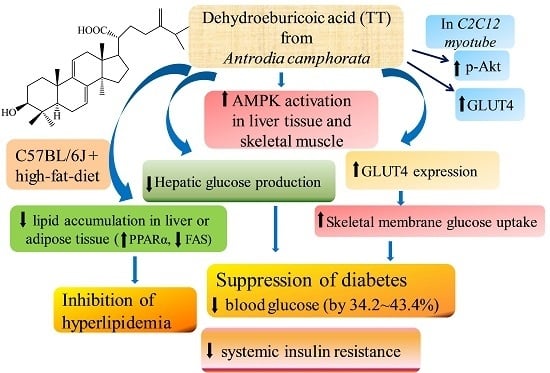
Dehydroeburicoic Acid from Antrodia camphorata Prevents the Diabetic and Dyslipidemic State via Modulation of Glucose Transporter 4, Peroxisome Proliferator-Activated Receptor α Expression and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice
Abstract
:1. Introduction
2. Results
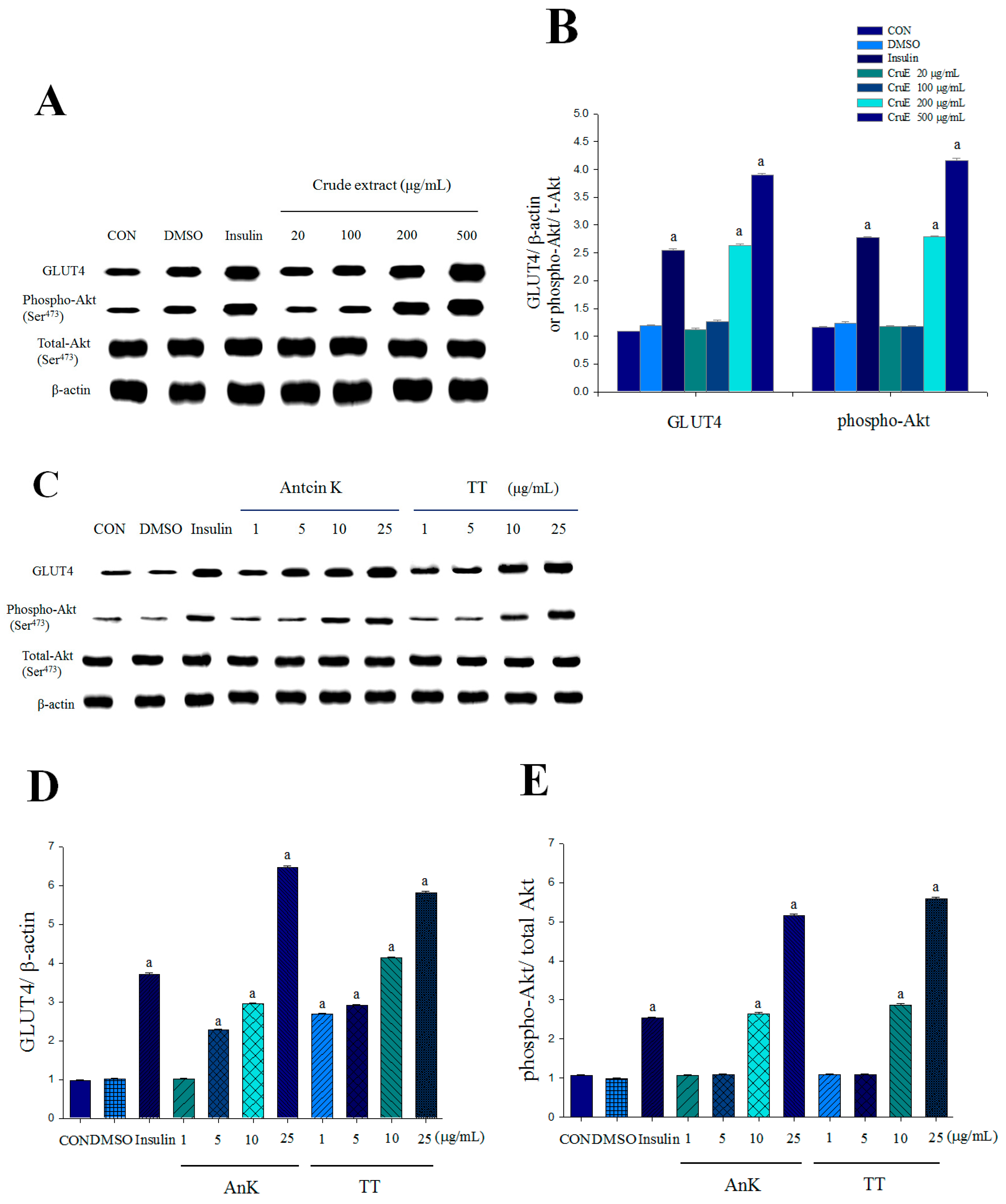
2.1. Expression Levels of Membrane GLUT4 and Akt Phosphorylation in Vitro
2.2. Metabolic Parameters
2.3. Blood Glucose, Insulin, and Leptin Levels
2.4. Blood Triglyceride, Total Cholesterol, and Hepatic Lipid
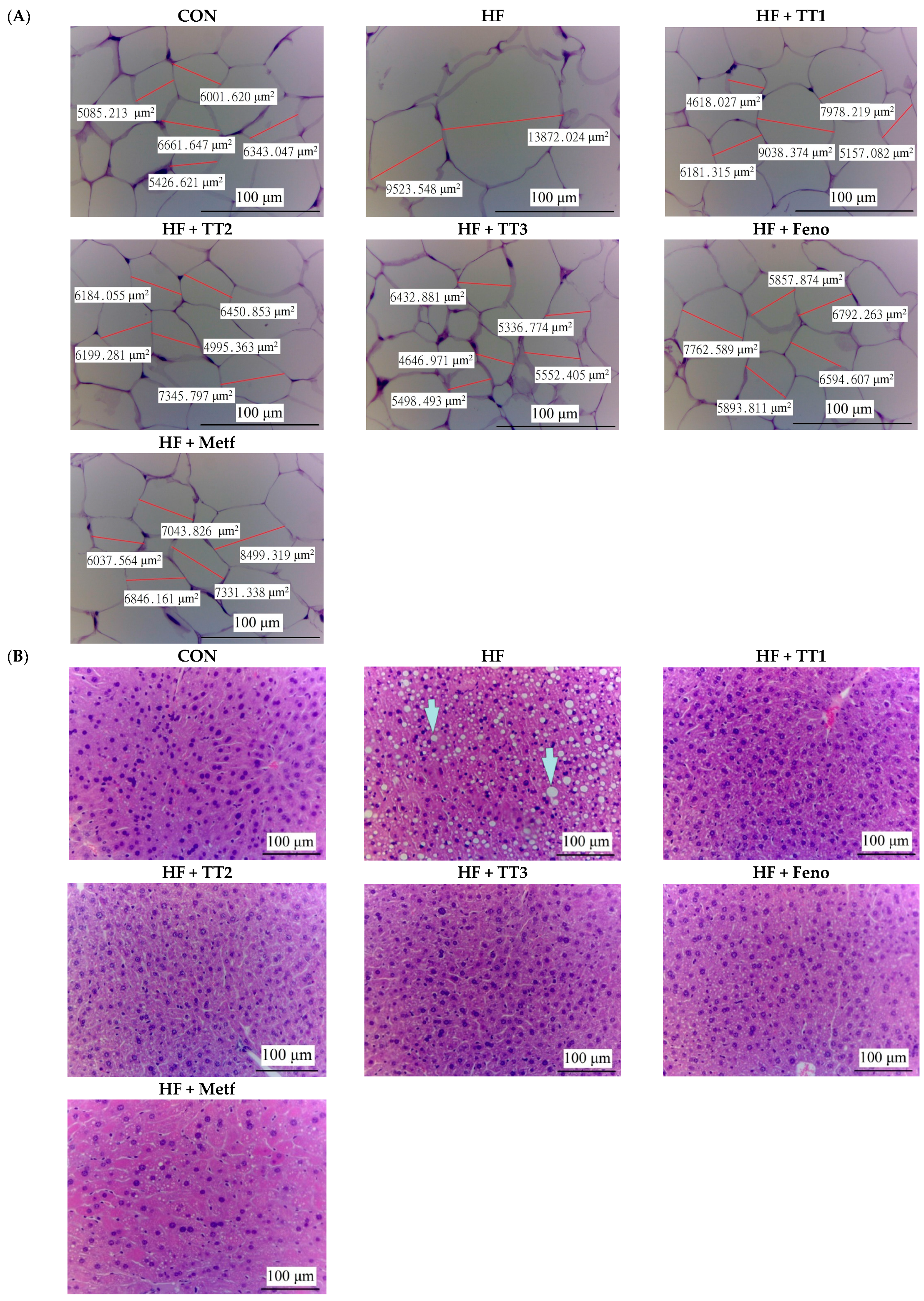
2.5. Pathological Examination
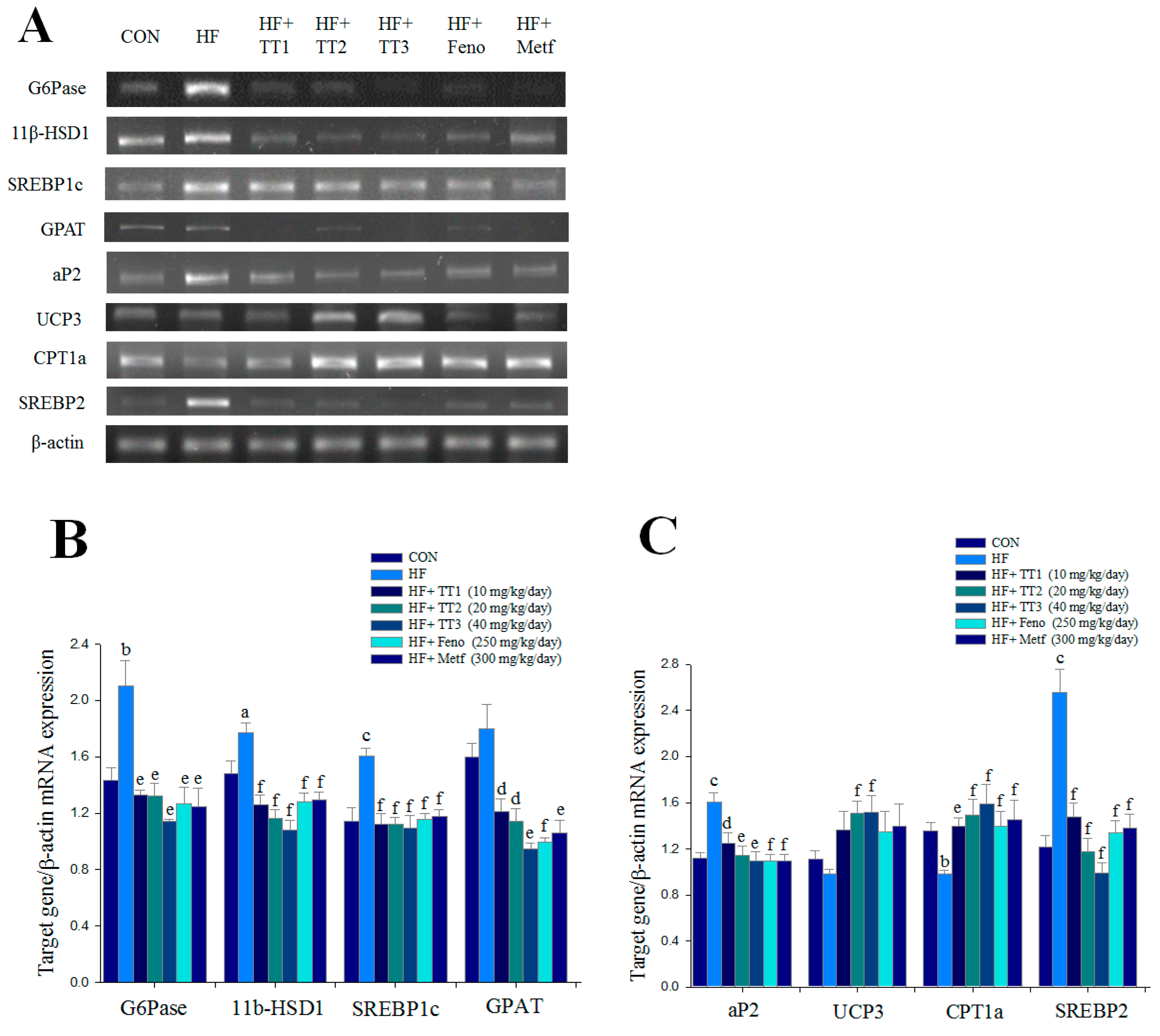
2.6. mRNA Levels of Targeted Hepatic Genes
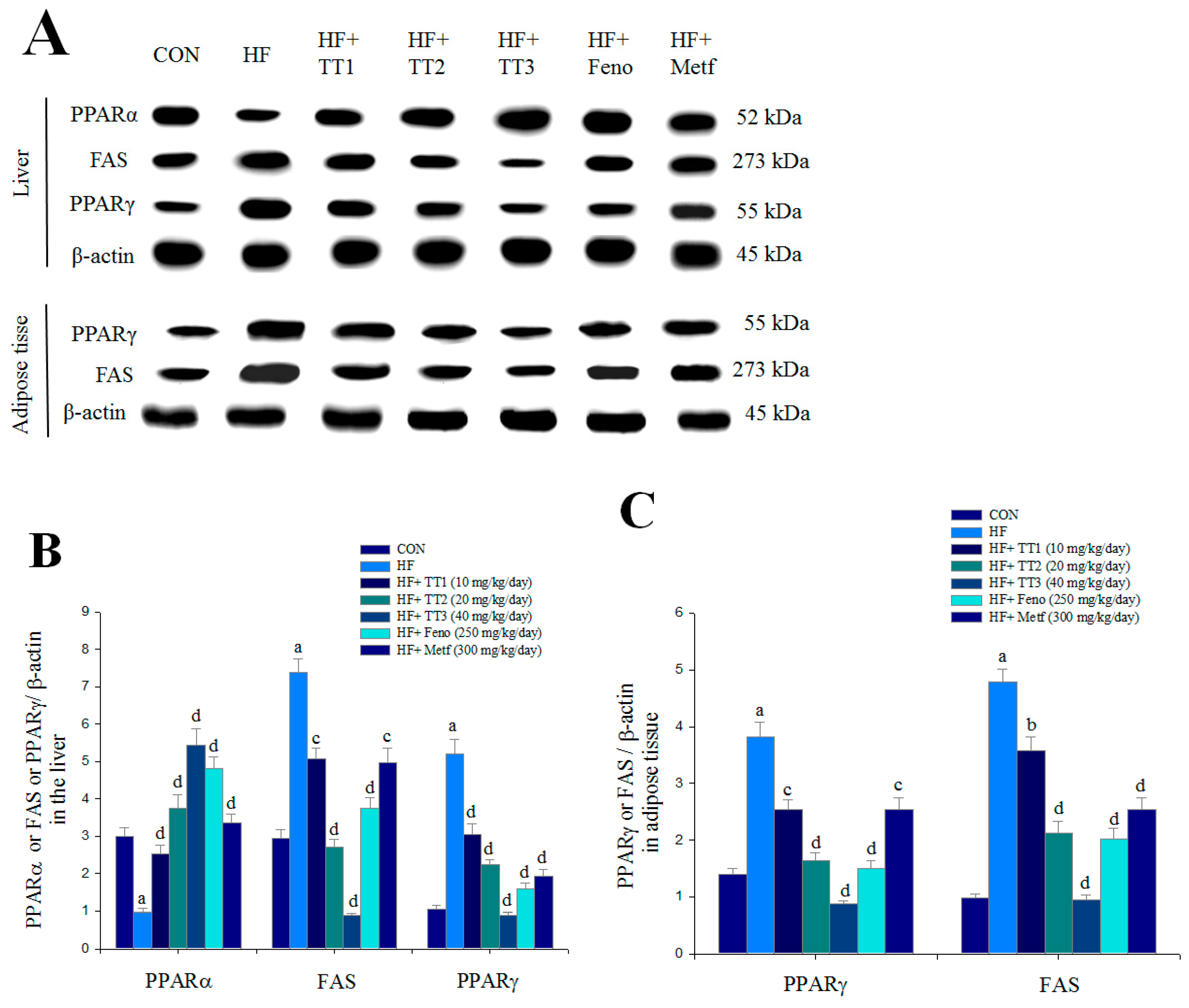
2.7. Targeted Gene Expression Levels in Different Tissues
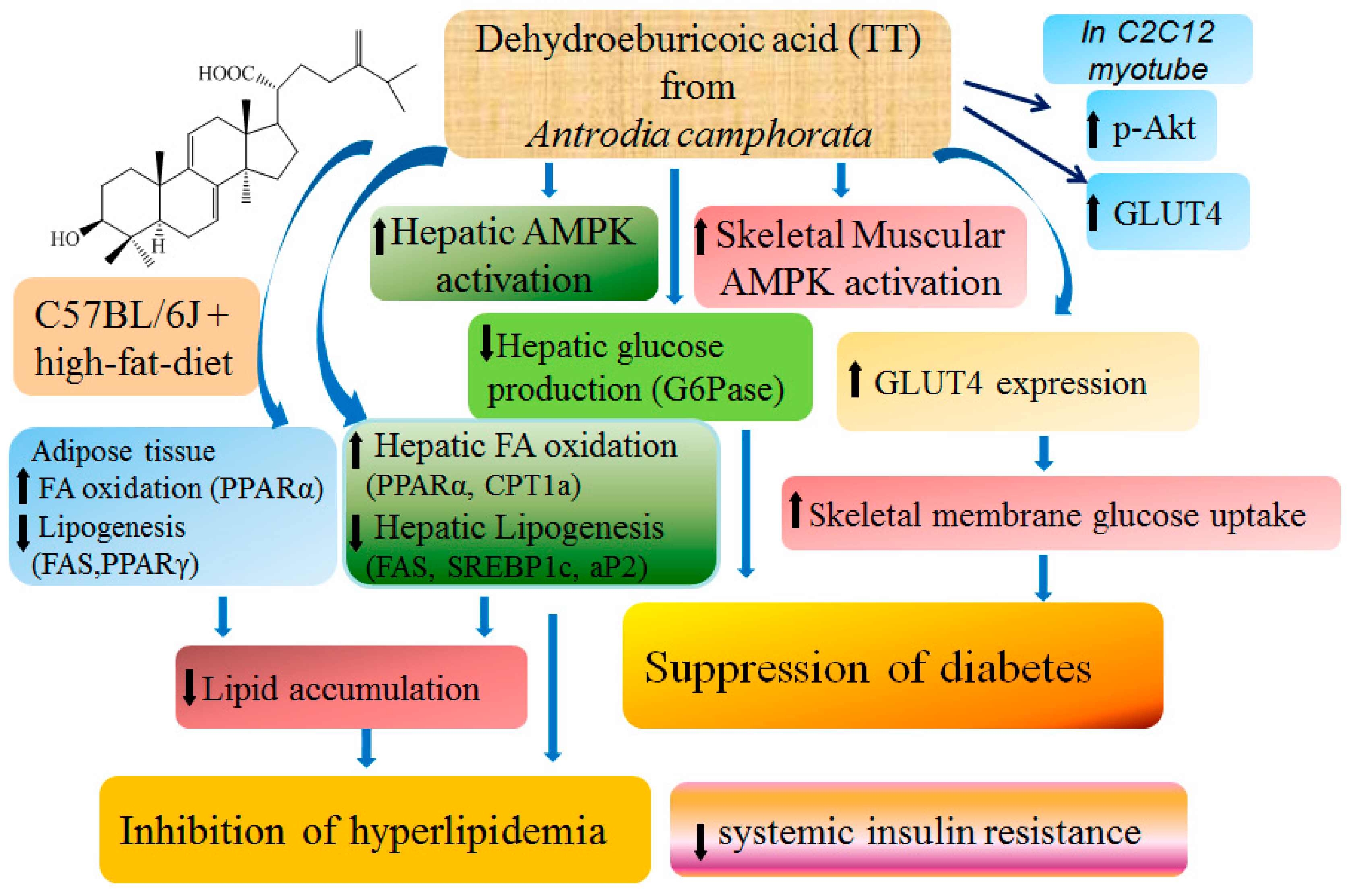
3. Discussion
4. Materials and Methods
4.1. Chemicals
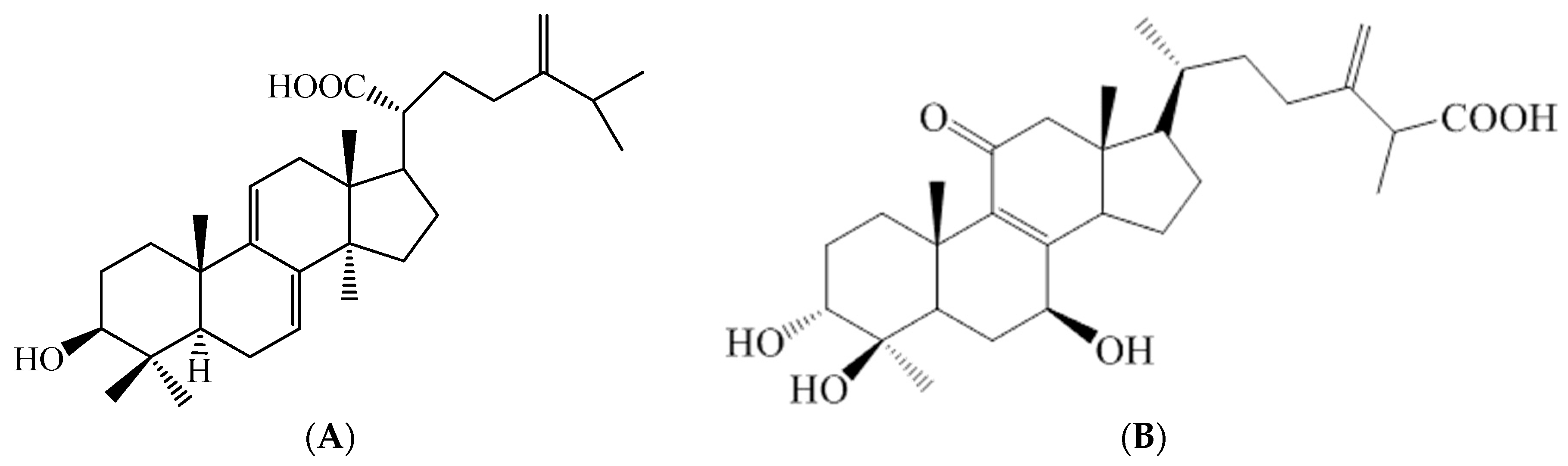
4.2. The Active Compound Determination
4.3. Cell Culture
4.4. Detection of Expression Levels of Membrane GLUT4 and Phosphorylation of Akt (Ser473) in Vitro
4.5. Animal Study
4.6. Measurements of Blood Glucose Levels and Biochemical Parameters
4.7. Histopathology Examination
4.8. Liver Lipids Analysis
4.9. Relative Quantization of mRNA and Western Blotting
4.10. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| aP2 | adipocyte fatty acid binding protein 2 |
| 11β-HSD1 | 11β hydroxysteroid dehydrogenase |
| BAT | brown adipose tissue |
| CON | control |
| CPT1a | carnitine palmitoyl transferase Ia |
| EWAT | epididymal white adipose tissue |
| FAS | fatty acid synthase |
| Feno | fenofibrate |
| FFA | free fatty acid |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| G6 Pase | glucose-6-phosphatase |
| GPAT | glycerol-3-phosphate acyltransferase |
| GLUT4 | glucose transporter 4 |
| HFD | high-fat-diet |
| Metf | metformin |
| MWAT | mesenteric white adipose tissue |
| PEPCK | phosphoenolpyruvate carboxykinase |
| phospho-AMPK | AMPK phosphorylation |
| PPAR | peroxisome proliferator-activated receptor |
