Analysis of 2-(2-Phenylethyl)chromones by UPLC-ESI-QTOF-MS and Multivariate Statistical Methods in Wild and Cultivated Agarwood
Abstract
:1. Introduction
2. Results
2.1. Characterization of 2-(2-Phenylethyl)chromone Derivatives by UPLC-ESI-QTOF-MS
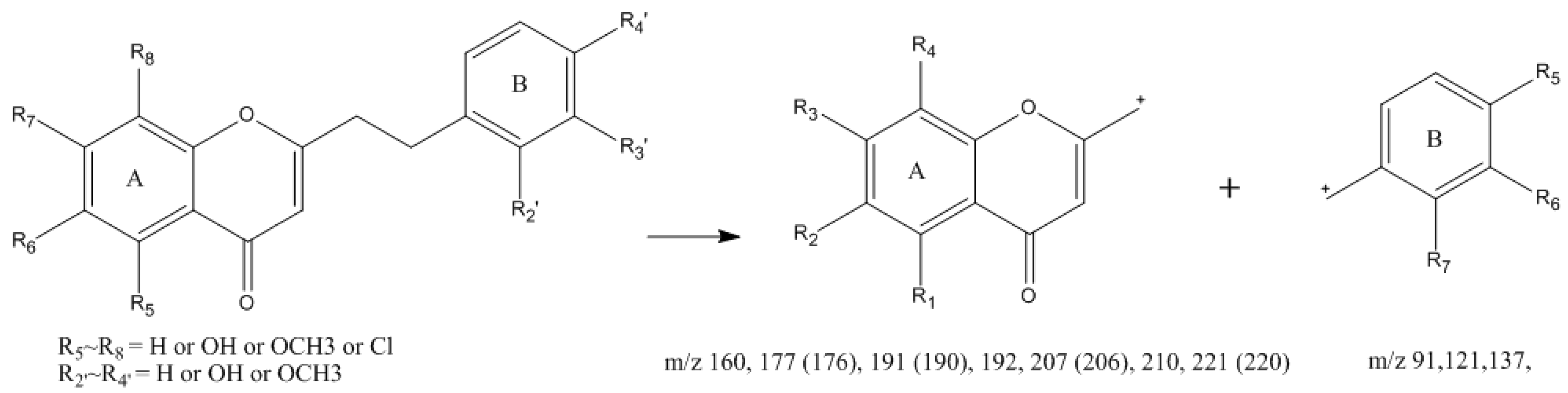
2.1.1. Identification of 2-(2-Phenylethyl)chromones (Unoxidized) in Agarwood
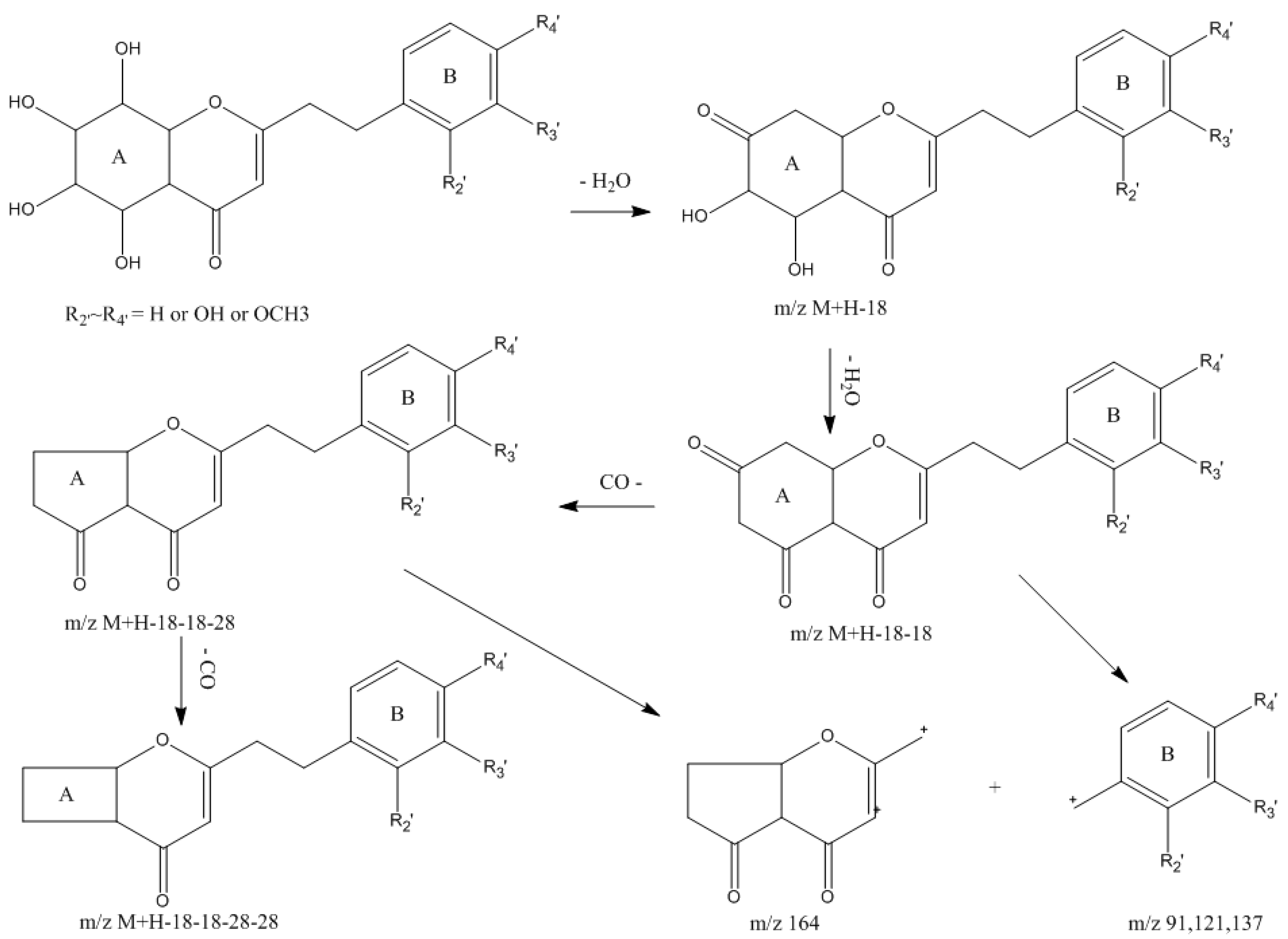
2.1.2. Identification of 5,6,7,8-Tetrahydro-2-(2-phenylethyl)chromones in Agarwood
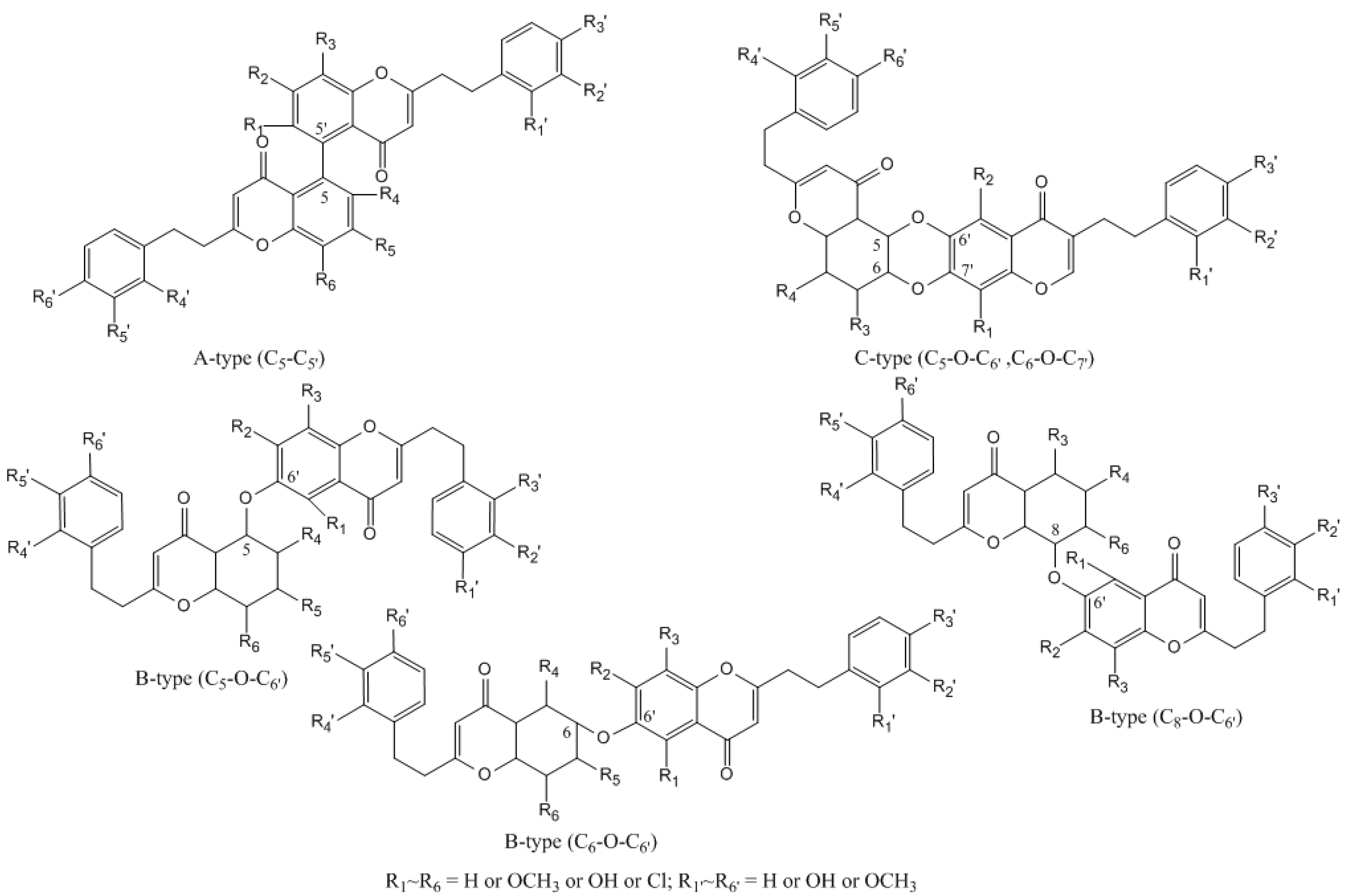
2.1.3. Identification of Bi-2-(2-phenylethyl)chromones in Agarwood
2.1.4. Identification of Tri-2-(2-phenylethyl)chromones in Agarwood
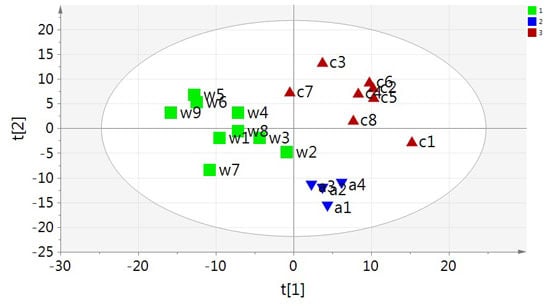
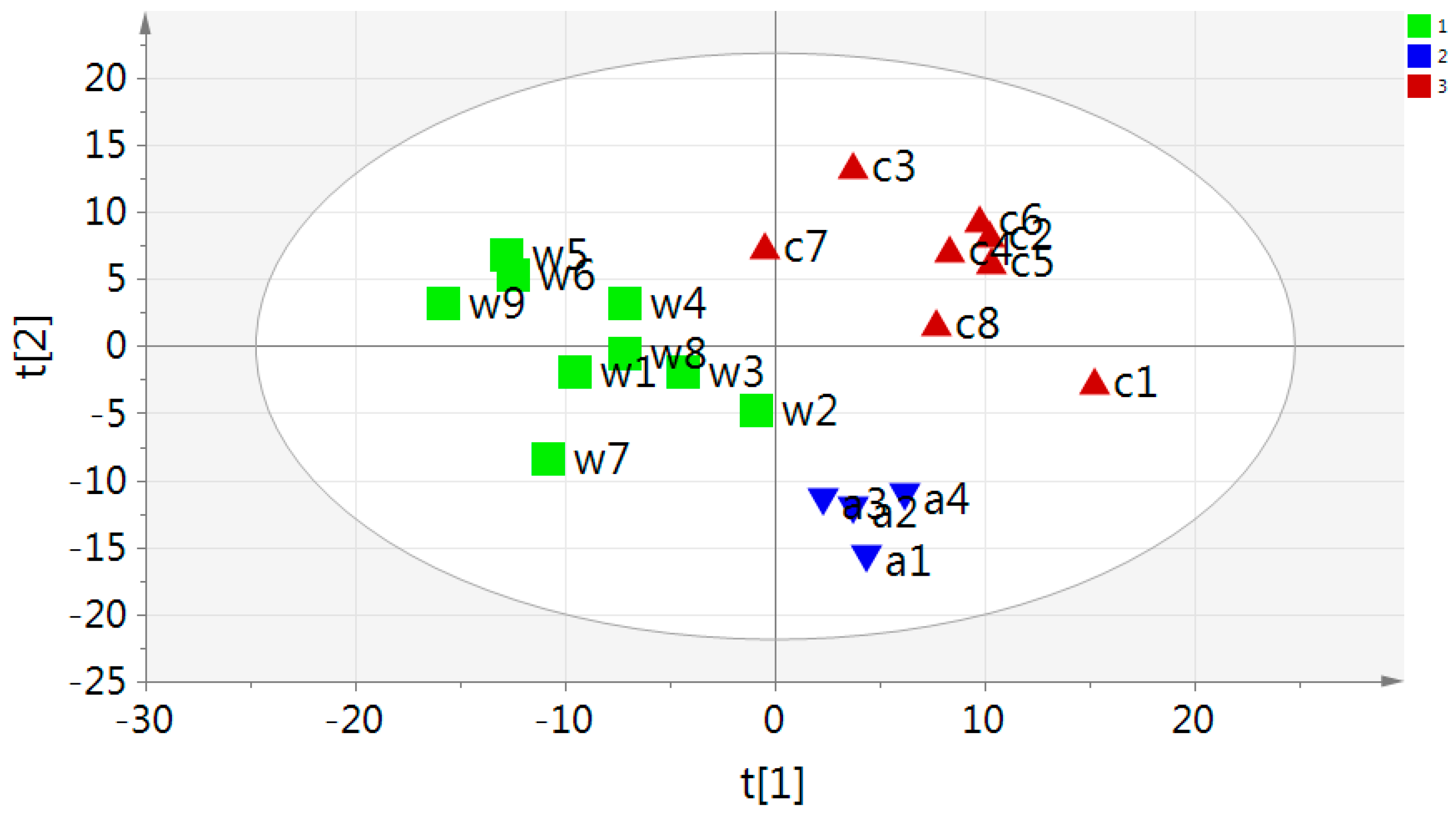
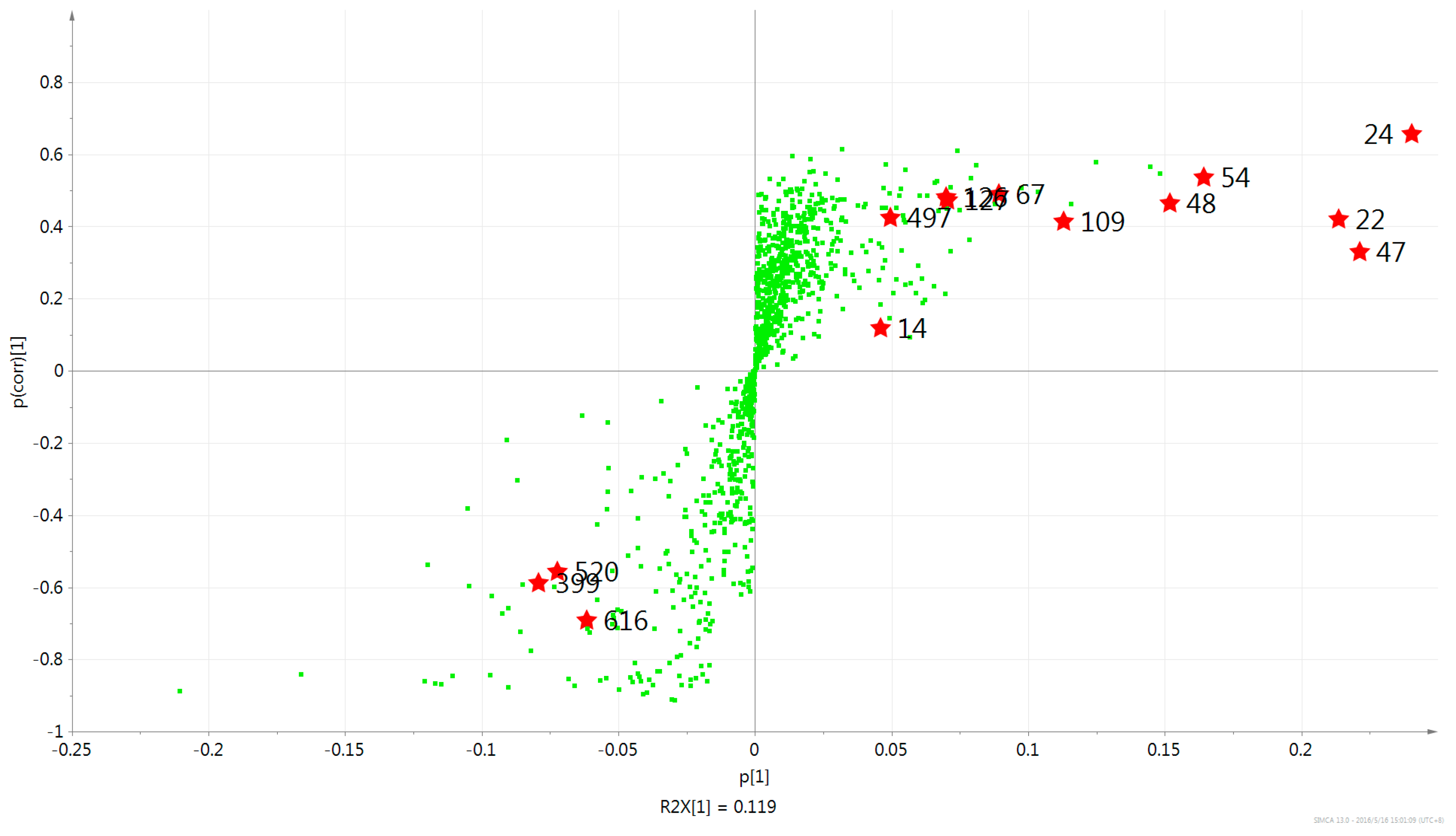
2.2. Multivariate PCA and OPLS-DA Analysis of UPLC-MS Data
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Plant Material
3.3. Sample Preparation
3.4. UPLC Condition
3.5. Mass Spectrometry
3.6. UPLC-MS Data Processing and Multivariate Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mei, W.L.; Yang, D.L.; Wang, H.; Yang, J.L.; Zeng, Y.B.; Guo, Z.K.; Dong, W.H.; Li, W.; Dai, H.F. Characterization and determination of 2-(2-phenylethyl)chromones in agarwood by GC-MS. Molecules 2013, 18, 12324–12345. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, C.; Espinoza, E. Evaluating agarwood products for 2-(2-phenylethyl)chromones using direct analysis in real time time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.; et al. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef] [PubMed]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Frag J. 2011, 26, 73–89. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of cgarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- IUCN Red List Committee. Red List of Threatened SpeciesTM Strategic Plan 2013-2020, Version 1.0. Available online: http://www.iucnredlist.org/documents/red_list_strategic_plan_2013_2020.pdf (accessed on 12 July 2015).
- Yang, L.; Qiao, L.R.; Xie, D.; Yuan, Y.H.; Chen, N.H.; Dai, J.G.; Guo, S.X. 2-(2-Phenylethyl)chromones from Chinese eaglewood. Phytochemistry 2012, 76, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kwon, S.W.; Hwang, G.S.; Park, J.H. Eight new 2-(2-phenylethyl)chromone (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv. Chim. Acta 2012, 95, 1657–1665. [Google Scholar] [CrossRef]
- Wu, B.; Lee, J.G.; Lim, C.J.; Jia, S.D.; Kwon, S.W.; Hwang, G.S.; Park, J.H. Sesquiterpenoids and 2-(2-phenylethyl)-4H-chromen-4-one (=2-(2-phenylethyl)-4H-1-benzopyran-4-one) derivatives from Aquilaria malaccensis agarwood. Helv. Chim. Acta 2012, 95, 636–642. [Google Scholar] [CrossRef]
- Gao, Y.H.; Liu, J.M.; Lu, H.X.; Wei, Z.X. Two new 2-(2-phenylethyl)chromen-4-ones from Aquilaria sinensis (Lour.) Gilg. Helv. Chim. Acta 2012, 95, 951–954. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Z.R.; Chai, X.Y.; Zeng, K.W.; Jia, Y.X.; Bi, D.; Ma, Z.Z.; Tu, P.F. Nine 2-(2-phenylethyl)chromone derivatives from the resinous wood of Aquilaria sinensis and their inhibition of LPS-induced NO production in RAW 264.7 Cells. Eur. J. Org. Chem. 2012, 27, 5389–5397. [Google Scholar] [CrossRef]
