.; Yoon, J.S.; Kim, S.J.; Kim, E.S.; Ahn, K.S.; Kim, D.S.; Yoon, S.S.; Kim, B.K.; Lee, Y.Y. Advanced glycation end product (AGE)-induced proliferation of HEL cells via receptor for AGE-related signal pathways. Int. J. Oncol. 2008, 33, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, H.; Matou-Nasri, S.; Wang, Q.; Rabhan, Z.; Al-Eidi, H.; Al Abdulrahman, A.; Ahmed, N. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochim. Biophys. Acta 2015, 1852, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Cai, M.; Zhu, J.; Geng, J.; Zhu, K.; Jin, X.; Ding, W. Matrix metalloproteinase activity in early-stage lung cancer. Onkologie 2013, 36, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.M.; Hsia, T.C.; Yin, M.C. Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-κB pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Guleria, R.; Mohan, A.; Singh, V.; Bharti, A.C.; Das, B.C. Efficacy of plasma TGF-β1 level in predicting therapeutic efficacy and prognosis in patients with advanced non-small cell lung cancer. Cancer Investig. 2011, 29, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, Y.; Abe, T.; Masuda, N.; Akahoshi, T. Progression of non-small cell lung cancer: Diagnostic and prognostic utility of matrix metalloproteinase-2, C-reactive protein and serum amyloid A. Oncol. Rep. 2013, 29, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Ehrhardt, C. The receptor for advanced glycation end products (RAGE) and the lung. J. Biomed. Biotechnol. 2010, 2010, 917108. [Google Scholar] [CrossRef] [PubMed]
- Marinakis, E.; Bagkos, G.; Piperi, C.; Roussou, P.; Diamanti-Kandarakis, E. Critical role of RAGE in lung physiology and tumorigenesis: A potential target of therapeutic intervention? Clin. Chem. Lab. Med. 2014, 52, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Takino, J.; Yamagishi, S.; Takeuchi, M. Cancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-products. J. Oncol. 2010, 2010, 739852. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, C.; Zhang, Y. Investigation of variations in the acrylamide and Nε-(carboxymethyl) lysine contents in cookies during baking. J. Food Sci. 2014, 79, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Smith, J.S. Determination of advanced glycation endproducts in cooked meat products. Food Chem. 2015, 68, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Ko, H.A.; Shieh, T.M.; Chang, W.C.; Chen, H.I.; Chang, S.S.; Lin, I.H. Cell migration is regulated by AGE-RAGE interaction in human oral cancer cells in vitro. PLoS ONE 2014, 9, e110542. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pei, C.; Yan, S.; Liu, G.; Liu, G.; Chen, W.; Cui, Y.; Liu, Y. NADPH oxidase 1-dependent ROS is crucial for TLR4 signaling to promote tumor metastasis of non-small cell lung cancer. Tumor Biol. 2015, 36, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Wakefield, L.M. The two faces of transforming growth factor β in carcinogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 8621–8623. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Nakamura, H.; Awut, I.; Kawasaki, N.; Hagiwara, M.; Ogata, A.; Hosaka, M.; Saijo, T.; Kato, Y.; Kato, H. Significance of expression of TGF-β in pulmonary metastasis in non-small cell lung cancer tissues. Ann. Thorac. Cardiovasc. Surg. 2003, 9, 295–300. [Google Scholar] [PubMed]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Mehta, H.J.; Patel, V.; Sadikot, R.T. Curcumin and lung cancer-a review. Target Oncol. 