| RT-PCR | reverse transcription-polymerase chain reaction |
| RWAT | retroperitoneal white adipose tissue |
| SREBP | sterol regulatory element binding protein |
| TC | total cholesterol |
| TG | triglyceride |
| UCP3 | uncoupling protein 3 |
| WAT | white adipose tissue |
References
- Reaven, G.M.; Laws, A. Insulin resistance, compensatory hyperinsulinemia, and coronary heart disease. Diabetologia 1994, 37, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–29. [Google Scholar] [PubMed]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.W.; Elliot, B.T. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Pessin, J.E. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog. Horm. Res. 2001, 56, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Tai, T.; Akahori, A.; Shingu, T. Triterpenes of Poria cocos. Phytochemistry 1993, 32, 1239–1244. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Ergostatrien-3β-ol from Antrodia camphorata inhibits diabetes and hyperlipidemia in high-fat-diet treated mice via regulation of hepatic related genes, glucose transporter 4, and AMP-Activated protein kinase phosphorylation. J. Agric. Food Chem. 2015, 63, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Antidiabetic and antihyperlipidemic properties of a triterpenoid compound, dehydroeburicoic acid, from Antrodia camphorata in vitro and in streptozotocin-induced mice. J. Agric. Food Chem. 2015, 63, 10140–10151. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.M. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid. Based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Lee, C.Y.; Hou, W.C.; Wang, S.Y.; Sung, P.J.; Kuo, P.J. Hepatoprotective effects of eburicoic acid and dehydroeburicoic acid from Antrodia camphorata in a mouse model of acute hepatic injury. Food Chem. 2013, 141, 3020–3027. [Google Scholar] [CrossRef] [PubMed]
