Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review
Abstract
:1. Introduction
2. Solar Radiation and Deleterious Cutaneous Effects
2.1. Ultraviolet Radiation (UVR) and Photoinduced-Skin Cancer Aging
2.1.1. Photooxidative Stress: Generation of Reactive Oxygen Species (ROS)
2.1.2. DNA Photodamage
2.1.3. Photoinflammation
2.1.4. Photoimmunosuppression
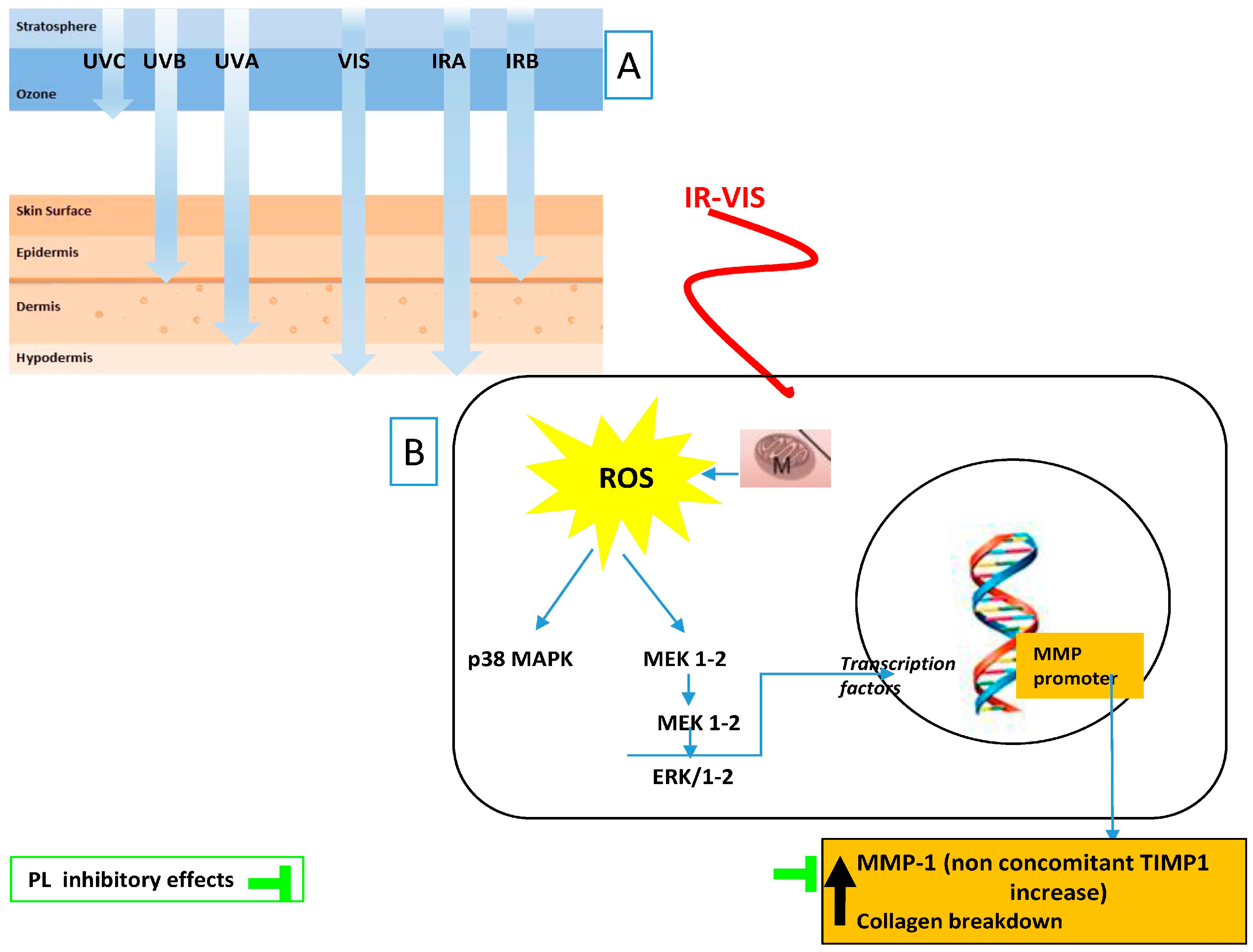
2.2. Solar Non-Ultraviolet (UV) (Visible and Infrared) Radiation and Photoaging
2.2.1. Infrared (IR) Radiation
2.2.2. Visible Light (VIS) Radiation
3. Polypodium leucotomos (Fernblock®) as Photoprotective Agents
3.1. Composition
3.2. Cellular and Molecular Evidence of the Photoprotective Properties of Fernblock®
3.3. Fernblock in Photodamage and DNA Repair and Cellular Homeostasis
3.4. Fernblock Effects on Inflammation
3.5. Fernblock and Immunosuppression
3.6. Fernblock, an Anti-UV-Induced Tumor Progression Agent
3.7. Fernblock and Extracellular Remodeling: Collagen, Elastin, and Matrix Metalloproteinases Network
3.8. Fernblock and VIS and IR Radiation
4. Potential Use of Fernblok in the Treatment in Other Skin Pathology
4.1. Melanoma High-Risk Patients
4.2. Idiopathic Photodermatosis
4.3. Actinic Keratosis
4.4. Pigmentary Disorders
4.4.1. Vitiligo
4.4.2. Melasma
4.5. Premature Aging
5. Fernblock: A Road to (Present and Future) Prevention of UV-, VIS-, and IR-Mediated Skin Damage
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 8-OH-dG | 8-Hydroxy-2′-deoxyguanosine |
| AP-1 | Activator protein 1 |
| COLIα1 | Collagen type I |
| CD | Common deletion |
| COX-2 | Cyclooxygenase-2 |
| CPDs | Cyclobutane pyrimidine dimers |
| DC | Dendritic cells |
| eLC | Epidermal Langerhans cells |
| GP | Glutathione peroxidase |
| GPx4 | Glutathione peroxidase 4 |
| GST | Glutathione S-transferase |
| HPLC | High-performance liquid chromatography |
| NB-UVB | Narrow-band UVB |
| NF-κB | Nuclear factor kappa beta |
| PL | Polypodium leucotomos |
| PMLE | Polymorphic light eruption |
| PUVA | Psoralens + UVA |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SSR | Solar simulated radiation |
| TGF-β | Transforming growth factor beta |
| Th1 | T helper 1 lymphocyte |
| TIMP | Tissue inhibitor of metalloproteinase |
| TNF-α | Tumor necrosis factor-alpha |
| UCA | Urocanic acid |
References
