The OsCYP19-4 Gene Is Expressed as Multiple Alternatively Spliced Transcripts Encoding Isoforms with Distinct Cellular Localizations and PPIase Activities under Cold Stress
Abstract
:1. Introduction
2. Results
2.1. OsCYP19-4 Produces Extensive AS Isoforms in a Cold-Specific Manner
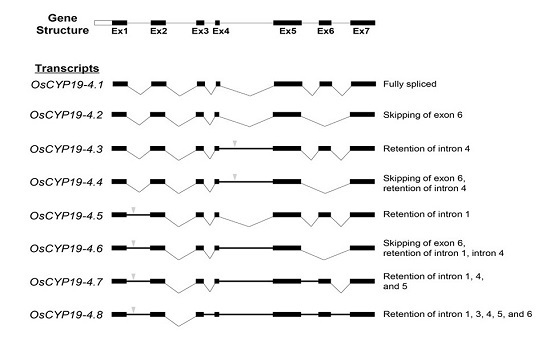
2.2. Gene Structures of Alternatively Spliced OsCYP19-4 Transcripts
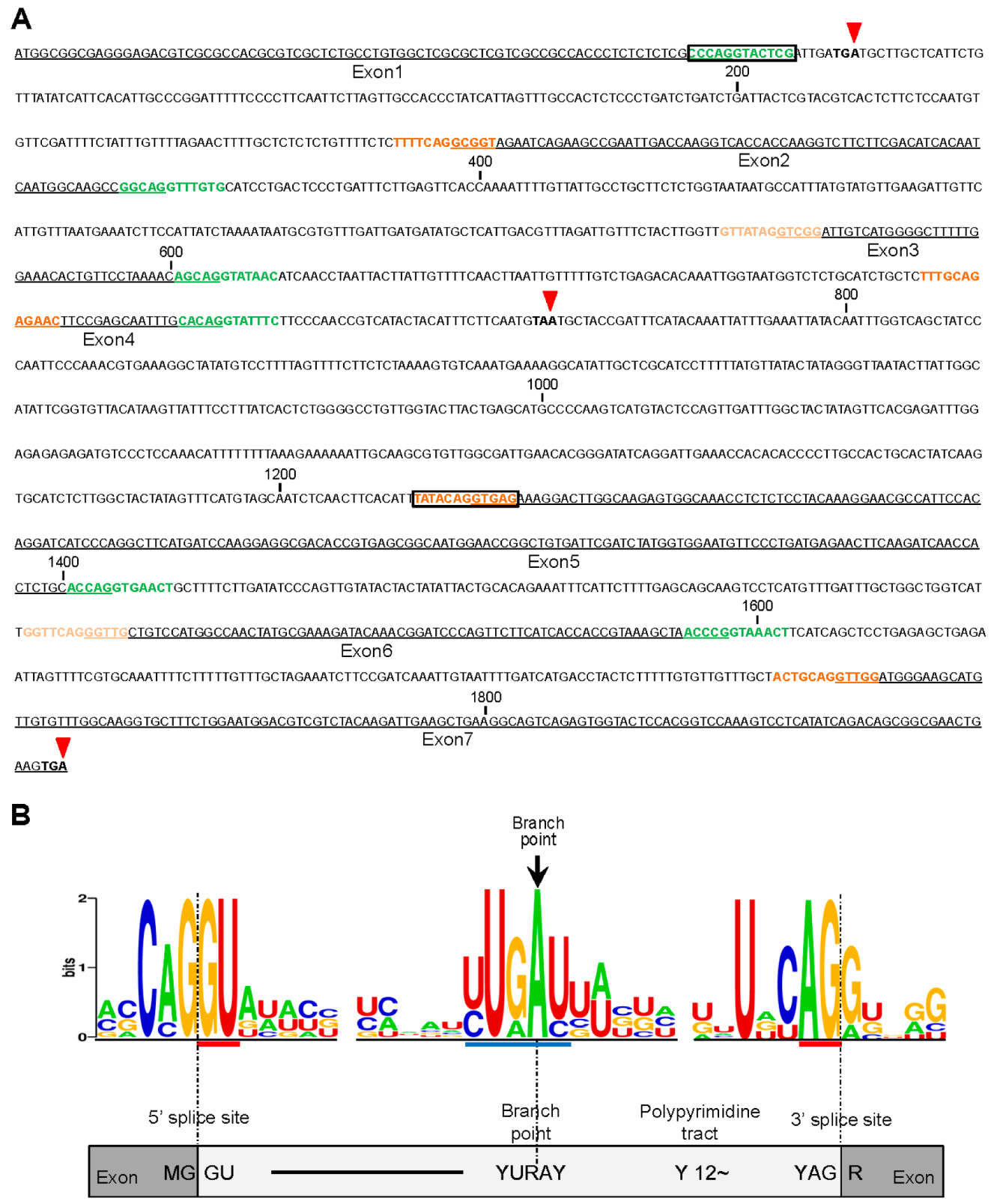
2.3. Analysis of the 5′and 3′ Splice Sites in All Introns of OsCYP19-4
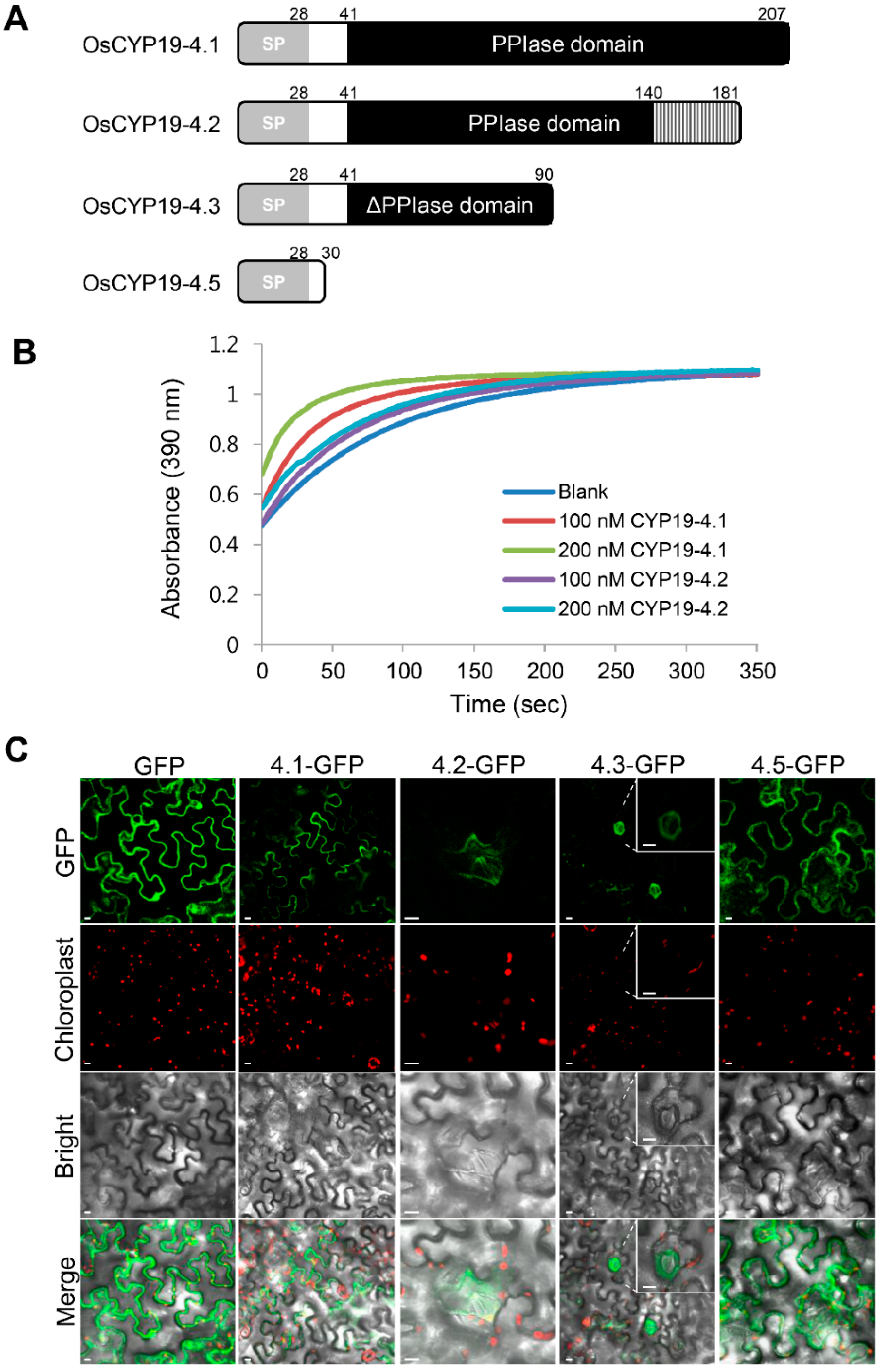
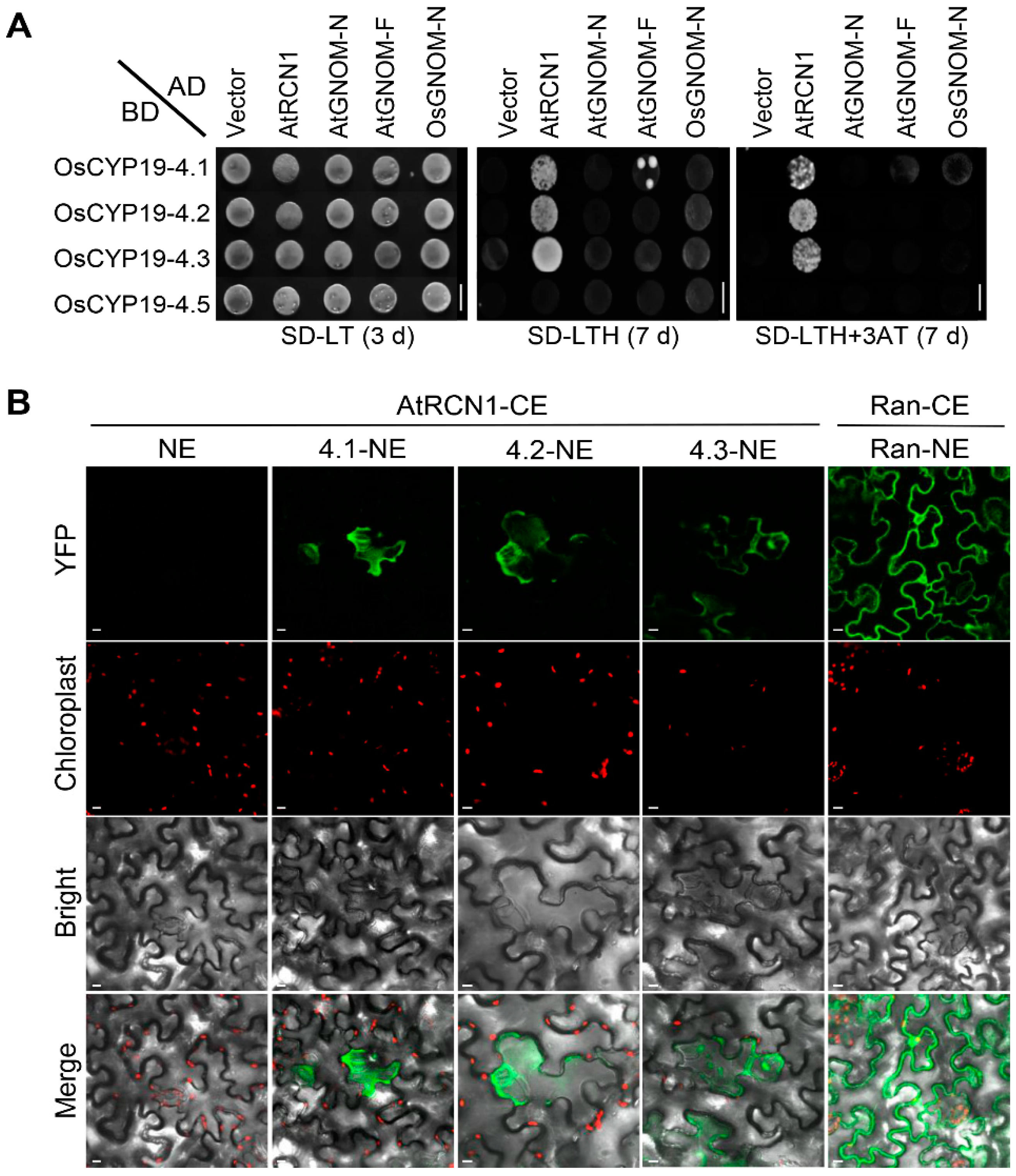
2.4. Biochemical Properties of Proteins Encoded by Alternatively Spliced Isoforms of OsCYP19-4
2.5. OsCYP19-4.2/3 AS Isoforms also Interact with AtRCN1in Guard and Subsidiary Cells
3. Discussion
4. Materials and Methods
4.1. Plant material, Growth Conditions and Cold Stress Treatments
4.2. RNA Extraction and Semi-Quantitative Reverse Transcription-Polymerase Chain Reaction
4.3. Analysis of the 5′ and 3′ Splice Site
4.4. Cellular Localization of OsCYP19-4 AS Isoforms
4.5. Y2H Assay
4.6. Bimolecular Fluorescence Complementation Assaay
4.7. Immunoblot