- Song, T.Y.; Yen, G.C. Protective effects of fermented filtrate from Antrodia camphorata in submerged culture against CCl4-induced hepatic toxicity in rats. J. Agric. Food Chem. 2003, 51, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Majid, E.; Male, K.B.; Tzeng, Y.M.; Omamogho, J.O.; Glennon, J.D.; Luong, J.H. Cyclodextrin-modified capillary electrophoresis for achiral and chiral separation of ergostane and lanostane compounds extracted from the fruiting body of Antrodia camphorata. Electrophoresis 2009, 30, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Tzeng, Y.M. Chemistry and DMPK studies of Antrodiam camphorata. In Fruiting Body’s Standards and the Correct Scientific Name of Niu-Chang Musroom Antrodia cinnamomea [on Cinnamomum kanehirai] Endemic in Taiwan & the Original Record of 2014 International Symposium and Workshop on Taiwan Medical Mushrooms: Antrodia cinnamomea; Academic Sinica: Taipei, Taiwan, 2014. [Google Scholar]
- Farnier, M.; Bonnefous, F.; Debbas, N.; Irvine, A. Comparative efficacy and safety of micronised fenofibrate and simvastatin in patients with primary type IIa or IIb hyperlipidemia. Arch. Intern. Med. 1994, 154, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.J.; Griesel, B.A.; King, C.D.; Josey, M.A.; Olson, A.L. Moderate GLUT4 overexpression improves insulin sensitivity and fasting triglyceridemia in high-fat-diet-fed transgenic mice. Diabetes 2013, 62, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Jocot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.R. The effect of insulin on the disposal of intravenous glucose. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Ryder, J.W.; Yang, J.; Galuska, D.; Rincόn, J.; Bjőrnholm, M.; Krook, A.; Lund, S.; Pedersen, O.; Wallberg-Henriksson, H.; Zieratgh, J.R.; et al. Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes 2000, 49, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kahn, C.R.; Kahn, C.R. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin and glucose homeostasis. J. Biol. Chem. 2003, 278, 33609–33612. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.W.; Patel, Y.M. GLUT4 expression in 3T3-L1 adipocytes is repressed by proteasome inhibition, but not by inhibition of calpains. Mol. Cell. Endocrinol. 2005, 232, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Damci, T.; Tatliagac, S.; Osar, Z.; Ilkova, H. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur. J. Intern. Med. 2003, 14, 357–360. [Google Scholar] [CrossRef]