2014, 9, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.M.; He, M.Y.; Liu, Y.W.; Lu, Y.J.; Hong, Y.Q.; Luo, H.H.; Ren, Z.L.; Zhao, S.C.; Jiang, Y. AGE/RAGE/Akt pathway contributes to prostate cancer cell proliferation by promoting Rb phosphorylation and degradation. Am. J. Cancer Res. 2015, 5, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.; Logsdon, C.D. S100P: A novel therapeutic target for cancer. Amino Acids 2011, 41, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, H.; Wang, T.; Sun, Y.; Ni, P.; Liu, Y.; Tian, S.; Amoah Barnie, P.; Shen, H.; Xu, W.; et al. Exogenous high-mobility group box 1 inhibits apoptosis and promotes the proliferation of lewis cells via RAGE/TLR4-dependent signal pathways. Scand. J. Immunol. 2014, 79, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Gho, Y.S.; Kim, P.N.; Li, H.C.; Elkin, M.; Kleinman, H.K. Stimulation of tumor growth by human soluble intercellular adhesion molecule-1. Cancer Res. 2001, 61, 4253–4257. [Google Scholar] [PubMed]
- Dowlati, A.; Gray, R.; Sandler, A.B.; Schiller, J.H.; Johnson, D.H. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—An Eastern Cooperative Oncology Group Study. Clin. Cancer Res. 2008, 14, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Guney, N.; Soydinc, H.O.; Derin, D.; Tas, F.; Camlica, H.; Duranyildiz, D.; Yasasever, V.; Topuz, E. Serum levels of intercellular adhesion molecule ICAM-1 and E-selectin in advanced stage non-small cell lung cancer. Med. Oncol. 2008, 25, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ritzenthaler, J.D.; Han, S.; Roman, J. Stimulation of lung carcinoma cell growth by fibronectin-integrin signaling. Mol. BioSyst. 2008, 4, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, S.C.; Nicolson, M.C.; Lloret, C.; McKay, J.A.; Ross, V.G.; Kerr, K.M.; Murray, G.I.; McLeod, H.L. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: Implications for MMP inhibition therapy. Oncol. Rep. 2001, 8, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Hamano, Y.; Zeisberg, M.; Sugimoto, H.; Lively, J.C.; Maeshima, Y.; Yang, C.; Hynes, R.O.; Werb, Z.; Sudhakar, A.; Kalluri, R. Physiological levels of tumstatin, a fragment of collagen IV α3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via αV β3 integrin. Cancer Cell 2003, 3, 589–601. [Google Scholar] [CrossRef]
- Hoonhorst, S.J.; Lo Tam Loi, A.T.; Pouwels, S.D.; Faiz, A.; Telenga, E.D.; van den Berge, M.; Koenderman, L.; Lammers, J.W.; Boezen, H.M.; van Oosterhout, A.J.; et al. Advanced glycation endproducts and their receptor in different body compartments in COPD. Respir. Res. 2016, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Misselwitz, J.; Franke, S.; Kauf, E.; John, U.; Stein, G. Advanced glycation end products in children with chronic renal failure and type 1 diabetes. Pediatr. Nephrol. 2002, 17, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Taneda, S.; Kawai, R.; Ueda, Y.; Horiuchi, S.; Hara, M.; Maeda, K.; Monnier, V.M. Identification of pentosidine as a native structure for advanced glycation end products in β2-microglobulin-containing amyloid fibrils in patients with dialysis-related amyloidosis. Proc. Natl. Acad. Sci. USA 1996, 93, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
| A549 Cells | Calu-6 Cells | |
|---|---|---|
| CML, 0 | 100 | 100 |
| 1 | 98 ± 4 | 101 ± 2 |
| 2 | 101 ± 5 | 97 ± 4 |
| 4 | 103 ± 3 | 100 ± 3 |
| 8 | 97 ± 5 | 103 ± 2 |