4.8. In Vitro PPIase Activity Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Witten, J.T.; Ule, J. Understanding splicing regulation through rna splicing maps. Trends Genet. 2011, 27, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.W.; Graveley, B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010, 463, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, P.; Yepiskoposyan, H.; Metze, S.; Orozco, R.Z.; Kleinschmidt, N.; Mühlemann, O. Nonsense-mediated mrna decay in human cells: Mechanistic insights, functions beyond quality control and the double-life of nmd factors. Cell. Mol. Life Sci. 2010, 67, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Marquez, Y.; Brown, J.W.; Simpson, C.; Barta, A.; Kalyna, M. Transcriptome survey reveals increased complexity of the alternative splicing landscape in arabidopsis. Genome Res. 2012, 22, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Lu, G.; Fan, D.; Zhu, C.; Li, W.; Zhao, Q.; Feng, Q.; Zhao, Y.; Guo, Y.; Li, W.; et al. Function annotation of the rice transcriptome at single-nucleotide resolution by RNA-seq. Genome Res. 2010, 20, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Staiger, D.; Brown, J.W. Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell 2013, 25, 3640–3656. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Ohta, M.; Nakazawa, M.; Ono, M.; Hasegawa, P.M. ICE1 Ser403 is necessary for protein stabilization and regulation of cold signaling and tolerance. Plant J. 