- NTP (National ToxiScology Program). Report on Carcinogens, Thirteenth Edition; Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2 October 2014. Available online: http://ntp.niehs.nih.gov/pubhealth/roc/roc13/ (accessed on 24 March 2016).
- De Vries, E.; Arnold, M.; Altsitsiadis, E.; Trakatelli, M.; Hinrichs, B.; Stockfleth, E.; Coebergh, J. Potential impact of interventions resulting in reduced exposure to ultraviolet (UV) radiation (UVA and UVB) on skin cancer incidence in four European countries, 2010–2050. Br. J. Dermatol. 2012, 167, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rundhaug, J.E.; Pavone, A.; Kim, E.; Fischer, S.M. The effect of cyclooxygenase-2 overexpression on skin carcinogenesis is context dependent. Mol. Carcinog. 2007, 46, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, C. Cellular aspects of photocarcinogenesis. Photochem. Photobiol. Sci. 2006, 5, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Wachtel-Galor, S.; Benzie, I.F.F. An introduction to its history, usage, regulation, current trends, and research needs. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 1–10. [Google Scholar]
- Paur, I.; Carlsen, M.H.; Halvorsen, B.L.; Blomhoff, R. Antioxidants in herbs and spices roles in oxidative stress and redox signaling. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 11–36. [Google Scholar]
- García, F.; Pivel, J.P.; Guerrero, A.; Brieva, A.; Martínez-Alcazar, M.P.; Caamano-Somoza, M.; González, S. Phenolic components and antioxidant activity of Fernblock, an aqueous extract of the aerial parts of the fern Polypodium leucotomos. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Gombau, L.; Garcia, F.; Lahoz, A.; Fabre, M.; Roda-Navarro, P.; Majano, P.; Alonso-Lebrero, J.L.; Pivel, J.P.; Castell, J.V.; Gómez-Lechon, M.J.; et al. Polypodium leucotomos extract, Antioxidant activity and disposition. Toxicol. in Vitro 2006, 20, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Wang, S.Q.; Burnett, M.; Osterwalder, U.; Lim, H.W. Photoprotection: Part I. Photoprotection by naturally occurring, physical, and systemic agents. J. Am. Acad. Dermatol. 2013, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- CIE. ILV: International Lighting Vocabulary; Standard CIE S 017/E:2011. Available online: http://cie.co.at/index.php?i_ca_id=827 (accessed on 24 March 2016).