- Yang, D.L.; Wang, H.; Guo, Z.K.; Dong, W.H.; Mei, W.L.; Dai, H.F. A new 2-(2-phenylethyl)chromone derivative in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2014, 16, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. Planta Med. 2013, 79, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, E.O.; Lancaster, C.A.; Kreitals, N.M.; Hata, M.; Cody, R.B.; Blanchette, R.A. Distinguishing wild from cultivated agarwood (Aquilaria spp.) using direct analysis in real time and time of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Konoshima, T.; Shimada, Y.; Kiyosawa, S. Six new 2-(2-phenylethyl)chromones from agarwood. Chem. Pharm. Bull. 2002, 50, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Yagura, T.; Ito, M.; Kiuchi, F.; Honda, G.; Shimada, Y. Four new 2-(2-phenylethyl)chromone derivatives from withered wood of Aquilaria sinensis. Chem. Pharm. Bull. 2003, 51, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Wang, Y.L.; Su, Y.L. Studies on the chemical constituents of Aquilaria sinensis (Lour.) Gilg. IV. Isolation and characterization of 2-(2-phenylethyl)chromone derivatives. Acta Pharm. Sin. 1989, 24, 678–683. [Google Scholar]
- Liu, J.M.; Gao, Y.H.; Xu, H.H.; Chen, H.Y. Chemical constituents of Aquilaria sinensis (I). Chin. Tradit. Herbal Drugs 2006, 37, 325–327. [Google Scholar]
- Li, J.; Chen, D.; Jiang, Y.; Zhang, Q.; Zhang, L.; Tu, P.F. Identification and quantification of 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones in Chinese eaglewood by HPLC with diode array detection and MS. J. Sep. Sci. 2013, 36, 3733–3740. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T.; Iwagoe, K.; Sugimoto, A.; Kiyosawa, S.; Fujiwara, Y.; Shimada, Y. Studies on agalwood Jinko X. structures of 2-(2-phenylethyl)chromone derivatives. Chem. Pharm. Bull. 1991, 39, 207–209. [Google Scholar] [CrossRef]
- Shimada, Y.; Konishi, T.; Kiyosawa, S. Studies on the agalwood (Jinko). VI. Structures of three 2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone derivatives, AH1A, AH2a and AH2b. Chem. Pharm. Bull. 1986, 34, 3033–3037. [Google Scholar] [CrossRef]
- Iwagoe, K.; Kakae, T.; Konishi, T.; Kiyosawa, S.; Fujiwara, Y.; Shimada, Y.; Miyahara, K.; Kawasaki, T. Studies on the agalwood Jinko VIII. structures of biphenylethylchromone derivatives. Chem. Pharm. Bull. 1989, 37, 124–128. [Google Scholar] [CrossRef]
- Konishi, T.; Iwagoe, K.; Kiyosawa, S.; Fujiwara, Y. Studies on the agalwood (Jinko). XI. structure of dioxan-linked bi-phenylethylchromone derivative. Chem. Pharm. Bull. 1991, 39, 1869–1871. [Google Scholar] [CrossRef]
- Konishi, T.; Iwagoe, K.; Kiyosawa, S.; Fujiwara, Y. Tri-2-(2-phenylethyl)chromones from agalwood. Phytochemistry 1989, 28, 3548–3550. [Google Scholar] [CrossRef]
- Gao, X.X.; Xie, M.R.; Liu, S.F.; Guo, X.L.; Chen, X.Y.; Zhong, Z.J.; Wang, L.; Zhang, W.M. Chromatographic fingerprint analysis of metabolites in natural and artificial agarwood using gas chromatography-mass spectrometry combined with chemometric methods. J. Chromatogr. B 2014, 967, 264–273. [Google Scholar] [CrossRef] [PubMed]
| Peak | RT (min) | Formula | [M + H]+ | Error ppm | MS/MS Fragments | Substituent Group | Proposed Compound | |
|---|---|---|---|---|---|---|---|---|
| A Ring | B Ring | |||||||
| 1 | 8.12 | C17H14O4 | 283.0964 | −0.2 | 192, 164, 91 | OH, OH | − | 6,8-dihydroxy-2-(2-phenylethyl)chromone (isomer 1) |
| 2 | 10.6 | C18H16O6 | 329.1011 | −2.52 | 137 | OH, OH | OH, OCH3 | 6,8-dihydroxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone (isomer 1) |