- Staels, B.; Fruchart, J.C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Nedachi, T.; Kanzaki, M. Regulation of glucose transporters by insulin transporter by insulin and extracellular glucose in C2C12 myotubes. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E817–E828. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. (Lond.) 2012, 122, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Kotelevtsev, Y.; Holmes, M.C.; Burchell, A.; Houston, P.M.; Schmoll, D.; Jamieson, P.; Best, R.; Brown, R.; Edwards, C.R.W.; Seckl, J.R.; et al. 11β-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl Acad. Sci. USA 1997, 94, 14924–14929. [Google Scholar] [CrossRef] [PubMed]
- Barthel, A.; Schmoll, D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E685–E692. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Tso, A.W.K.; Cheung, B.M.Y.; Wang, Y.; Wat, N.M.S.; Fong, C.H.Y.; Yeung, D.C.Y.; Janus, E.D.; Sham, P.C.; Lam, K.S.L. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome. Circulation 2007, 115, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.D.; Knight, B.L.; Wiggins, D.; Humphreys, S.M.; Gibbons, G.F. Disturbances in the normal regulation of SREBP-sensitive genes in PPAR α-deficient mice. J. Lipid Res. 2001, 42, 328–337. [Google Scholar] [PubMed]
- Lewin, T.M.; Granger, D.A.; Kim, J.H.; Coleman, R.A. Regulation of mitochondrial sn-glycerol-3-phosphate acyltransferase activity: Response to feeding status is unique in various rat tissues and is discordant with protein expression. Arch. Biochem. Biophys. 2001, 396, 119–127. [Google Scholar] [CrossRef] [PubMed]
- McGarry, J.D.; Brown, N.F. The mitochondrial carnitine palmitoyl-transferase system from concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, E.; de Lange, P.; Moreno, M.; Lombardi, A.; Ragni, M.; Feola, A.; Schiavo, L.; Goglia, F.; Lanni, A. Fenofibrate activates the biochemical pathways and the de novo expression of genes related to lipid handing and uncoupling protein-3 