- Holick, M.F. Sunlight, UV-radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2008, 624, 1–15. [Google Scholar] [PubMed]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Lohan, S.B.; Müller, R.; Albrecht, S.; Mink, K.; Tscherch, K.; Ismaeel, F.; Lademann, J.; Rohn, S.; Meinke, M.C. Free radicals induced by sunlight in different spectral regions—In vivo vs. ex vivo study. Exp. Dermatol. 2016, 25, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation, a review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.H.; Hexsel, C.L.; Hamzavi, I.H.; Lim, H.W. Effects of Visible Light on the Skin. Photochem. Photobiol. 2008, 84, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.A.; Riley, F.C.; Fitzpatrick, T.B. Melanogenesis in human skin following exposure to long-wave ultraviolet and visible light. J. Investig. Dermatol. 1962, 39, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Baqer, A. An experimental study of the changes in pigmentation in human skin in vivo with visible and near infrared light. Photochem. Photobiol. 1984, 39, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Liebel, F.; Kaur, S.; Ruvolo, E.; Kollias, N.; Southall, M.D. Irradiation of Skin with Visible Light Induces Reactive Oxygen Species and Matrix-Degrading Enzymes. J. Investig. Dermatol. 2012, 132, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Akhalaya, M.Y.; Maksimova, G.V.; Rubina, A.B.; Lademannb, J.; Darvinb, M.E. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res. Rev. 2014, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Pohl, C.; Calles, C.; Marks, C.; Wild, S.; Krutmann, J. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic. Biol. Med. 2007, 43, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative inhibition of receptor-type protein-tyrosine phosphatase κ by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. J. Biol. Chem. 2006, 281, 27389–27397. [Google Scholar] [CrossRef] [PubMed]
- Hildesheim, J.; Fornace, A.J., Jr. The dark side of light, the damaging effects of UV rays and the protective efforts of MAP kinase signaling in the epidermis. DNA Repair 2004, 3, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.; Piva, T.J. The UV response of the skin, a review of the MAPK, NFκB and TNFα signal transduction pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing, mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Tornaletti, S.; Pfeifer, G.P. Slow repair of pyrimidine dimers at p53 mutation hotspots in skin cancer. Science 1994, 263, 1436–1438. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G-T and A-C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [PubMed]
- Villa, A.; Viera, M.H.; Amini, S.; Huo, R.; Pérez, O.; Ruiz, P.; Amador, A.; Elgart, G.; Berman, B. Decrease of ultraviolet A light-induced “common deletion” in healthy volunteers after oral Polypodium leucotomos extract supplement in a randomized clinical trial. J. Am. Acad. Dermatol. 2010, 62, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Balupillai, A.; Prasad, R.N.; Ramasamy, K.; Muthusamy, G.; Shanmugham, M.; Govindasamy, K.; Gunaseelan, S. Caffeic Acid Inhibits UVB-induced Inflammation and Photocarcinogenesis through Activation of Peroxisome Proliferator-activated Receptor-γ in Mouse Skin. Photochem. Photobiol. 2015, 91, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of ultraviolet B exposure-mediated activation of NF-κB in normal human keratinocytes by resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Prescott, S.M.; Fitzpatrick, F.A. Cyclooxygenase-2 and carcinogenesis. Biochim. Biophys. Acta 2000, 1470, 69–78. [Google Scholar] [CrossRef]
- Janczyk, A.; Garcia-Lopez, M.A.; Fernández-Penas, P.; Alonso-Lebrero, J.L.; Benedicto, I.; Lopez-Cabrera, M.; González, S. A Polypodium leucotomos extract inhibits solar-simulated radiation-induced TNF-α and iNOS expression, transcriptional activation and apoptosis. Exp. Dermatol. 2007, 16, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wu, S. Mechanism for dynamic regulation of iNOS expression after UVB-irradiation. Mol. Carcinog. 2013, 52, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Edelbaum, D.; Bergstresser, P.R.; Cruz, P.D., Jr. Distorted antigen-presenting function of Langerhans cells induced by tumor necrosis factor α via a mechanism that appears different from that induced by ultraviolet B radiation. Photodermatol. Photoimmunol. Photomed. 1991, 8, 90–94. [Google Scholar]