| 3 | 10.8 | C17H14O4 | 283.0967 | 0.82 | 177, 107 | OH | OH | 6-hydroxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 2) |
| 4 | 11.22 | C17H14O4 | 283.0966 | 0.37 | 177, 107 | OH | OH | 6-hydroxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 3) |
| 5 | 11.25 | C18H16O6 | 329.1017 | −0.86 | 137 | OH, OH | OH, OCH3 | 6,8-dihydroxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone (isomer 2) |
| 6 | 12.08 | C17H14O4 | 283.0963 | −0.74 | 177, 107 | OH | OH | 6-hydroxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 4) |
| 7 | 12.11 | C18H16O5 | 313.1069 | −0.37 | 207, 192, 107 | OH, OCH3 | OH | 6-hydroxy-7-methoxy-2-[2-(4′-hydroxyphenyl)ethyl]chromone (isomer 1) |
| 8 | 12.49 | C19H18O6 | 343.1174 | −0.76 | 207, 137 | OCH3, OH | OCH3, OH | 6-hydroxy-7-methoxy-2-(4′-hydroxy-3′-methoxyphenethyl) chromone (isomer 1) |
| 9 | 12.71 | C17H14O4 | 283.0963 | −0.78 | 177, 107 | OH | OH | 6-hydroxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 5) |
| 10 | 12.78 | C19H18O6 | 343.1173 | −0.8 | 206, 137 | OCH3, OH | OCH3, OH | 6-hydroxy-7-methoxy-2-(4′-hydroxy-3′-methoxyphenethyl) chromone (isomer 2) |
| 11 | 12.94 | C19H18O6 | 343.1173 | −0.77 | 206, 137 | OCH3, OH | OCH3, OH | 6-hydroxy-7-methoxy-2-(4′-hydroxy-3′-methoxyphenethyl) chromone (isomer 3) |
| 12 | 12.99 | C18H16O5 | 313.1067 | −0.98 | 177, 137 | OH | OH, OCH3 | 6-hydroxy-2-[2-(4′-hydroxy-3′-methoxy phenyl)ethyl]chromone (isomer 1) |
| 13 | 13.1 | C17H14O4 | 283.0962 | −0.87 | 192, 176, 91 | OH, OH | − | 6,8-dihydroxy-2-(2-phenylethyl)chromone (isomer 2) |
| 14 | 13.45 | C18H16O5 | 313.1067 | −0.96 | 177, 137 | OH | OH, OCH3 | 6-hydroxy-2-[2-(4′-hydroxy-3′-methoxy phenyl)ethyl]chromone (isomer 2) |
| 15 | 13.67 | C18H16O5 | 313.1071 | 0.17 | 121 | OH, OH | OCH3 | 6,7-dihydroxy-2-[2-(4′-methoxyphenyl)ethyl]chromone (isomer 1) |
| 16 | 13.69 | C19H18O5 | 327.1224 | −0.78 | 221, 177, 107 | OCH3, OCH3 | OH | qinanone g (isomer 1) |
| 17 | 13.84 | C17H14O4 | 283.0965 | 0.02 | 192, 164, 91 | OH, OH | − | 6,8-dihydroxy-2-(2-phenylethyl)chromone (isomer 3) |
| 18 | 13.87 | C18H16O4 | 297.112 | −0.6 | 206, 167, 91 | OH, OCH3 | − | 6-hydroxy-7-methoxy-2-[2-phenylethyl] chromone (isomer 1) |
| 19 | 14.36 | C17H14O4 | 283.0965 | 0.2 | 177, 107 | OH | OH | 6-hydroxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 1) |
| 20 | 14.42 | C17H14O3 | 267.1016 | 0.16 | 161, 107 | − | OH | qinanone d (isomer 1) |
| 21 | 14.5 | C19H18O5 | 327.1228 | 0.4 | 221, 177, 107 | OCH3, OCH3 | OH | qinanone g (isomer 2) |
| 22 | 14.74 | C20H20O6 | 357.1333 | 0.06 | 221, 137 | OCH3, OCH3 | OH, OCH3 | 6,7-dimethoxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone (isomer 1) |
| 23 | 14.9 | C19H18O5 | 327.1226 | −0.3 | 236, 220, 207, 192, 91 | OH, OCH3, OCH3 | − | 8-hydroxy-6,7-dimethoxy-2-(2-phenethyl)chromone |
| 24 | 14.92 | C18H16O5 | 313.1072 | 0.63 | 121 | OH, OH | OCH3 | 6,7-dihydroxy-2-[2-(4′-methoxyphenyl)ethyl]chromone (isomer 2) |
| 25 | 15.14 | C17H14O4 | 283.0966 | 0.27 | 192, 153, 91 | OH, OH | − | 6,8-dihydroxy-2-(2-phenylethyl)chromone (isomer 4) |
| 26 | 15.31 | C20H20O6 | 357.133 | −0.64 | 220, 137 | OCH3, OCH3 | OH, OCH3 | 6,7-dimethoxy-2-[2-(3′-hydroxy-4′-methoxyphenyl)ethyl]chromone (isomer 2) |
| 27 | 15.58 | C17H14O3 | 267.1014 | −0.54 | 161, 107 | − | OH | qinanone d (isomer 2) |