2011, 67, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Henderson, D.A.; Zhu, J.K. The arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 2005, 17, 3155–3175. [Google Scholar] [CrossRef] [PubMed]
- Hannah, M.A.; Heyer, A.G.; Hincha, D.K. A global survey of gene regulation during cold acclimation in arabidopsis thaliana. PLoS Genet. 2005, 1, e26. [Google Scholar] [CrossRef] [PubMed]
- Palusa, S.G.; Ali, G.S.; Reddy, A.S. Alternative splicing of pre-mrnas of arabidopsis serine/arginine-rich proteins: Regulation by hormones and stresses. Plant J. 2007, 49, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Kim, M.J.; Ryu, J.Y.; Jeong, E.Y.; Park, C.M. Two splice variants of the IDD14 transcription factor competitively form nonfunctional heterodimers which may regulate starch metabolism. Nat. Commun. 2011, 2, 303. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Park, M.-J.; Lim, M.-H.; Kim, S.-G.; Lee, M.; Baldwin, I.T.; Park, C.-M. A self-regulatory circuit of circadian clock-associated1 underlies the circadian clock regulation of temperature responses in arabidopsis. Plant Cell 2012, 24, 2427–2442. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.M.; Figueiredo, D.D.; Tepperman, J.; Borba, A.R.; Lourenco, T.; Abreu, I.A.; Ouwerkerk, P.B.; Quail, P.H.; Margarida Oliveira, M.; Saibo, N.J. Rice phytochrome-interacting factor protein OsPIF14 represses OsDREB1B gene expression through an extended N-box and interacts preferentially with the active form of phytochrome B. Biochim. Biophys. Acta 2016, 1859, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Leviatan, N.; Alkan, N.; Leshkowitz, D.; Fluhr, R. Genome-wide survey of cold stress regulated alternative splicing in arabidopsis thaliana with tiling microarray. PLoS ONE 2013, 8, e66511. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Lee, S.S.; Park, H.J.; Lyu, J.I.; Chong, W.S.; Liu, J.R.; Kim, B.-G.; Ahn, J.C.; Cho, H.S. Overexpression of OsCYP19–4 increases tolerance to cold stress and enhances grain yield in rice (Oryza sativa L.). J. Exp. Bot. 2015. [Google Scholar] [CrossRef]
- Wang, M.; Marin, A. Characterization and prediction of alternative splice sites. Gene 2006, 366, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Hebsgaard, S.M.; Korning, P.G.; Tolstrup, N.; Engelbrecht, J.; Rouze, P.; Brunak, S. Splice site prediction in Arabidopsis Thaliana pre-mRNA by combining local and global sequence information. Nucleic Acids Res. 1996, 24, 3439–3452. [Google Scholar] [CrossRef] [PubMed]