- Prater, M.R.; Blaylock, B.L.; Holladay, S.D. Molecular mechanisms of cis-urocanic acid and permethrin-induced alterations in cutaneous immunity. Photodermatol. Photoimmunol. Photomed. 2003, 19, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Tejedor, R.; de la Fuente, H.; García-López, M.A.; Ursa, A.; Peñas, P.F.; García-Díez, A.; Alonso-Lebrero, J.L.; Pivel, J.P.; González, S.; et al. Solar-simulated ultraviolet radiation induces abnormal maturation and defective chemotaxis of dendritic cells. J. Investig. Dermatol. 2005, 125, 334–342. [Google Scholar] [PubMed]
- Schroeder, P.; Calles, C.; Krutmann, J. Prevention of infrared-A radiation mediated detrimental effects in human skin. Skin Ther. Lett. 2009, 14, 4–5. [Google Scholar]
- Kochevar, I.E.; Taylor, C.R.; Krutmann, J. Fundamentals of cutaneous photobiology and photoimmunology. In Fitzpatrick’s Dermatology in General Medicine, 8th ed.; Wolff, K., Goldsmith, L.A., Katz, S., Paller, G.A., Leffell, D.J., Eds.; McGraw-Hill: New York, NY, USA, 2012; Volume 1, pp. 1031–1047. [Google Scholar]
- Kligman, LH. Intensification of ultraviolet-induced dermal damage by infrared radiation. Arch. Dermatol. Res. 1982, 272, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Haag, S.; Meinke, M.; Zastrow, L.; Sterry, W.; Lademann, J. Radical production by infrared A irradiation in human tissue. Skin Pharmacol. Physiol. 2010, 23, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Höhn, A.; Piazena, H.; Grune, T. Effects of water-filtered infrared A irradiation on human fibroblasts. Free Radic. Biol. Med. 2010, 48, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, L.; Groth, N.; Klein, F.; Kockott, D.; Lademann, J.; Renneberg, R.; Ferrero, L. The missing link—Light-induced (280–1600 nm) free radical formation in human skin. Skin Pharmacol. Physiol. 2009, 22, 31–44. [Google Scholar] [PubMed]
- Krutmann, J.; Schroeder, P. Role of mitochondria in photoaging of human skin, the defective power- house model. J. Investig. Dermatol. 2009, 14, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Schieke, S.; Stege, H.; Kürten, V.; Grether-Beck, S.; Sies, H.; Krutmann, J. Infrared-A radiation-induced matrix metalloproteinase 1 expression is mediated through extracellular signal-regulated kinase 1/2 activation in human dermal fibroblasts. J. Investig. Dermatol. 2002, 119, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Moon, Y.J.; Seo, J.E.; Lee, Y.; Kim, K.H.; Chung, J.H. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic. Biol. Med. 2008, 44, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Morita, A.; Chung, J.H. Sun exposure, what molecular photodermatology tells us about its good and bad sides. J. Investig. Dermatol. 2012, 132, 976–998. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, Y.K.; Cho, K.H.; Chung, J.H. Regulation of type I procollagen and MMP-1 expression after single or repeated exposure to infrared radiation in human skin. Mech. Ageing Dev. 2006, 127, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared radiation-induced matrix metalloproteinase in human skin, implications for protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Dorr, S.; Greinert, R.; Volkmer, B.; Epe, B. Visible light (>395 nm) causes micronuclei formation in mammalian cells without generation of cyclobutane pyrimidine dimers. Mutat. Res. 2005, 572, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, M.J.; Kim, M.S.; Lee, S.; Kim, Y.K.; Lee, D.H.; Kang, S.; Cho, W.G.; Park, H.J.; Oh, K.W.; et al. Infrared plus visible light and heat from natural sunlight participate in the expression of MMPs and type I procollagen as well as infiltration of inflammatory cell in human skin in vivo. J. Dermatol. Sci. 2008, 50, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Vandersee, S.; Beyer, M.; Lademann, J.; Darvin, M.E. Blue-violet light irradiation dose dependently decreases carotenoids in human skin, which indicates the generation of free radicals. Oxid. Med. Cell. Longev. 