| 28 | 15.87 | C17H14O3 | 267.1013 | −0.99 | 176, 91 | OH | − | 7-hydroxy-2-(2-phenylethyl)chromone (isomer 1) |
| 29 | 15.87 | C18H16O4 | 297.1119 | −0.63 | 191, 107 | OCH3 | OH | 6-methoxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 2) |
| 30 | 16.37 | C19H18O5 | 327.1227 | 0.02 | 220, 177, 107 | OCH3, OCH3 | OH | qinanone g (isomer 3) |
| 31 | 17.05 | C18H16O4 | 297.1121 | −0.03 | 191, 107 | OCH3 | OH | 6-methoxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 3) |
| 32 | 17.15 | C17H14O3 | 267.1013 | −0.85 | 176, 91 | OH | − | 7-hydroxy-2-(2-phenylethyl)chromone (isomer 2) |
| 33 | 17.32 | C19H18O5 | 327.1228 | 0.35 | 191, 137 | OCH3 | OH, OCH3 | 6-methoxy-2-[2-(4′-hydroxy-3′-methoxyphenyl)ethyl]chromone (isomer 1) |
| 34 | 17.55 | C18H16O4 | 297.1121 | −0.06 | 206, 167, 91 | OH, OCH3 | − | 6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone (isomer 2) |
| 35 | 17.6 | C17H14O3 | 267.1014 | −0.46 | 161, 107 | − | OH | qinanone d (isomer 3) |
| 36 | 17.8 | C18H16O4 | 297.112 | −0.49 | 206, 167, 91 | OH, OCH3 | − | 6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone (isomer 4) |
| 37 | 17.97 | C19H18O5 | 327.1224 | −0.92 | 191, 137 | OCH3 | OH, OCH3 | 6-methoxy-2-[2-(4′-hydroxy-3′-methoxyphenyl)ethyl]chromone (isomer 2) |
| 38 | 18.37 | C19H18O6 | 343.1174 | −0.69 | 207, 137 | OCH3, OH | OCH3, OH | 6-hydroxy-7-methoxy-2-(4′-hydroxy-3′-methoxyphenethyl) chromone (isomer 4) |
| 39 | 18.38 | C18H16O4 | 297.1121 | −0.15 | 176, 121 | OH | OCH3 | 6-hydroxy-2-[2-(4′-methoxyphenyl)ethyl] chromone |
| 40 | 18.38 | C19H18O5 | 327.1225 | −0.5 | 121 | OH, OCH3 | OCH3 | 7-hydroxy-6-methoxy-2-(4′-methoxyphenethyl)chromone (isomer 1) |
| 41 | 18.45 | C18H16O5 | 313.107 | −0.09 | 206, 191, 107 | OH, OCH3 | OH | 6-hydroxy-7-methoxy-2-[2-(4′-hydroxyphenyl)ethyl]chromone (isomer 2) |
| 42 | 18.69 | C17H14O3 | 267.1018 | 0.68 | 176, 91 | OH | − | 7-hydroxy-2-(2-phenylethyl)chromone (isomer 3) |
| 43 | 18.99 | C19H18O6 | 343.1173 | −0.93 | 137 | OCH3, OH | OCH3, OH | 6-hydroxy-7-methoxy-2-(4′-hydroxy-3′-methoxyphenethyl) chromone (isomer 5) |
| 44 | 19.13 | C18H16O4 | 297.112 | −0.51 | 191, 107 | OCH3 | OH | 6-methoxy-2-[2-(4′-hydroxyphenyl)ethyl] chromone (isomer 1) |
| 45 | 19.35 | C18H16O5 | 313.1073 | 0.74 | 121 | OH, OH | OCH3 | 6,7-dihydroxy-2-[2-(4′-methoxyphenyl)ethyl]chromone (isomer 3) |
| 46 | 19.58 | C17H14O4 | 283.0962 | −0.96 | 192, 153, 91 | OH, OH | − | 6,8-dihydroxy-2-(2-phenylethyl)chromone (isomer 5) |
| 47 | 20.35 | C18H16O5 | 313.1067 | −0.99 | 207, 192, 107 | OH, OCH3 | OH | 6-hydroxy-7-methoxy-2-[2-(4′-hydroxyphenyl)ethyl]chromone (isomer 3) |
| 48 | 20.63 | C20H20O5 | 341.1386 | 0.83 | 220, 121 | OCH3, OCH3 | OCH3 | 6,7-dimethoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone |
| 49 | 20.91 | C18H16O5 | 313.1076 | 1.6 | 207, 178, 91 | OH, OH, OCH3 | − | 6,8-dihydroxy-7-methoxy-2-(2-phenylethyl)chromone |
| 50 | 20.99 | C19H18O4 | 311.1281 | 0.97 | 220, 205, 177, 91 | OCH3, OCH3 | − | 6,7-dimethoxy-2-(2-phenylethyl)chromone |
| 51 | 21.36 | C17H14O4 | 283.0967 | 0.81 | 192, 164, 153, 91 | OH, OH | − | 6,8-dihydroxy-2-(2-phenylethyl)chromone (isomer 6) |
| 52 | 22.51 | C18H16O3 | 281.1173 | 0.25 | 160, 121 | − | OCH3 | 2-[2-(4′-methoxyphenyl)ethyl]chromone |
| 53 | 22.89 | C17H14O2 | 251.1068 | 0.44 | 160, 91 | − | − | 2-(2-phenylethyl)chromone |