- Dogan, R.I.; Getoor, L.; Wilbur, W.J.; Mount, S.M. SplicePort—An interactive splice-site analysis tool. Nucleic Acids Res. 2007, 35, W285–W291. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhang, P.; Liu, L.; Li, T.; Peng, Y.; Li, G.; Li, Q. Sequence-specific flexibility organization of splicing flanking sequence and prediction of splice sites in the human genome. Chromosome Res. 2014, 22, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Ner-Gaon, H.; Halachmi, R.; Savaldi-Goldstein, S.; Rubin, E.; Ophir, R.; Fluhr, R. Intron retention is a major phenomenon in alternative splicing in arabidopsis. Plant J. 2004, 39, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. Meme: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. Weblogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
 ) indicates premature termination codons (PTCs). AS types are represented at the right.
) indicates premature termination codons (PTCs). AS types are represented at the right.
 ) indicates premature termination codons (PTCs). AS types are represented at the right.
) indicates premature termination codons (PTCs). AS types are represented at the right.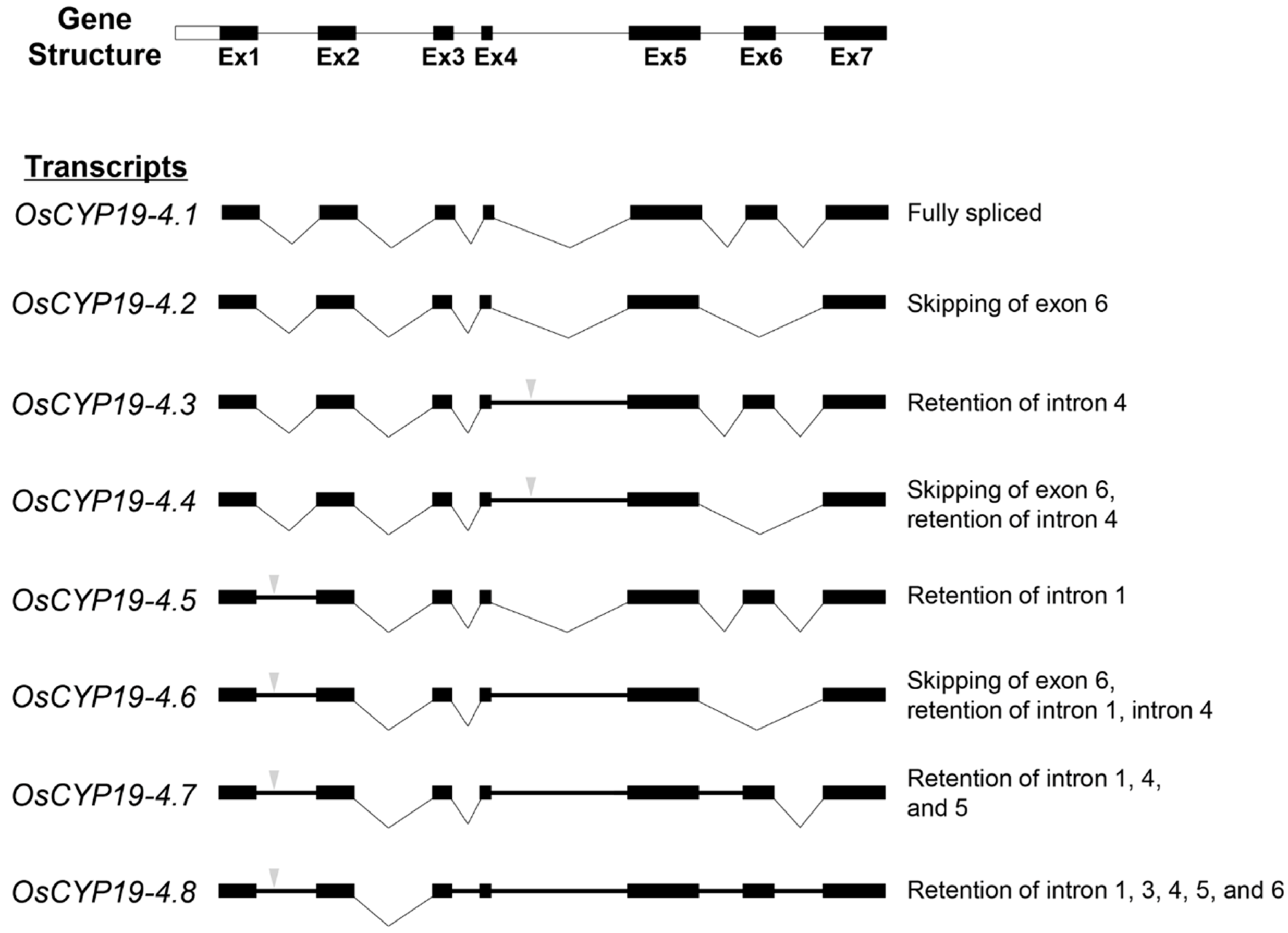
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.; Lee, S.S.; Jung, W.Y.; Park, H.J.; Lim, B.R.; Kim, H.-S.; Ahn, J.C.; Cho, H.S. The OsCYP19-4 Gene Is Expressed as Multiple Alternatively Spliced Transcripts Encoding Isoforms with Distinct Cellular Localizations and PPIase Activities under Cold Stress. Int. J. Mol. Sci. 2016, 17, 1154. https://doi.org/10.3390/ijms17071154