functions in liver of normal rats. Biochim. Biophys. Acta 2006, 1757, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Lanni, A.; Mancini, F.; Sabatino, L.; Silvestri, E.; Franco, R.; De Rosa, G.; Goglia, F.; Colantuoni, V. De nevo expression of uncoupling protein 3 is associated to enhanced mitochondrial thioesterase-1 expression and fatty acid metabolism in liver of fenofibrate-treated rats. FEBS Lett. 2002, 525, 7–12. [Google Scholar] [CrossRef]
- Camara, Y.; Mampe, T.; Armengol, J.; Villarroya, F.; Dejean, L. UCP3 expression in liver modulates gene expression and oxidative metabolism in response to fatty acids, and sensitizes mitochondria to permeability transition. Cell. Physiol. Biochem. 2009, 24, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Segal, K.R.; Landt, M.; Klein, S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes 1996, 45, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Thomas, T.C.; Storlien, T.C.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Kim, Y.B.; Peroni, O.D.; Fryer, L.G.; Mȕller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Saladin, R.; Falas, L.; Dana, S.; Halvorsen, Y.D.; Auwerx, J.; Briggs, M. Differential regulation of peroxisome proliferator activated receptor gamma1 (PPARγ1) and PPARγ2 messenger RNA expression in the early stages of adipogenesis. Cell Growth Differ. 1999, 10, 43–48. [Google Scholar] [PubMed]
- Kersten, S. Peroxisome proliferator activated receptors and obesity. Eur. J. Pharmacol. 2002, 440, 223–234. [Google Scholar] [CrossRef]
- Shimano, H.; Shimomura, I.; Hammer, R.E.; Herz, J.; Goldstein, J.L.; Brown, M.S.; Horton, J.D. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J. Clin. Investig. 1997, 100, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Kuo, Y.C.; Huang, R.L.; Lin, L.C.; Don, M.J.; Chang, T.T. New ergostane and lanostane from Antrodia camphorata. J. Chin. Med. 2003, 14, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Klip, A.; Ramlal, T.; Young, D.A.; Holloszy, J.O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987, 224, 224–230. [Google Scholar] [CrossRef]