| 54 | 23.41 | C18H16O3 | 281.1172 | −0.15 | 190, 91 | OCH3 | − | 6-methoxy-2-(2-phenylethyl)chromone (isomer 1) |
| 55 | 23.43 | C18H15ClO4 | 331.0733 | 0.46 | 121 | OH, Cl | OCH3 | 8-chloro-6-hydroxy-2-(4′-methoxyphenethyl)chromone |
| 56 | 23.98 | C17H13ClO3 | 301.0626 | 0.16 | 210, 170, 91 | OH, Cl | − | 8-chloro-6-hydroxy-2-(2-phenethyl) chromone |
| 57 | 24.1 | C19H18O4 | 311.128 | 0.69 | 190, 121 | OCH3 | OCH3 | 6-methoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone |
| 58 | 24.51 | C18H16O3 | 281.1175 | 0.94 | 190, 91 | OCH3 | − | 6-methoxy-2-(2-phenylethyl)chromone (isomer 2) |
| 59 | 25.63 | C19H18O5 | 327.1229 | 0.59 | 206, 121 | OH, OCH3 | OCH3 | 7-hydroxy-6-methoxy-2-(4′-methoxyphenethyl)chromone (isomer 2) |
| 60 | 26.14 | C18H16O4 | 297.1124 | 0.92 | 206, 167, 91 | OH, OCH3 | − | 6-hydroxy-7-methoxy-2-(2-phenylethyl)chromone (isomer 3) |
| 61 | 6.21 | C18H20O8 | 365.1223 | −2.08 | 137 | OH, OH, OH, OH | OH, OCH3 | aquilarone a |
| 62 | 6.52 | C17H18O7 | 335.1126 | 0.21 | 317, 299, 271, 243, 107 | OH, OH, OH, OH | OH | aquilarone f (isomer 1) |
| 63 | 7.23 | C17H18O7 | 335.1126 | 0.09 | 317, 299, 271, 243, 107 | OH, OH, OH, OH | OH | aquilarone f (isomer 2) |
| 64 | 7.8 | C17H16O5 | 301.107 | −0.3 | 283, 255, 227, 192, 164, 91 | OH, OH, –O– | − | 2,3-dihydroxy-5-phenethyl-2,3-dihydro-1ah-oxireno[2,3-f]chromen-7(7bh)-one (isomer 1) |
| 65 | 8.11 | C17H16O5 | 301.1072 | 0.49 | 283,255,227,192,164,91 | OH, OH, –O– | − | 2,3-dihydroxy-5-phenethyl-2,3-dihydro-1ah-oxireno[2,3-f]chromen-7(7bh)-one (isomer 2) |
| 66 | 8.12 | C17H18O6 | 319.1178 | 0.6 | 301, 283, 255, 227, 164, 91 | OH, OH, OH, OH | − | agarotetrol |
| 67 | 8.37 | C18H20O7 | 349.1282 | −0.03 | 331, 313, 285, 121 | OH, OH, OH, OH | OCH3 | 5,6,7,8-tetrahydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone (isomer 1) |
| 68 | 8.47 | C17H16O5 | 301.1065 | −1.83 | 283, 255, 227, 192, 164, 91 | OH, OH, –O– | _ | 2,3-dihydroxy-5-phenethyl-2,3-dihydro-1ah-oxireno[2,3-f]chromen-7(7bh)-one (isomer 3) |
| 69 | 8.65 | C17H18O6 | 319.1178 | 0.61 | 301, 283, 255, 227, 164, 91 | OH, OH, OH, OH | _ | aquilarone b |
| 70 | 8.66 | C17H16O5 | 301.107 | −0.04 | 283, 255, 227, 192, 164, 91 | OH, OH, –O– | _ | 2,3-dihydroxy-5-phenethyl-2,3-dihydro-1ah-oxireno[2,3-f]chromen-7(7bh)-one (isomer 4) |
| 71 | 8.85 | C18H20O7 | 349.1279 | −0.83 | 331, 313, 285, 121 | OH, OH, OH, OH | OCH3 | 5,6,7,8-tetrahydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone (isomer 2) |
| 72 | 8.92 | C18H20O6 | 333.1333 | 0.01 | 301, 283, 255, 227, 164, 91 | OH, OH, OH, OCH3 | _ | 5,6,7-trihydroxy-8-methoxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone (isomer 1) |
| 73 | 8.95 | C17H18O5 | 303.1225 | −0.75 | 285, 267, 239, 211, 194, 176, 91 | OH, OH, OH | 5,6,7-trihydroxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone (isomer 1) | |
| 74 | 9.11 | C18H20O6 | 333.133 | −0.89 | 315, 121 | OH, OH, OH | OCH3 | 5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone (isomer 1) |
| 75 | 9.61 | C18H20O6 | 333.1334 | 0.44 | 301, 283, 255, 227, 164, 91 | OH, OH, OH, OCH3 | _ | 5,6,7-trihydroxy-8-methoxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone (isomer 2) |
| 76 | 9.64 | C17H18O5 | 303.1224 | −0.86 | 285, 267, 239, 211, 194, 176, 91 | OH, OH, OH | _ | 5,6,7-trihydroxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone (isomer 2) |
