Effects of a Bovine Lactoferrin Formulation from Cow’s Milk on Menstrual Distress in Volunteers: A Randomized, Crossover Study
Abstract
:1. Introduction
2. Results
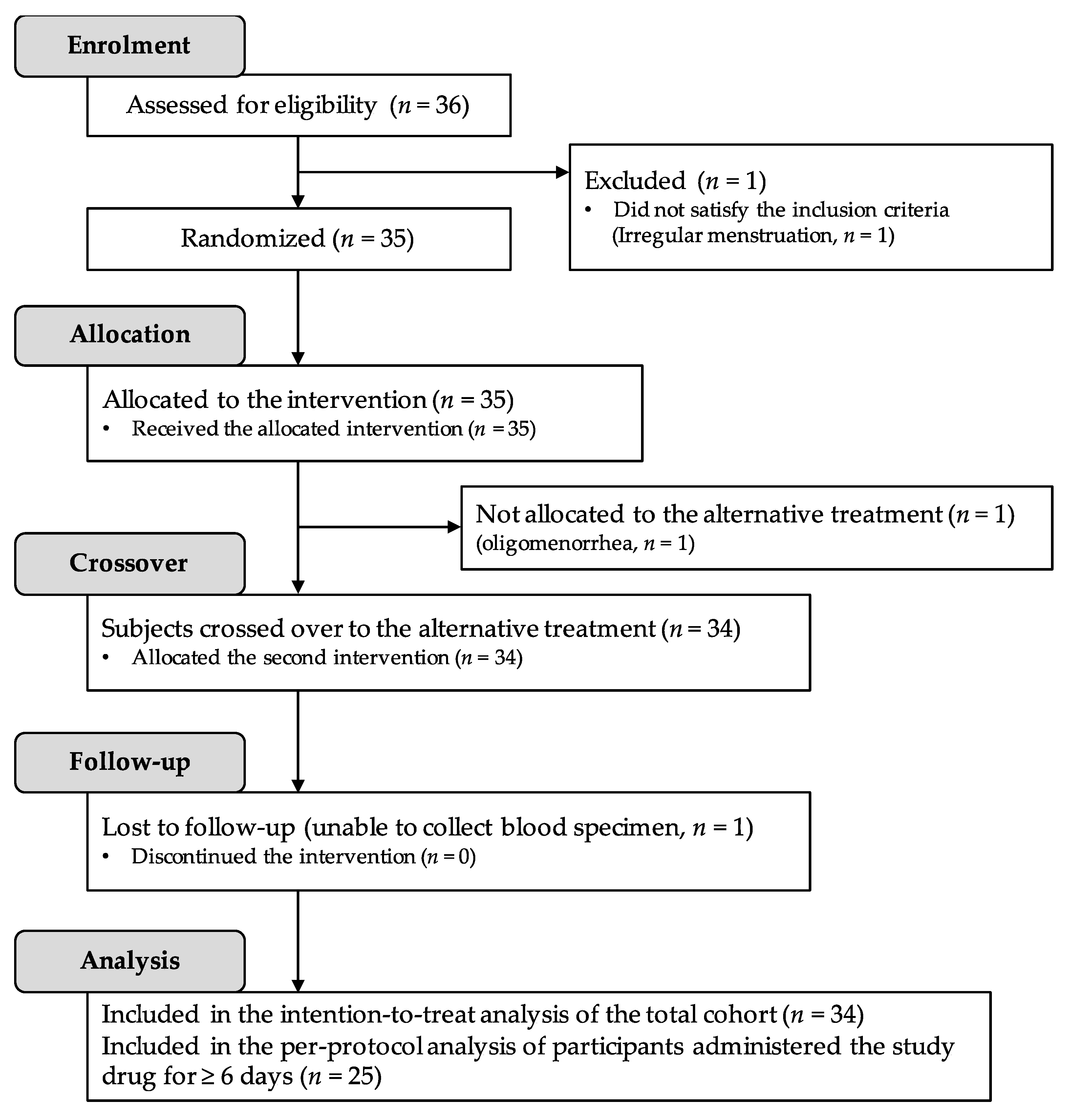
2.1. Participant Disposition and Baseline Characteristics
2.2. Subjective Symptoms
2.3. Heart Rate Variability
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Procedure
4.3. Sample Size, Data Analyses, and Statistical Tests
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Lf | lactoferrin |
| FeLf | iron-lactoferrin complex |
| MDQ | Moos menstrual distress questionnaire |
| NSAID | nonsteroidal anti-inflammatory drug |
| VAS | visual analogue scale |
| VRS | verbal rating scale |
| HFA | heart rate variability index of high-frequency area |
| LFA | heart rate variability index of low-frequency area |
| H% | power value of the high-frequency relative to the total power area |
| L% | power value of the low-frequency relative to the total power area |
References
- Ju, H.; Jones, M.; Mishra, G. The prevalence and risk factors of dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.L.; Murphy, P.A. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database Syst. Rev. 2001, CD002124. [Google Scholar] [CrossRef]
- Osuga, Y.; Hayashi, K.; Kobayashi, Y.; Toyokawa, S.; Momoeda, M.; Koga, K.; Yoshino, O.; Tsutsumi, O.; Hoshiai, H.; Terakawa, N.; et al. Dysmenorrhea in japanese women. Int. J. Gynaecol. Obstet. 2005, 88, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Ohde, S.; Tokuda, Y.; Takahashi, O.; Yanai, H.; Hinohara, S.; Fukui, T. Dysmenorrhea among japanese women. Int. J. Gynaecol. Obstet. 2008, 100, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Momoeda, M.; Osuga, Y.; Rossi, B.; Nomoto, K.; Hayakawa, M.; Kokubo, K.; Wang, E.C. Burden of menstrual symptoms in japanese women—An analysis of medical care-seeking behavior from a survey-based study. Int. J. Womens Health 2014, 6, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fjerbæk, A.; Knudsen, U.B. Endometriosis, dysmenorrhea and diet—What is the evidence? Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 132, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Razzak, K.K.; Ayoub, N.M.; Abu-Taleb, A.A.; Obeidat, B.A. Influence of dietary intake of dairy products on dysmenorrhea. J. Obstet. Gynaecol. Res. 2010, 36, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Lönnerdal, B. Nutritional roles of lactoferrin. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.; Roy, K.; Patel, Y.; Zhou, S.-F.; Singh, M.; Singh, D.; Nasir, M.; Sehgal, R.; Sehgal, A.; Singh, R.; et al. Multifunctional iron bound lactoferrin and nanomedicinal approaches to enhance its bioactive functions. Molecules 2015, 20, 9703–9731. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Novel function of bovine milk-derived lactoferrin on antinociception mediated by μ-opioid receptor in the rat spinal cord. Brain Res. 2003, 965, 239–245. [Google Scholar] [CrossRef]