- Shih, C.C.; Lin, C.H.; Lin, W.L.; Wu, J.B. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J. Ethnopharmacol. 2009, 123, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Ye, J.M.; Turner, N.; Hohnen-Behtens, C.; Ke, C.Q.; Tang, C.P.; Chen, T.; Weiss, H.C.; Gesing, E.R.; Rowland, A.; et al. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Cell Chem. Biol. 2008, 15, 263–273. [Google Scholar]
- Shih, C.C.; Wu, J.B.; Jian, J.Y.; Lin, C.H.; Ho, H.Y. (–)-Epicatechin-3-O-β-d-allopyranoside from Davallia formosana, prevents diabetes and hyperlipidemia by regulation of glucose transporter 4 and AMP-activated protein kinase phosphorylation in high-fat-fed mice. Int. J. Mol. Sci. 2015, 16, 24983–25001. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.C.; Lin, C.H.; Wu, J.B. Eriobotrya japonica improves hyperlipidaemia and reverses insulin resistance in high-fat-fed mice. Phytother. Res. 2010, 24, 1769–1780. [Google Scholar] [CrossRef] [PubMed]

| Parameter | CON | HF | HF + TT1 | HF + TT2 | HF + TT3 | HF + Feno | HF + Metf |
|---|---|---|---|---|---|---|---|
| Dose (mg/kg/day) | – | – | 10 | 20 | 40 | 250 | 300 |
| Absolute tissue weight (mg) | |||||||
| EWAT | 423.6 ± 22.4 | 963.1 ± 68.7 c | 798.9 ± 43.2 c | 784.3 ± 102.0 b | 667.8 ± 53.2 c,e | 663.1 ±47.0 b,e | 482.1 ± 31.5 f |
| MWAT | 246.3 ± 7.3 | 369.5 ± 24.2 b | 361.3 ± 41.1 a | 340.9 ± 28.4 | 304.6 ± 29.5 | 275.9 ± 26.1 d | 198.2 ± 24.2 f |
| RWAT | 133.5 ± 11.8 | 470.4 ± 45.6 c | 343.9 ± 23.4 c | 331.9 ± 47.5 c | 311.4 ± 33.6 c,e | 268.6 ± 26.2 b,e | 180.4 ± 15.7 f |
| Visceral fat | 557.0 ± 31.5 | 1433.4 ± 112.9 c | 1142.9 ± 32.4 b | 1116.2 ± 131.5 c,d | 979.3 ± 86.2 c,e | 931.7 ± 70.6 b,e | 662.4 ± 44.0 f |
| Liver | 878.5 ± 28.0 | 889.0 ± 15.3 | 848.3 ± 30.6 d | 841.8 ± 13.4 a,d | 802.8 ± 24.4 d | 882.4 ± 24.1 | 1959.0 ± 105.6 c,f |
| Spleen | 76.0 ± 2.6 | 86.1 ± 1.7 | 77.7 ± 3.0 | 81.5 ± 10.1 | 82.3 ± 3.7 | 74.6 ± 4.5 | 81.0 ± 3.5 |
| BAT | 135.6 ± 9.7 | 249.0 ± 15.3 c | 228.6 ± 23.9 c | 197.3 ± 12.9 c,a | 198.1 ± 14.2 c,a | 170.4 ± 11.0 a,e | 134.2 ± 5.1 f |