| 77 | 10.22 | C17H18O5 | 303.1224 | −0.84 | 285, 267, 239, 211, 194, 176, 91 | OH, OH, OH | _ | 5,6,7-trihydroxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone (isomer 3) |
| 78 | 10.32 | C18H20O6 | 333.1331 | −0.58 | 315, 121 | OH, OH, OH | OCH3 | 5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone (isomer 1) |
| 79 | 10.61 | C17H18O4 | 287.1276 | −0.7 | 269, 251, 178, 160, 91 | OH, OH | _ | 6,7-dihydroxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone |
| 80 | 10.77 | C17H18O5 | 303.1221 | −2.02 | 285, 267, 239, 211, 194, 176, 91 | OH, OH, OH | _ | 5,6,7-trihydroxy-5,6,7,8-tetrahydro-2-(2-phenylethyl)chromone (isomer 4) |
| 81 | 10.8 | C17H16O5 | 301.107 | −0.04 | 283, 255, 227, 192, 164, 91 | OH, OH, –O– | _ | 2,3-dihydroxy-5-phenethyl-2,3-dihydro-1ah-oxireno[2,3-f]chromen-7(7bh)-one (isomer 5) |
| 82 | 11.52 | C17H17ClO5 | 337.0837 | −0.08 | 319, 301, 283, 265, 192, 91 | OH, OH, OH, Cl | _ | 8-chloro-5,6,7-trihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone (isomer 1) |
| 83 | 11.64 | C18H19ClO6 | 367.0945 | 0.44 | 349, 121 | OH, OH, OH, Cl | OCH3 | 8-chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromene (isomer 1) |
| 84 | 11.68 | C17H17ClO5 | 337.0835 | −0.66 | 319, 301, 283, 265, 192, 91 | OH, OH, OH, Cl | _ | 8-chloro-5,6,7-trihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone (isomer 2) |
| 85 | 12.01 | C17H16O5 | 301.107 | -0.32 | 283, 255, 227, 192, 164, 91 | OH, OH, –O– | _ | 2,3-dihydroxy-5-phenethyl-2,3-dihydro-1ah-oxireno[2,3-f]chromen-7(7bh)-one (isomer 6) |
| 86 | 12.82 | C18H19ClO6 | 367.093 | −3.41 | 349, 121 | OH, OH, OH, Cl | OCH3 | 8-chloro-5,6,7-trihydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromene (isomer 2) |
| 87 | 12.91 | C17H17ClO5 | 337.0839 | 0.53 | 319, 301, 283, 265, 192, 9 | OH, OH, OH, Cl | _ | 8-chloro-5,6,7-trihydroxy-2-(2-phenylethyl)-5,6,7,8-tetrahydrochromone (isomer 3) |
| Peak | RT (min) | Formula | [M + H]+ | Error ppm | MS/MS Fragments | Proposed Compound |
|---|---|---|---|---|---|---|
| 88 | 16.68 | C34H30O9 | 583.1965 | 0.37 | 565, 547, 355, 302, 283 | AH15 (isomer 1) (B-type) |
| 89 | 17.39 | C34H30O9 | 583.1966 | 0.59 | 565, 547, 519, 459, 441, 283, 255, 228, 177 | AH15 (isomer 2) (B-type) |
| 90 | 18.3 | C34H30O9 | 583.1951 | −1.97 | 565, 547, 519, 415, 283, 192, 91 | AH15 (isomer 3) (B-type) |
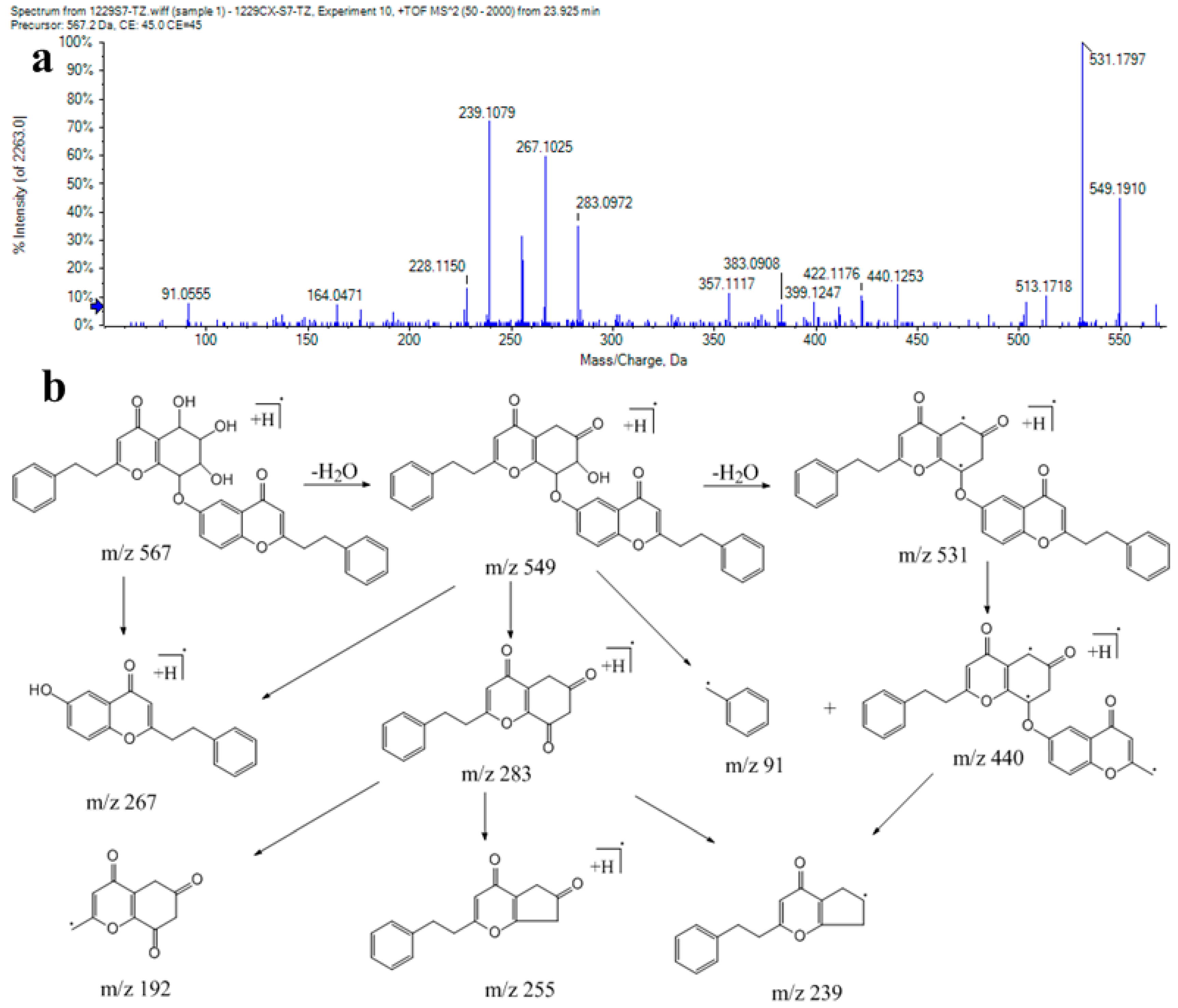
| 91 | 18.85 | C34H30O8 | 567.2014 | 0.14 | 549, 531, 503, 283, 267, 239, 91 | AH13 (isomer 1) (B-type) |
| 92 | 18.86 | C34H30O9 | 583.1959 | −0.68 | 565, 547, 519, 302, 283, 192, 91 | AH15 (isomer 4) (B-type) |
| 93 | 19.36 | C35H32O9 | 597.2112 | −1.21 | 579, 561, 297, 255, 121 | AH12 (isomer 1) (B-type) |
| 94 | 19.44 | C34H28O8 | 565.1847 | −1.81 | 547, 519, 283, 91 | AH21 (isomer 1) (C-type) |
| 95 | 19.47 | C34H30O9 | 583.195 | −2.14 | 547, 519, 283, 255, 192, 91 | AH15 (isomer 5) (B-type) |
| 96 | 19.52 | C34H30O8 | 567.2005 | −1.51 | 549, 531, 503, 283, 267, 239, 228, 192, 91 | AH13 (isomer 2)(B-type) |
| 97 | 19.84 | C34H30O9 | 583.1954 | −1.51 | 565, 547, 519, 301, 283, 192, 91 | AH15 (isomer 6) (B-type) |
| 98 | 19.95 | C35H32O9 | 597.2115 | −0.7 | 579, 561, 297, 283, 255, 206, 192, 121 | AH12 (isomer 2) (B-type) |
| 99 | 20.16 | C34H30O8 | 567.2008 | −0.89 | 549, 531, 513, 440, 283, 267, 255, 192, 176, 91 | AH13 (isomer 3) (B-type) |