2015, 579675. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramaniam, R.; Roy, A.; Sharma, B.; Nagalakshmi, S. Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochem. Photobiol. Sci. 2011, 10, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, B.H.; Ruvolo, E.; Hexsel, C.L.; Liu, Y.; Owen, M.R.; Kollias, N.; Lim, H.W.; Hamzavi, I.H. Impact of long-wavelength UVA and visible light on melanocompetent skin. J. Investig. Dermatol. 2010, 130, 2092–2097. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Gilaberte, Y.; Philips, N.; Juarranz, A. Fernblock, a nutriceutical with photoprotective properties and potential preventive agent for skin photoaging and photoinduced skin cancers. Int. J. Mol. Sci. 2011, 12, 8466–8475. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, H.; Tejedor, R.; García-López, M.A.; Mittelbrunn, M.; Alonso-Lebrero, J.L.; Sánchez-Madrid, F.; García-Díez, A.; Pivel, J.P.; Peñas, P.F.; González, S. Polypodium leucotomos induces protection of UV-induced apoptosis in human skin cells. J. Investig. Dermatol. 2005, 124, A121. [Google Scholar]
- Bhatia, N. Polypodium leucotomos, a potential new photoprotective agent. Am. J. Clin. Dermatol. 2015, 16, 73–79. [Google Scholar] [CrossRef] [PubMed]
- El-Haj, N.; Goldstein, N. Sun protection in a pill, the photoprotective properties of Polypodium leucotomos extract. Int. J. Dermatol. 2015, 54, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Murbach, T.S.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Clewell, A.E.; Szakonyiné, I.P. A comprehensive toxicological safety assessment of an aqueous extract of Polypodium leucotomos (Fernblock®). Food Chem. Toxicol. 2015, 86, 328–341. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Gilaberte, Y.; Philips, N. Mechanistic insights in the use of a Polypodium leucotomos extract as an oral and topical photoprotective agent. Photochem. Photobiol. Sci. 2010, 9, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Yanes, E.; Cuevas, J.; González, S.; Mallol, J. Oral administration of Polypodium leucotomos delays skin tumor development and increases epidermal p53 expression and the anti-oxidant status of UV-irradiated hairless mice. Exp. Dermatol. 2014, 23, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, P.; Carrera, C.; Puig-Butille, J.A.; Badenas, C.; Lecha, M.; González, S.; Malvehy, J.; Puig, S. Benefits of oral Polypodium leucotomos extract in MM high-risk patients. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Zattra, E.; Coleman, C.; Arad, S.; Helms, E.; Levine, D.; Bord, E.; Guillaume, A.; El-Hajahmad, M.; Zwart, E.; van Steeg, H.; et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am. J. Pathol. 2009, 175, 1952–1961. [Google Scholar]
- Mulero, M.; Rodríguez-Yanes, E.; Nogues, M.R.; Giralt, M.; Romeu, M.; González, S.; Mallol, J. Polypodium leucotomos extract inhibits glutathione oxidation and prevents Langerhans cell depletion induced by UVB/UVA radiation in a hairless rat model. Exp. Dermatol. 2008, 17, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Garcia-Caballero, T.; Rius-Díaz, F.; Fitzpatrick, T.B.; González, S. Orally administered Polypodium leucotomos extract decreases psoralen-UVA induced phototoxicity, pigmentation, and damage of human skin. J. Am. Acad. Dermatol. 2004, 50, 41–49. [Google Scholar] [CrossRef]
- Middelkamp-Hup, M.A.; Pathak, M.A.; Parrado, C.; Goukassian, D.; Rius-Díaz, F.; Mihm, M.C.; Fitzpatrick, T.B.; González, S. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J. Am. Acad. Dermatol. 2004, 51, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, S.Z.; Bhatia, N.; Ceilley, R.; Hougeir, F.; Lieberman, R.; Hamzavi, I.; Lim, H.W. Role of oral Polypodium leucotomos extract in dermatologic diseases, a review of the literature. J. Drugs Dermatol. 2014, 13, 148–153. [Google Scholar] [PubMed]