- Hayashida, K.; Takeuchi, T.; Shimizu, H.; Ando, K.; Harada, E. Lactoferrin enhances opioid-mediated analgesia via nitric oxide in the rat spinal cord. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R306–R312. [Google Scholar] [CrossRef] [PubMed]
- Kamemori, N.; Takeuchi, T.; Hayashida, K.; Harada, E. Suppressive effects of milk-derived lactoferrin on psychological stress in adult rats. Brain Res. 2004, 1029, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Harada, E. Relieving effects of enteric lactoferrin on menstrual pain in women and their quality of life. Jpn. J. Matern. Health 2007, 48, 239–245. (In Japanese) [Google Scholar]
- Yoshise, R.; Ueda, N.; Matsuyama, H.; Serizawa, A. Suppressive effect of lactoferrin with 70 irons via oral administration on dysmenorrheal. Milk Sci. 2010, 59, 115–123. (In Japanese) [Google Scholar]
- Vitetta, L.; Coulson, S.; Beck, S.L.; Gramotnev, H.; Du, S.; Lewis, S. The clinical efficacy of a bovine lactoferrin/whey protein Ig-Rich fraction (Lf/IgF) for the common cold: A double blind randomized study. Complement. Ther. Med. 2013, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Pan, F.; Sawano, Y.; Makino, T.; Kakehi, Y.; Komiyama, M.; Kawakami, H.; Tanokura, M. Studies of the structure of multiferric ion-bound lactoferrin: A new antianemic edible material. Int. Dairy J. 2008, 18, 1051–1056. [Google Scholar] [CrossRef]
- Ueno, H.M.; Kato, K.; Ueda, N.; Matsui, H.; Nakajima, H. Native, but not thermally denatured lactoferrin solubilizes iron in the presence of bicarbonate ions. Dairy Sci. Technol. 2012, 92, 25–35. [Google Scholar] [CrossRef]
- Ueno, H.M.; Ueda, N.; Morita, M.; Kakehi, Y.; Kobayashi, T. Thermal stability of the iron–lactoferrin complex in aqueous solution is improved by soluble soybean polysaccharide. Food Biophys. 2012, 7, 183–189. [Google Scholar] [CrossRef]
- Takada, M.; Ebara, T.; Sakai, Y. The acceleration plethysmography system as a new physiological technology for evaluating autonomic modulations. Health Eval. Promot. 2008, 35, 373–377. [Google Scholar] [CrossRef]
- Yoshise, R.; Matsuyama, H.; Hosoya, T.; Ogawa, A.; Kadooka, Y. Effects of Fe-lactoferrin via oral administration on mental stress. Milk Sci. 2010, 59, 93–101. (In Japanese) [Google Scholar]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Agelink, M.W.; Boz, C.; Ullrich, H.; Andrich, J. Relationship between major depression and heart rate variability. Clinical consequences and implications for antidepressive treatment. Psychiatr. Res. 2002, 113, 139–149. [Google Scholar] [CrossRef]