| 100 | 20.42 | C34H28O8 | 565.184 | −3.02 | 547, 519, 301, 283, 192, 91 | AH21 (isomer 2) (C-type) |
| 101 | 20.77 | C34H30O9 | 583.1959 | −0.69 | 565, 547, 519, 267, 255, 91 | AH15 (isomer 7) (B-type) |
| 102 | 21.09 | C34H28O8 | 565.1855 | −0.42 | 547, 283, 192, 91 | AH21 (isomer 3) (C-type) |
| 103 | 21.48 | C35H32O9 | 597.2115 | −0.69 | 597, 561, 297, 255, 206 | AH12 (isomer 3) (B-type) |
| 104 | 21.61 | C34H30O9 | 583.1962 | −0.07 | 565, 547, 456, 283, 192, 91 | AH15 (isomer 8) (B-type) |
| 105 | 22.11 | C34H26O7 | 547.1742 | −1.76 | 529, 456, 282, 267, 91 | hydroxy AH11 (isomer 1)(A-type) |
| 106 | 22.19 | C34H30O9 | 583.1957 | −0.88 | 547, 519, 302, 283, 256, 91 | AH15 (isomer 9) (B-type) |
| 107 | 22.29 | C35H32O9 | 597.2113 | −1.08 | 579, 561, 297, 283, 267, 239, 206, 192, 121 | AH12 (isomer 4) (B-type) |
| 108 | 22.44 | C34H30O8 | 567.2004 | −1.69 | 549, 531, 513, 283, 255, 176, 91 | AH13 (isomer 4) (B-type) |
| 109 | 22.47 | C34H28O8 | 565.1853 | −0.73 | 547, 519, 283, 192, 91 | AH21 (isomer 4) (C-type) |
| 110 | 22.6 | C35H32O9 | 597.2108 | −1.9 | 579, 561, 297, 255, 239, 192, 121 | AH12 (isomer 5) (B-type) |
| 111 | 22.86 | C34H30O9 | 583.1959 | −0.58 | 547, 519, 302, 281, 192, 91 | AH15 (isomer 10) (B-type) |
| 112 | 23.13 | C34H26O7 | 547.1746 | −0.89 | 529, 456, 282, 267, 91 | hydroxy AH11 (isomer 2 ) (A-type) |
| 113 | 23.17 | C34H30O8 | 567.2008 | −0.96 | 549, 531, 440, 283, 267, 239, 192, 176, 91 | AH13 (isomer 5) (B-type) |
| 114 | 23.38 | C34H30O9 | 583.1955 | −1.24 | 547, 519, 302, 281, 267, 91 | AH15 (isomer 11) (B-type) |
| 115 | 23.53 | C35H32O9 | 597.2108 | −1.89 | 579, 561, 313, 269, 121 | AH12 (isomer 6) (B-type) |
| 116 | 23.55 | C34H28O8 | 565.1851 | −0.97 | 547, 519, 409, 283, 255, 192, 91 | AH21 (isomer 5) (C-type) |
| 117 | 23.98 | C34H30O8 | 567.2008 | −0.89 | 549, 531, 513, 440, 283, 267, 239, 91 | AH13 (isomer 6) (B-type) |
| 118 | 24.47 | C34H30O9 | 583.196 | −0.53 | 547, 415, 399, 303, 283 | AH15 (isomer 12) (B-type) |
| 119 | 25.15 | C34H30O9 | 583.1963 | 0.1 | 547, 301, 283, 192, 91 | AH15 (isomer 13) (B-type) |
| 120 | 25.21 | C35H30O8 | 579.201 | −0.54 | 561, 543, 389, 371, 280 | methoxy AH21 (isomer 1) (C-type) |
| 121 | 25.25 | C34H28O8 | 565.1853 | −0.74 | 547, 519, 474, 456, 283, 91 | AH21 (isomer 6) (C-type) |
| 122 | 25.88 | C35H28O7 | 561.1905 | −0.58 | 470, 401, 121 | methoxy AH11 (isomer 1) (A-type) |
| 123 | 26.37 | C34H26O6 | 531.1798 | −0.79 | 440, 267, 91 | AH11 (isomer 1) (A-type) |
| 124 | 26.46 | C35H28O7 | 561.1903 | −0.94 | 470, 401, 121 | methoxy AH11 (isomer 2)(A-type) |
| 125 | 26.93 | C35H30O8 | 579.2006 | −1.36 | 561, 488, 470 | methoxy AH21(isomer 2) (C-type) |
| 126 | 27.06 | C34H26O7 | 547.1747 | −0.85 | 529, 456, 282, 267, 91 | hydroxy AH11(isomer 3)(A-type) |
| 127 | 27.37 | C34H28O8 | 565.1844 | −2.22 | 547, 529, 474, 439, 373, 283, 176, 91 | AH21 (isomer 7) (C-type) |
| 128 | 27.7 | C35H30O8 | 579.2007 | −1.13 | 561, 543, 529, 470, 283 | methoxy AH21 (isomer 3) (C-type) |
| 129 | 28.07 | C34H26O7 | 547.1743 | −1.5 | 529, 456, 282, 267, 91 | hydroxy AH11(isomer 4)(A-type) |
| 130 | 28.42 | C34H26O6 | 531.1797 | −0.9 | 440, 267, 91 | AH11 (isomer 2) (A-type) |
| 131 | 28.45 | C35H30O8 | 579.201 | −0.55 | 561, 543, 487, 458, 283, 121 | methoxy AH21(isomer 4) (C-type) |
| 132 | 28.64 | C34H28O7 | 549.1903 | −0.93 | 531, 458 | dehydroxy AH21 (C-type) |