| Weight gain (g) | 1.56 ± 0.19 | 2.89 ± 0.27 c | 2.24 ± 0.14 a,d | 0.89 ± 0.26 f | 0.83 ± 0.21 a,f | 0.46 ± 0.24 b,f | 0.52 ± 0.29 a,f |
| Final body weight (g) | 24.72 ± 0.48 | 28.55 ± 0.64 c | 27.52 ± 0.67 b | 26.47 ± 0.57 d | 26.53 ± 0.91 d | 26.04 ± 0.60 d | 26.35 ± 0.85 d |
| Food intake (g/day/mouse) | 2.45 ± 0.05 | 2.26 ± 0.06 a | 2.21 ± 0.05 b | 2.16 ± 0.05 c | 2.07 ± 0.04 c,d | 2.05 ± 0.06 c,d | 2.25 ± 0.06 a |
| Liver lipids | |||||||
| total lipid (mg/g) | 56.9 ± 1.4 | 89.7 ± 2.1 c | 64.4 ± 1.8 b,f | 61.7 ± 1.9 f | 57.9 ± 1.4 f | 62.0 ± 2.1 f | 61.1 ± 1.9 f |
| triacylglycerol (μmol/g) | 45.9 ± 3.0 | 78.4 ± 4.6 c | 53.0 ± 2.6 f | 48.0 ± 2.4 f | 47.1± 2.0 f | 48.6 ± 2.0 f | 49.0 ± 2.1 f |
| Blood profiles | |||||||
| FFA (meq/L) | 0.96 ± 0.13 | 1.28 ± 0.19 c | 0.95 ± 0.09 f | 0.87 ± 0.120 f | 0.81 ± 0.06 b,f | 0.86 ± 0.08 a,f | 0.85 ± 0.09 a,f |
| Blood glucose (mg/dL) | 78.56 ± 1.71 | 137.44 ± 2.62 c | 90.44 ± 1.80 c,f | 82.89 ±1.91 f | 77.78 ± 2.44 f | 86.56 ± 3.23 a,f | 87.33 ± 2.78 a,f |
| TG (mg/dL) | 83.82 ± 2.14 | 105.27 ± 1.10 c | 84.85 ± 1.36 f | 83.63 ± 2.42 f | 82.31 ± 1.60 f | 83.35 ± 2.09 f | 82.07 ± 1.53 f |
| TC (mg/dL) | 101.70 ± 1.17 | 152.71 ± 4.15 c | 120.55 ± 0.92 c,f | 114.93 ±2.74 c,f | 112.46 ±1.76 c,f | 115.25 ± 3.05 c,f | 117.20 ± 3.16 c,f |
| Insulin (μg/L) | 2.247 ± 0.010 | 3.563 ± 0.004 c | 2.982 ± 0.024 b,f | 2.625 ± 0.017 b,f | 2.190 ± 0.025 f | 2.402 ± 0.029 b,f | 2.467 ± 0.025 b,f |
| Leptin(ng/mL) | 6.640 ± 0.168 | 14.116 ± 0.244 c | 9.827 ± 0.052 c,f | 8.468 ± 0.182 c,f | 6.439 ± 0.091 f | 7.507 ± 0.144 b,f | 7.359 ± 0.1325 b,f |
| Gene | Accession Number | Forward Primer and Reverse Primer | PCR Product (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| Liver | ||||
| G6Pase | NM_008061.3 | F: GAACAACTAAAGCCTCTGAAAC | 350 | 50 |
| R: TTGCTCGATACATAAAACACTC | ||||
| SREBP1c | NM_011480 | F: GGCTGTTGTCTACCATAAGC | 219 | 48 |
| R: AGGAAGAAACGTGTCAAGAA | ||||
| GPAT | BC019201.1 | F: CAGTCCTGAATAAGAGGT | 441 | 51 |
| R: TGGACAAAGATGGCAGCAGA | ||||
| apo C-III | NM_023114.3 | F: CAGTTTTATCCCTAGAAGCA | 349 | 47 |
| R: TCTCACGACTCAATAGCTG | ||||
| CPT1a | BC054791.1 | F: CTTGTGACCCTACTACATCC | 332 | 51 |