| 16 | 102 ± 4 | 99 ± 4 |
| Pentosidine, 0 | 100 | 100 |
| 1 | 102 ± 3 | 99 ± 5 |
| 2 | 103 ± 4 | 102 ± 2 |
| 4 | 100 ± 2 | 104 ± 3 |
| 8 | 97 ± 3 | 101 ± 4 |
| 16 | 101 ± 5 | 98 ± 5 |
| A549 Cells | Calu-6 Cells | |||
|---|---|---|---|---|
| Invasion | Migration | Invasion | Migration | |
| CML, 0 | 100 a | 100 a | 100 a | 100 a |
| 1 | 102 ± 3 a | 98 ± 4 a | 99 ± 2 a | 98 ± 5 a |
| 2 | 107 ± 4 a | 106 ± 5 a | 127 ± 4 b | 132 ± 3 b |
| 4 | 135 ± 3 b | 140 ± 7 b | 155 ± 5 c | 158 ± 4 c |
| 8 | 142 ± 5 b | 144 ± 5 b | 182 ± 4 d | 187 ± 6 d |
| 16 | 166 ± 7 c | 174 ± 4 c | 190 ± 6 d | 195 ± 5 d |
| Pentosidine, 0 | 100 a | 100 a | 100 a | 100 a |
| 1 | 99 ± 2 a | 104 ± 5 a | 103 ± 4 a | 107 ± 4 a |
| 2 | 103 ± 5 a | 108 ± 3 a | 133 ± 2 b | 147 ± 3 b |
| 4 | 138 ± 4 b | 142 ± 4 b | 164 ± 5 c | 173 ± 6 c |
| 8 | 157 ± 5 c | 168 ± 6 c | 191 ± 7 d | 205 ± 5 d |
| 16 | 184 ± 7 d | 201 ± 4 d | 225 ± 6 e | 232 ± 3 e |
| A549 Cells | Calu-6 Cells | |||||||
|---|---|---|---|---|---|---|---|---|
| ROS | TNF-α | IL-6 | TGF-β1 | ROS | TNF-α | IL-6 | TGF-β1 | |
| CML, 0 | 1.97 ± 0.18 a | 156 ± 13 a | 133 ± 10 a | 141 ± 8 a | 2.09 ± 0.21 a | 160 ± 18 a | 136 ± 8 a | 130 ± 7 a |
| 1 | 2.06 ± 0.21 a | 161 ± 9 a | 142 ± 8 a | 147 ± 5 a | 2.14 ± 0.15 a | 158 ± 12 a | 142 ± 14 a | 139 ± 10 a |
| 2 | 2.18 ± 0.25 a | 167 ± 17 a | 150 ± 16 a | 163 ± 11 a | 2.23 ± 0.24 a | 166 ± 9 a | 148 ± 10 a | 145 ± 12 a |
| 4 | 2.65 ± 0.17 b | 194 ± 14 b | 187 ± 11 b | 197 ± 9 b | 2.71 ± 0.17 b | 201 ± 15 b | 191 ± 16 b | 186 ± 8 b |
| 8 | 2.84 ± 0.20 b | 237 ± 22 c | 224 ± 19 c | 245 ± 13 c | 3.18 ± 0.23 c | 242 ± 13 c | 247 ± 19 c | 217 ± 14 c |
| 16 | 3.39 ± 0.28 c | 280 ± 19 d | 275 ± 23 d | 291 ± 17 d | 3.30 ± 0.28 c | 293 ± 20 d | 258 ± 25 c | 266 ± 21 d |
| Pentosidine, 0 | 2.08 ± 0.11 a | 149 ± 15 a | 136 ± 7 a | 139 ± 9 a | 2.21 ± 0.14 a | 152 ± 12 a | 130 ± 11 a | 134 ± 9 a |
| 1 | 2.13 ± 0.16 a | 157 ± 12 a | 145 ± 14 a | 148 ± 10 a | 2.18 ± 0.19 a | 159 ± 8 a | 127 ± 14 a | 143 ± 13 a |
| 2 | 2.62 ± 0.09 b | 188 ± 10 b | 176 ± 18 b | 182 ± 13 b | 2.57 ± 0.12 b | 195 ± 16 b | 178 ± 15 b | 175 ± 15 b |
| 4 | 2.75 ± 0.21 b | 226 ± 19 c | 213 ± 15 c | 234 ± 8 c | 3.06 ± 0.20 c | 240 ± 23 c | 201 ± 19 b | 223 ± 10 c |
| 8 | 3.55 ± 0.18 c | 270 ± 22 d | 259 ± 17 d | 290 ± 14 d | 3.59 ± 0.16 d | 291 ± 26 d | 263 ± 25 c | 279 ± 14 d |
| 16 | 3.69 ± 0.25 c | 331 ± 27 e | 304 ± 22 e | 359 ± 19 e | 3.74 ± 0.25 d | 355 ± 21 e | 310 ± 27 d | 348 ± 16 e |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsia, T.-C.; Yin, M.-C.; Mong, M.-C. RETRACTED: Advanced Glycation End-Products Enhance Lung Cancer Cell Invasion and Migration. Int. J. Mol. Sci. 2016, 17, 1289. https://doi.org/10.3390/ijms17081289
Hsia T-C, Yin M-C, Mong M-C. RETRACTED: Advanced Glycation End-Products Enhance Lung Cancer Cell Invasion and Migration. International Journal of Molecular Sciences. 2016; 17(8):1289. https://doi.org/10.3390/ijms17081289
Chicago/Turabian StyleHsia, Te-Chun, Mei-Chin Yin, and Mei-Chin Mong. 2016. "RETRACTED: Advanced Glycation End-Products Enhance Lung Cancer Cell Invasion and Migration" International Journal of Molecular Sciences 17, no. 8: 1289. https://doi.org/10.3390/ijms17081289
APA StyleHsia, T.-C., Yin, M.-C., & Mong, M.-C. (2016). RETRACTED: Advanced Glycation End-Products Enhance Lung Cancer Cell Invasion and Migration. International Journal of Molecular Sciences, 17(8), 1289. https://doi.org/10.3390/ijms17081289