- Tanaka, E.; Momoeda, M.; Osuga, Y.; Rossi, B.; Nomoto, K.; Hayakawa, M.; Kokubo, K.; Wang, E.C. Burden of menstrual symptoms in Japanese women: Results from a survey-based study. J. Med. Econ. 2013, 16, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Steijns, J.M.; van Hooijdonk, A.C. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br. J. Nutr. 2000, 84 (Suppl. 1), S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Wakabayashi, H.; Shin, K.; Yamauchi, K.; Yaeshima, T.; Iwatsuki, K. Twenty-Five years of research on bovine lactoferrin applications. Biochimie 2009, 91, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-Y.; Chen, W.-J.; Lee, W.-Y.; Lo, S.-T.; Lee, T.-W.; Lo, J.-M. In vitro and in vivo evaluation of lactoferrin-conjugated liposomes as a novel carrier to improve the brain delivery. Int. J. Mol. Sci. 2013, 14, 2862–2874. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Moore, R.A.; McQuay, H.J. The visual analogue pain intensity scale: What is moderate pain in millimetres? Pain 1997, 72, 95–97. [Google Scholar] [CrossRef]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Goolsbee, W.; Pacifici, E.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron disorders in pregnant and non-pregnant women. Int. J. Immunopathol. Pharmacol. 2010, 23, 577–587. [Google Scholar] [PubMed]
- Brutsaert, T.D.; Hernandez-Cordero, S.; Rivera, J.; Viola, T.; Hughes, G.; Haas, J.D. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am. J. Clin. Nutr. 2003, 77, 441–448. [Google Scholar] [PubMed]
- Verdon, F.; Burnand, B.; Stubi, C.L.F.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; de Vevey, M.; Studer, J.P.; Herzig, L.; et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124. [Google Scholar] [CrossRef] [PubMed]
- Overview of Dietary reference intakes for Japanese. Available online: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf (accessed on 9 May 2016).
- National Health and Nutrition Survey in Japan. 2015. Available online: http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h26-houkoku-04.pdf (accessed on 9 May 2016).
- Moos, R.H. The development of a menstrual distress questionnaire. Psychosom. Med. 1968, 30, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, E.P. The use of visual analog scales in mood disorders: A critical review. J. Psychiatr. Res. 1997, 31, 569–579. [Google Scholar] [CrossRef]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Ebara, T.; Kamijima, M. Heart rate variability assessment in japanese workers recovered from depressive disorders resulting from job stress: Measurements in the workplace. Int. Arch. Occup. Environ. Health 2010, 83, 521–529. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value (Range) |
|---|---|
| Age (year) | 36.9 ± 3.9 (27–45) |
| Body weight (kg) | 57.3 ± 9.7 (44.8–93.7) |
| Body mass index (kg·m−2) | 22.3 ± 3.6 (18.4–35.7) |
| Serum iron (μg·dL−1) | 94.9 ± 54.1 (20–219) |
| Serum ferritin (ng·mL−1) | 28.5 ± 29.0 (5.0–115.1) |
| Red blood cell count (×104 μL−1) | 444 ± 31 (369–494) |
| Hemoglobin (g·dL−1) | 12.8 ± 1.2 (9.8–15.2) |
| Hematocrit (%) | 38.2 ± 2.8 (30.1–44.4) |
| Measurements | FeLf (n = 34) | Placebo (n = 34) | |
|---|---|---|---|
| MDQ subscale | Pain | 6.2 ± 4.0 | 6.5 ± 4.3 |
| Concentration | 2.6 ± 4.7 | 3.3 ±5.0 | |
| Autonomic nervous system | 1.1 ± 1.7 * | 1.8 ± 2.2 | |