- Nestor, M.S.; Berman, B.; Swenson, N. Safety and Efficacy of Oral Polypodium leucotomos Extract in Healthy Adult Subjects. J. Clin. Aesthet. Dermatol. 2015, 8, 19–23. [Google Scholar] [PubMed]
- González, S.; Pathak, M.A. Inhibition of ultraviolet-induced formation of reactive oxygen species, lipid peroxidation, erythema and skin photosensitization by Polypodium leucotomos. Photodermatol. Photoimmunol. Photomed. 1996, 12, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo Antitumoral Effects of Combinations of Polyphenols, or Polyphenols and Anticancer Drugs, Perspectives on Cancer Treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. 2010, 30, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Creasey, W.A. Antitumoral activity of the fern Cibotium schiedei. Nature 1969, 222, 1281–1282. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.V.; Pathak, M.A.; Rius, F.; Kollias, N.; González, S. An extract of Polypodium leucotomos appears to minimize certain photoaging changes in a hairless albino mouse animal model. Photodermatol. Photoimmunol. Photomed. 1999, 15, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kholi, I.; Griffith, J.L.; Isedeh, P.; Silpa-Archa, N.; Al-Jamal, M.; Lim, H.W.; Hamzavi, I.H. The Effect of Oral Polypodium leucotomos Extract (PLE) on Ultraviolet-induced Changes in the Skin. In Proceedings of the 34th Fall Clinical Dermatology Conference, Las Vegas, NV, USA, 1–4 October 2015.
- González, S.; Pathak, M.A.; Cuevas, J.; Villarubia, V.G.; Fitzpatrick, T.B. Topical or oral administration with an extract of Polypodium leucotomos prevents acute sunburn and psolaren-induced phototoxic reactions as well as depletion of Langerhans cells in human skin. Photodermatol. Photoimmunol. Photomed. 1997, 13, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Conte, J.; Chen, Y.J.; Natrajan, P.; Taw, M.; Keller, T.; Givant, J.; Tuason, M.; Dulaj, L.; Leonardi, D.; et al. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-β by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Arch. Dermatol. Res. 2009, 301, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lebrero, J.L.; Domínguez-Jiménez, C.; Tejedor, R.; Brieva, A.; Pivel, J.P. Photoprotective properties of a hydrophilic extract of the fern Polypodium leucotomos on human skin cells. J. Photochem. Photobiol. 2003, 70, 31–37. [Google Scholar] [CrossRef]
- González, S.; Alcaraz, M.V.; Cuevas, J.; Pérez, M.; Jaen, P.; Alvarez-Mon, M.; Villarrubia, V.G. An extract of the fern Polypodium leucotomos (Difur) modulates Th1/Th2 cytokines balance in vitro and appears to exhibit anti-angiogenic activities in vivo, pathogenic relationships and therapeutic implications. Anticancer Res. 2000, 20, 1567–1575. [Google Scholar] [PubMed]
- Capote, R.; Alonso-Lebrero, J.L.; García, F.; Brieva, A.; Pivel, J.P.; González, S. Polypodium leucotomos extract inhibits transurocanic acid photoisomerization and photodecomposition. J. Photochem. Photobiol. 2006, 82, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Yanes, E.; Juarranz, A.; Cuevas, J.; Gonzalez, S.; Mallol, J. Polypodium leucotomos decreases UV-induced epidermal cell proliferation and enhances p53 expression and plasma antioxidant capacity in hairless mice. Exp. Dermatol. 2012, 21, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Smith, J.; Keller, T.; González, S. Predominant effects of Polypodium leucotomos on membrane integrity, lipid peroxidation, and expression of elastin and matrixmetalloproteinase-1 in ultraviolet radiation exposed fibroblasts, and keratinocytes. J. Dermatol. Sci. 2003, 32, 1–9. [Google Scholar] [CrossRef]
- Truchuelo, M.T.; Jiménez, N.; Mascaraque, M.; Lucena, S.; Días, I.J.; Juarranz, A.; González, S. Pilot study to assess the effects of a new oral photoprotector against infrared-visible radiations. J. Investig. Dermatol. 2016, 136, S106. [Google Scholar]
- Mohammad, A. Vitiligo repigmentation with Anapsos (Polypodium leucotomos). Int. J. Dermatol. 1989, 28, 479. [Google Scholar] [PubMed]