| 133 | 28.7 | C34H29O7Cl | 585.1675 | 0.11 | 319, 301, 267, 176 | 6-((8-chloro-6,7-dihydroxy-4-oxo-2-phenethyl-5,6,7,8-tetrahydro-4H-chromen-5-yl)oxy)-2-phenethylchromone (B-type) |
| 134 | 28.87 | C34H26O7 | 547.1739 | −2.24 | 529, 456, 282, 267, 91 | hydroxy AH11(isomer 5) (A-type) |
| 135 | 29.07 | C34H26O6 | 531.1792 | −1.84 | 440, 267, 91 | AH11 (isomer 3) (A-type) |
| 136 | 29.37 | C35H28O7 | 561.1905 | −0.55 | 470, 401, 121 | methoxy AH11 (isomer 3) (A-type) |
| 137 | 29.52 | C34H26O7 | 547.1741 | −1.79 | 529, 456, 282, 267, 91 | hydroxy AH11(isomer 6) (A-type) |
| 138 | 29.56 | C34H26O6 | 531.1799 | −0.65 | 440, 267, 91 | AH11 (isomer 4) (A-type) |
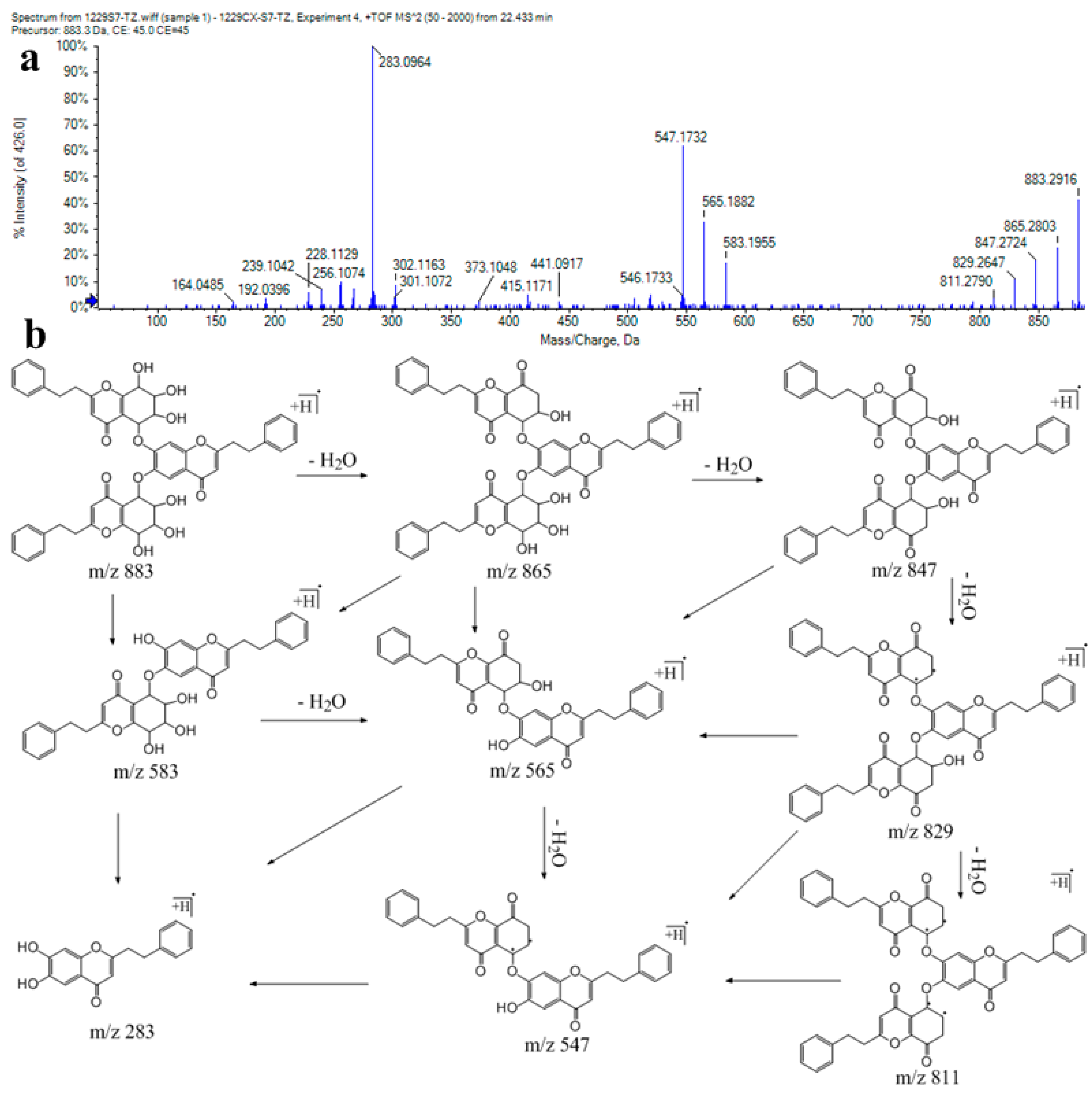
| 139 | 22.43 | C51H46O14 | 883.2958 | −0.29 | 865, 847, 829, 811, 583, 565, 547, 283 | tri-2-(2-phenylethyl)chromone (isomer 1) |
| 140 | 22.93 | C51H46O14 | 883.2957 | −0.4 | 865, 847, 829, 811, 583, 565, 547, 283 | tri-2-(2-phenylethyl)chromone (isomer 2) |
| 141 | 24.5 | C51H46O14 | 883.296 | −0.07 | 865, 847, 829, 811, 583, 565, 547, 301, 283 | tri-2-(2-phenylethyl)chromone (isomer 3) |
| Peak | Variable ID | VIP | Formula | Proposed Compound |
|---|---|---|---|---|
| 50 | 47 | 7.70 | C19H18O4 | 6,7-dimethoxy-2-(2-phenylethyl)chromone |
| 13 | 24 | 7.50 | C17H14O4 | 6,8-dihydroxy-2-(2-phenylethyl)chromone |
| 58 | 22 | 6.83 | C17H14O3 | 6-methoxy-2-(2-phenylethyl)chromone |
| 57 | 48 | 4.81 | C19H18O4 | 6-methoxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
| 41 | 54 | 4.52 | C18H16O5 | 6-hydroxy-7-methoxy-2-[2-(4′-hydroxyphenyl)ethyl]chromone |
| 48 | 109 | 4.01 | C20H20O5 | 6,7-dimethoxy-2-[2-(4-methoxyphenyl)ethyl]chromone |
| 42 | 14 | 3.06 | C17H14O3 | 7-hydroxy-2-(2-phenylethyl)chromone |
| 69 | 67 | 2.62 | C17H18O6 | Aquilarone B |
| 123 | 399 | 2.13 | C34H26O6 | AH11 |
| 113 | 520 | 2.11 | C34H30O8 | AH13 |
| 71 | 127 | 1.94 | C18H20O7 | 5,6,7,8-tetrahydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone |
| 67 | 126 | 1.91 | C18H20O7 | 5,6,7,8-tetrahydroxy-2-(4-methoxyphenethyl)-5,6,7,8-tetrahydrochromone |
| 110 | 616 | 1.68 | C35H32O9 | AH12 |
| 94 | 497 | 1.49 | C34H28O8 | AH21 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sheng, N.; Wang, L.; Li, S.; Chen, J.; Lai, X. Analysis of 2-(2-Phenylethyl)chromones by UPLC-ESI-QTOF-MS and Multivariate Statistical Methods in Wild and Cultivated Agarwood. Int. J. Mol. Sci. 2016, 17, 771. https://doi.org/10.3390/ijms17050771
Li Y, Sheng N, Wang L, Li S, Chen J, Lai X. Analysis of 2-(2-Phenylethyl)chromones by UPLC-ESI-QTOF-MS and Multivariate Statistical Methods in Wild and Cultivated Agarwood. International Journal of Molecular Sciences. 2016; 17(5):771. https://doi.org/10.3390/ijms17050771
Chicago/Turabian StyleLi, Yuanbin, Nan Sheng, Lingli Wang, Shijie Li, Jiannan Chen, and Xiaoping Lai. 2016. "Analysis of 2-(2-Phenylethyl)chromones by UPLC-ESI-QTOF-MS and Multivariate Statistical Methods in Wild and Cultivated Agarwood" International Journal of Molecular Sciences 17, no. 5: 771. https://doi.org/10.3390/ijms17050771
APA StyleLi, Y., Sheng, N., Wang, L., Li, S., Chen, J., & Lai, X. (2016). Analysis of 2-(2-Phenylethyl)chromones by UPLC-ESI-QTOF-MS and Multivariate Statistical Methods in Wild and Cultivated Agarwood. International Journal of Molecular Sciences, 17(5), 771. https://doi.org/10.3390/ijms17050771