| Behavior change | 4.1 ± 4.6 | 4.0 ± 4.6 | |
| Fluid accumulation | 2.9 ± 2.6 | 3.2 ± 3.1 | |
| Negative feeling | 1.9 ± 3.1 | 2.5 ± 4.1 | |
| Hyperthymia | 1.6 ± 3.4 | 1.5 ± 3.2 | |
| Control | 0.7 ± 1.5 | 1.0 ± 1.8 | |
| Finger photoplethysmographic waveform variability | LFA 2 | 709.1 ± 669.2 | 836.1 ± 772.7 |
| L% 3 | 32.5 ± 15.5 | 33.7 ± 14.7 | |
| HFA 4 | 941.6 ± 918.4 | 695.7 ± 598.8 | |
| H% 5 | 41.1 ± 18.8 * | 31.7 ± 15.0 | |
| TP 6 | 2175.2 ± 1587.6 | 2449.2 ± 2530.4 | |
| LFA/HFA 7 | 1.3 ± 1.6 | 1.9 ± 2.7 | |
| Menstrual pain | 53.5 ± 32.7 | 50.6 ± 35.5 | |
| Quality of life | 2.1 ± 2.0 | 2.0 ± 1.9 | |
| Measurements | FeLf (n = 25) | Placebo (n = 25) | |
|---|---|---|---|
| MDQ subscale | Pain | 6.1 ± 4.3 | 6.8 ± 4.7 |
| Concentration | 2.5 ± 4.7 | 3.5 ± 5.7 | |
| Autonomic nervous system | 1.0 ± 1.7 ** | 1.9 ± 2.4 | |
| Behavior change | 4.0 ± 4.6 | 4.8 ± 5.0 | |
| Fluid accumulation | 2.4 ± 2.5 † | 3.0 ± 3.1 | |
| Negative feeling | 1.8 ± 3.4 † | 3.2 ± 4.6 | |
| Hyperthymia | 2.2 ± 3.9 | 1.9 ± 3.6 | |
| Control | 0.8 ± 1.7 | 1.1 ± 2.0 | |
| Finger photoplethysmographic waveform variability | LFA | 627.4 ± 623.2 | 760.5 ± 829.5 |
| L% | 30.3 ± 13.4 | 32.3 ± 15.3 | |
| HFA | 940.5 ± 991.3 | 610.8 ± 552.0 | |
| H% | 41.9 ± 18.9 † | 32.1 ± 16.9 | |
| TP | 2118.2 ± 1750.9 | 2023.1 ± 1291.3 | |
| LFA/HFA | 1.1 ± 1.0 † | 2.1 ± 3.1 | |
| Menstrual pain | 53.9 ± 33.7 | 54.0 ± 36.2 | |
| Quality of life | 2.0 ± 2.0 | 2.2 ± 1.7 | |
| Nutrients | FeLf | Placebo |
|---|---|---|
| Iron (mg) | 6.7 | 0.0 |
| Lactoferrin (mg) | 123.8 | 0.0 |
| Energy (kcal) | 2.5 | 2.6 |
| Protein (g) | 0.1 | 0.0 |
| Fat (g) | 0.0 | 0.0 |
| Carbohydrate (g) | 0.5 | 0.6 |
| Sodium (mg) | 5.6 | 2.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, H.M.; Yoshise, R.E.; Sugino, T.; Kajimoto, O.; Kobayashi, T. Effects of a Bovine Lactoferrin Formulation from Cow’s Milk on Menstrual Distress in Volunteers: A Randomized, Crossover Study. Int. J. Mol. Sci. 2016, 17, 845. https://doi.org/10.3390/ijms17060845
Ueno HM, Yoshise RE, Sugino T, Kajimoto O, Kobayashi T. Effects of a Bovine Lactoferrin Formulation from Cow’s Milk on Menstrual Distress in Volunteers: A Randomized, Crossover Study. International Journal of Molecular Sciences. 2016; 17(6):845. https://doi.org/10.3390/ijms17060845
Chicago/Turabian StyleUeno, Hiroshi M., Ran Emilie Yoshise, Tomohiro Sugino, Osami Kajimoto, and Toshiya Kobayashi. 2016. "Effects of a Bovine Lactoferrin Formulation from Cow’s Milk on Menstrual Distress in Volunteers: A Randomized, Crossover Study" International Journal of Molecular Sciences 17, no. 6: 845. https://doi.org/10.3390/ijms17060845
APA StyleUeno, H. M., Yoshise, R. E., Sugino, T., Kajimoto, O., & Kobayashi, T. (2016). Effects of a Bovine Lactoferrin Formulation from Cow’s Milk on Menstrual Distress in Volunteers: A Randomized, Crossover Study. International Journal of Molecular Sciences, 17(6), 845. https://doi.org/10.3390/ijms17060845