| R: TCATAGCAGAACCTTAATCC | ||||
| SREBP2 | AF289715.2 | F: ATATCATTGAAAAGCGCTAC | 256 | 48 |
| R: ATTTTCAAGTCCACATCACT | ||||
| aP2 | NM_024406 | F: TCACCTGGAAGACAGCTCCT | 142 | 52 |
| R: TGCCTGCCACTTTCCTTGT | ||||
| UCP3 | NM_009464 | F: GAGGTGACTACAGCCTTCTG | 242 | 51 |
| R: TAGGAAGTGCTTCCATGTCT | ||||
| 11β-HSD1 | NM_008288.2 | F: AAGCAGAGCAATGGCAGCAT | 300 | 50 |
| R: GAGCAATCATAGGCTGGGTCA | ||||
| β-actin | NM_007392 | F: TCTCCACCTTCCAGCAGATGT | 92 | 60 |
| R: AGCTCAGTAACAGTCCGCCTAGA | ||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-H.; Lin, C.-H.; Shih, C.-C. Dehydroeburicoic Acid from Antrodia camphorata Prevents the Diabetic and Dyslipidemic State via Modulation of Glucose Transporter 4, Peroxisome Proliferator-Activated Receptor α Expression and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. Int. J. Mol. Sci. 2016, 17, 872. https://doi.org/10.3390/ijms17060872
Kuo Y-H, Lin C-H, Shih C-C. Dehydroeburicoic Acid from Antrodia camphorata Prevents the Diabetic and Dyslipidemic State via Modulation of Glucose Transporter 4, Peroxisome Proliferator-Activated Receptor α Expression and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. International Journal of Molecular Sciences. 2016; 17(6):872. https://doi.org/10.3390/ijms17060872
Chicago/Turabian StyleKuo, Yueh-Hsiung, Cheng-Hsiu Lin, and Chun-Ching Shih. 2016. "Dehydroeburicoic Acid from Antrodia camphorata Prevents the Diabetic and Dyslipidemic State via Modulation of Glucose Transporter 4, Peroxisome Proliferator-Activated Receptor α Expression and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice" International Journal of Molecular Sciences 17, no. 6: 872. https://doi.org/10.3390/ijms17060872
APA StyleKuo, Y.-H., Lin, C.-H., & Shih, C.-C. (2016). Dehydroeburicoic Acid from Antrodia camphorata Prevents the Diabetic and Dyslipidemic State via Modulation of Glucose Transporter 4, Peroxisome Proliferator-Activated Receptor α Expression and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. International Journal of Molecular Sciences, 17(6), 872. https://doi.org/10.3390/ijms17060872