- Padilla, H.C.; Lainez, H.; Pacheco, J.A. A new agent (hydrophilic fraction of Polypodium leucotomos) for management of psoriasis. Int. J. Dermatol. 1974, 13, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Calzavara-Pinton, P.G.; Rossi, M.T.; Zanca, A.; Arisi, M.; González, S.; Venturini, M. Oral Polypodium leucomotos increases the anti-inflammatory and melanogenic responses of the skin to different modalities of sun exposures: A pilot study. Photodermatol. Photoimmunol. Photomed. 2016, 32, 22–27. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, H.; Lamana, A.; Mittelbrunn, M.; Pérez-Gala, S.; González, S.; García-Diez, A.; Vega, M.; Sánchez-Madrid, F. Identification of genes responsive to solar simulated UV radiation in human monocyte-derived dendritic cells. PLoS ONE 2009, 4, e6735. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kaur, C.D.; Jangdey, M.; Saraf, S. Matrix metalloproteinase enzymes and their naturally derived inhibitors, novel targets in photocarcinoma therapy. Ageing Res. Rev. 2014, 13, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wilkins-Port, C.E.; Higgins, P.J. Regulation of extracellular matrix remodeling following transforming growth factor-β1/epidermal growth factor-stimulated epithelial-mesenchymal transition in human premalignant keratinocytes. Cells Tissues Organs 2007, 185, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Tanew, A.; Radakovic, S.; González, S.; Venturini, M.; Calzavara-Pinton, P. Oral administration of a hydrophilic extract of Polypodium leucotomos for the prevention of polymorphic light eruption. J. Am. Acad. Dermatol. 2012, 66, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, M.; Recalcati, S.; Piccinno, R. Oral Polypodium leucotomos extract photoprotective activity in 57 patients with idiopathic photodermatoses. G. Ital. Dermatol. Venereol. 2011, 146, 85–87. [Google Scholar] [PubMed]
- Caccialanza, M.; Percivalle, S.; Piccinno, R.; Brambilla, R. Photoprotective activity of oral Polypodium leucotomos extract in 25 patients with idiopathic photodermatoses. Photodermatol. Photoimmunol. Photomed. 2007, 23, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, M.; Di Nicola, M.; González, S.; Piaserico, S.; Capo, A.; Amerio, P. Polypodium leucotomos supplementation in the treatment of scalp actinic keratosis, could it improve the efficacy of photodynamic therapy? Dermatol. Surg. 2015, 41, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp-Hup, M.A.; Bos, J.D.; Rius-Diaz, F.; González, S.; Westerhof, W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: A randomized double blind placebo-controlled study. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Reyes, E.; Jaen, P.; de las Heras, E.; Carrion, F.; Alvarez-Mon, M.; de Eusebio, E.; Cuevas, J.; González, S.; Villarrubia, V.G. Systemic immunomodulatory effects of Polypodium leucotomos as an adjuvant to PUVA therapy in generalized vitiligo: A pilot study. J. Dermatol. Sci. 2006, 41, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.; Bucay, V.; Callender, V.; Cohen, J.L.; Sadick, N.; Waldorf, H. Polypodium leucotomos as an Adjunct Treatment of Pigmentary Disorders. J. Clin. Aesthet. Dermatol. 2014, 7, 13–17. [Google Scholar] [PubMed]
- Pacifico, A.; Vidoli, P.; Leone, G.; Iacovelli, P. Combined treatment of narrowband ultraviolet B light (NBUVB) phototherapy and oral Polypodium leucotomos extract versus NB UVB phototherapy alone in the treatment of patients with vitiligo. J. Am. Acad. Dermatol. 2009, 60 (Suppl. S1), AB154. [Google Scholar]
- Martin, L.K.; Caperton, C.; Woolery-Lloyd, H. A randomized double-blind placebo controlled study evaluating the effectiveness and tolerability of oral Polypodium leucotomos in patients with melasma. J. Am. Acad. Dermatol. 2012, 66 (Suppl. S1), AB21. [Google Scholar]
- Sheth, V.M.; Pandya, A.G. Melasma, a comprehensive update, part I. J. Am. Acad. Dermatol. 2011, 65, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Sheth, V.M.; Pandya, A.G. Melasma, a comprehensive update, part II. J. Am. Acad. Dermatol. 2011, 65, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Gan, W.; Shi, F.; Hu, G.X.; Chen, L.G.; Hayakawa, H.; Sekiguchi, M.; Cai, J.P. Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxid. Med. Cell. Longev. 2013, 2013, 433–454. [Google Scholar] [CrossRef] [PubMed]