Lee A, Lee SS, Jung WY, Park HJ, Lim BR, Kim H-S, Ahn JC, Cho HS. The OsCYP19-4 Gene Is Expressed as Multiple Alternatively Spliced Transcripts Encoding Isoforms with Distinct Cellular Localizations and PPIase Activities under Cold Stress. International Journal of Molecular Sciences. 2016; 17(7):1154. https://doi.org/10.3390/ijms17071154
Chicago/Turabian StyleLee, Areum, Sang Sook Lee, Won Yong Jung, Hyun Ji Park, Bo Ra Lim, Hyun-Soon Kim, Jun Cheul Ahn, and Hye Sun Cho. 2016. "The OsCYP19-4 Gene Is Expressed as Multiple Alternatively Spliced Transcripts Encoding Isoforms with Distinct Cellular Localizations and PPIase Activities under Cold Stress" International Journal of Molecular Sciences 17, no. 7: 1154. https://doi.org/10.3390/ijms17071154
APA StyleLee, A., Lee, S. S., Jung, W. Y., Park, H. J., Lim, B. R., Kim, H.-S., Ahn, J. C., & Cho, H. S. (2016). The OsCYP19-4 Gene Is Expressed as Multiple Alternatively Spliced Transcripts Encoding Isoforms with Distinct Cellular Localizations and PPIase Activities under Cold Stress. International Journal of Molecular Sciences, 17(7), 1154. https://doi.org/10.3390/ijms17071154