- Sauvaigo, S.; Caillat, S.; Odin, F.; Nkengne, A.; Bertin, C.; Oddos, T. Effect of aging on DNA excision/synthesis repair capacities of human skin fibroblasts. J. Investig. Dermatol. 2010, 130, 1739–1741. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.M. Skin ageing. J. Eur. Acad. Dermatol. 2011, 25, 873–884. [Google Scholar] [CrossRef] [PubMed]
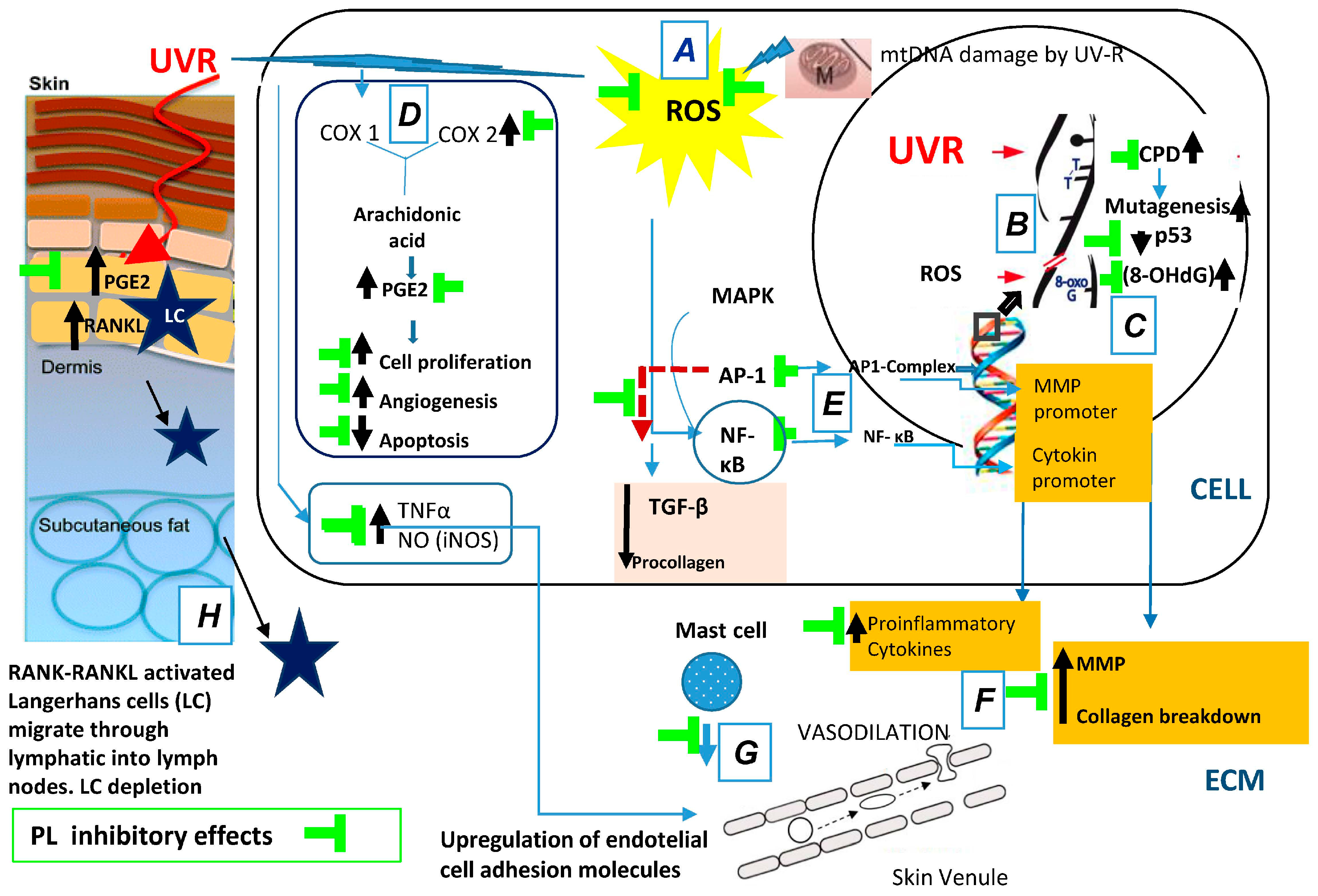
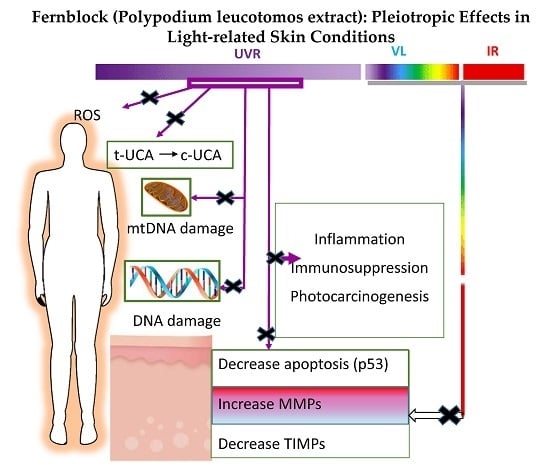
| UV Effects Tissue/Cellular/Molecular Target | PL Counteracts UV-Effects | References |
|---|---|---|
| DNA damage | Inhibit of DNA mutations | [65,68] |
| Inhibit accumulation of CPD | [65,68,76] | |
| Inhibit of 8-hydroxy-2′-deoxyguanosine | [65] | |
| Inhibit of mtDNA mutations | [31] | |
| Inflammation | Inhibit of TNF-α, iNOS, NF-κB, AP-1, COX-2 | [35,57,65] |
| Decrease mast cell, neutrophils, and macrophage infiltration | [59,61,62] | |
| Inhibit of PUVA induced vasodilation | [71] | |
| Immunosuppression | Inhibit of UVR-mediated Langerhans cell depletion | [67,68,77] |
| Protect DCs from UV-induced apoptosis | [66,67,68] | |
| Induce DCs production of anti-inflammatory cytokines (IL-12) | [80] | |
| Reduce of glutathione oxidation in blood and epidermis | [66] | |
| Interfere the cis-UCA isomerization | [81] | |
| Photo Carcinogenesis | Reduce the number of mice showing skin tumors at 8 weeks after the cessation of chronic UVB exposure | [75] |
| Increase the number of p53(+) cells | [63,65,82] | |
| Increase TIMP | [65] | |
| Inhibit of angiogenesis | [77] | |
| Inhibit of epidermal cell proliferation | [68,76] | |
| Enhance the antioxidant plasma capacity | [71,82] | |
| UV–ECM damage | Inhibit MMP-1 (also in melanoma cells) | [78,83] |
| Increase TIMP (also in melanoma cells) | [78] | |
| Increase the synthesis of types I, III, and V collagen | [78] | |
| IR–VIS Effects Tissue/Cellular/Molecular Target | PL Counteracts IR–VIS Effects | [84] |
| UV–ECM damage | Inhibit MMP-1 | [84] |
| Pathology | Potential Clinical Use of PL | References |
|---|---|---|
| Melanoma | PL extract improves systemic photoprotection in patients at risk of MM | [64] |
| The strongest effect of PL in patients with familial MM, those exhibiting a mutated CDKN2A and/or polymorphisms in MC1R | [64] | |
| Idiopathic Photodermatosis | PL significant reduces skin reactions and subjective symptoms. | [92,93,94] |
| Actinic Keratosis | PL improves PDT clearance and decreases AK recurrence rate at 6 months | [95] |
| Pigmentary Disorders | Vitiligo | [96,97,98,99] |
| Addition of PL to the treatment with NB-UVB shows an increased repigmentation mainly in the head and neck area | ||
| Melasma | [100] | |
| PL had decreased Mean Melasma Area and Severity Index. Photographic assessment and patient self-assessments revealed mild and marked improvement by PL | ||
| Aging | PL decreased the proposed skin aging oxidative damage | See Table 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parrado, C.; Mascaraque, M.; Gilaberte, Y.; Juarranz, A.; Gonzalez, S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. Int. J. Mol. Sci. 2016, 17, 1026. https://doi.org/10.3390/ijms17071026
Parrado C, Mascaraque M, Gilaberte Y, Juarranz A, Gonzalez S. Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. International Journal of Molecular Sciences. 2016; 17(7):1026. https://doi.org/10.3390/ijms17071026
Chicago/Turabian StyleParrado, Concepcion, Marta Mascaraque, Yolanda Gilaberte, Angeles Juarranz, and Salvador Gonzalez. 2016. "Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review" International Journal of Molecular Sciences 17, no. 7: 1026. https://doi.org/10.3390/ijms17071026
APA StyleParrado, C., Mascaraque, M., Gilaberte, Y., Juarranz, A., & Gonzalez, S. (2016). Fernblock (Polypodium leucotomos Extract): Molecular Mechanisms and Pleiotropic Effects in Light-Related Skin Conditions, Photoaging and Skin Cancers, a Review. International Journal of Molecular Sciences, 17(7), 1026. https://doi.org/10.3390/ijms17071026