Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence
Abstract
:1. Introduction
2. Results
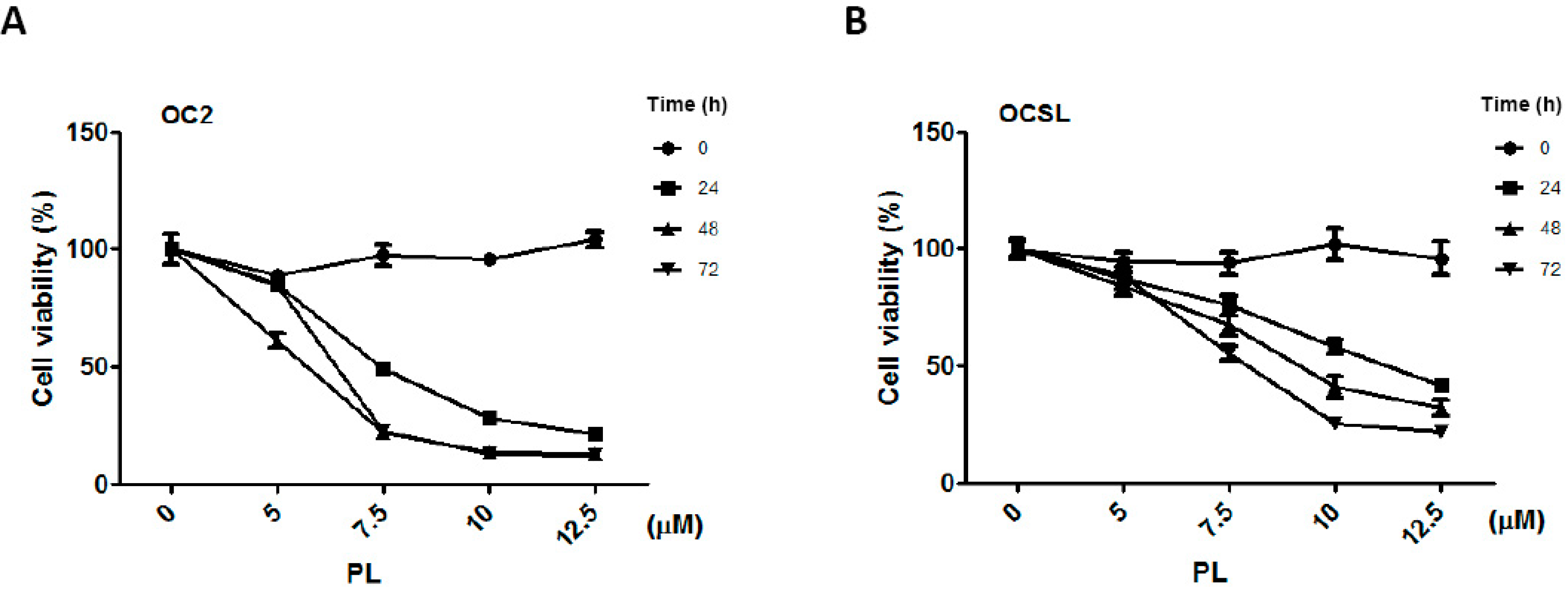
2.1. Piperlongumine Suppresses the Growth of Human Oral Squamous Cell Carcinoma
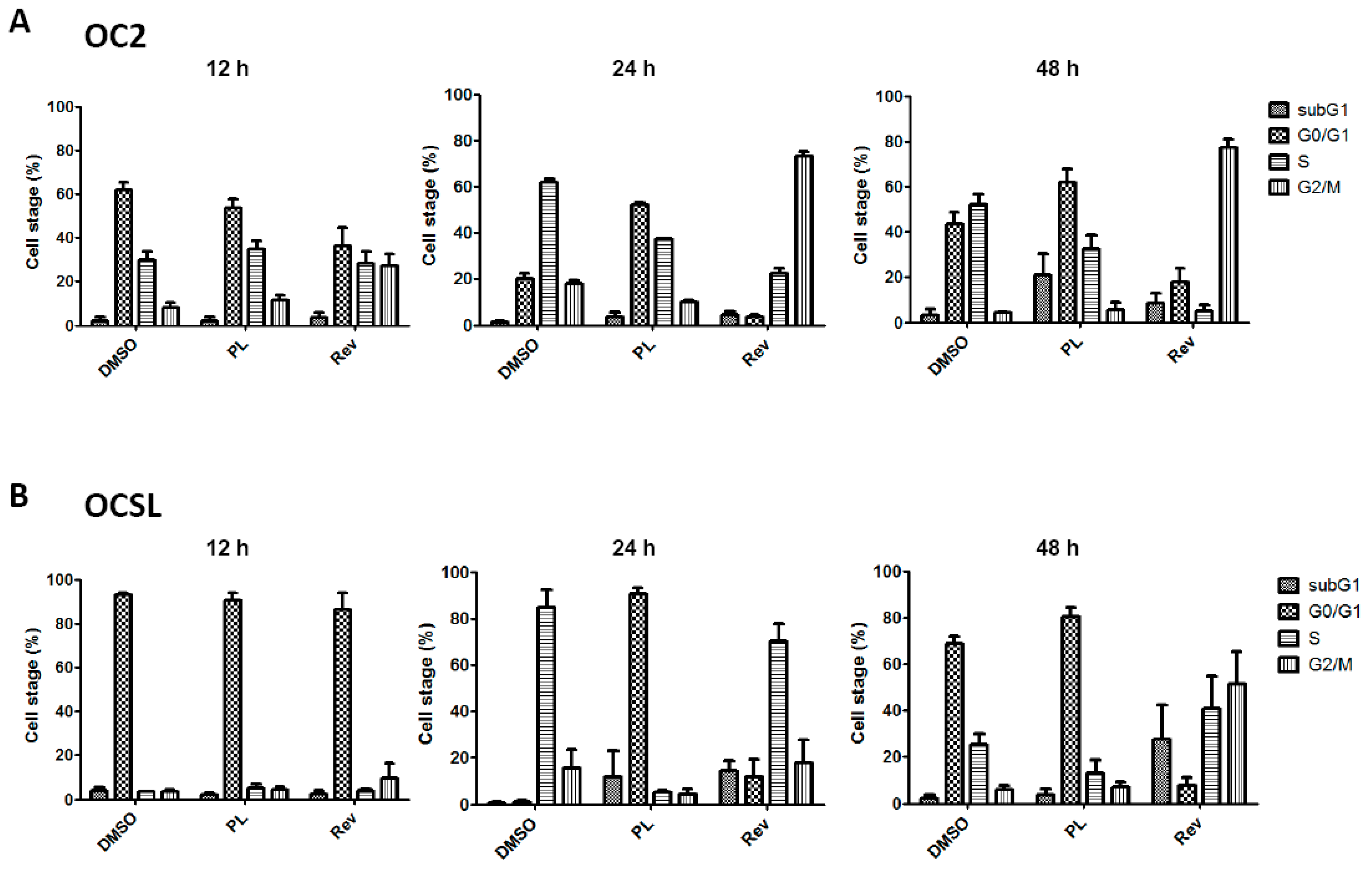
2.2. Piperlongumine Induces G1 Phase Arrest in Human Oral Squamous Cell Carcinoma
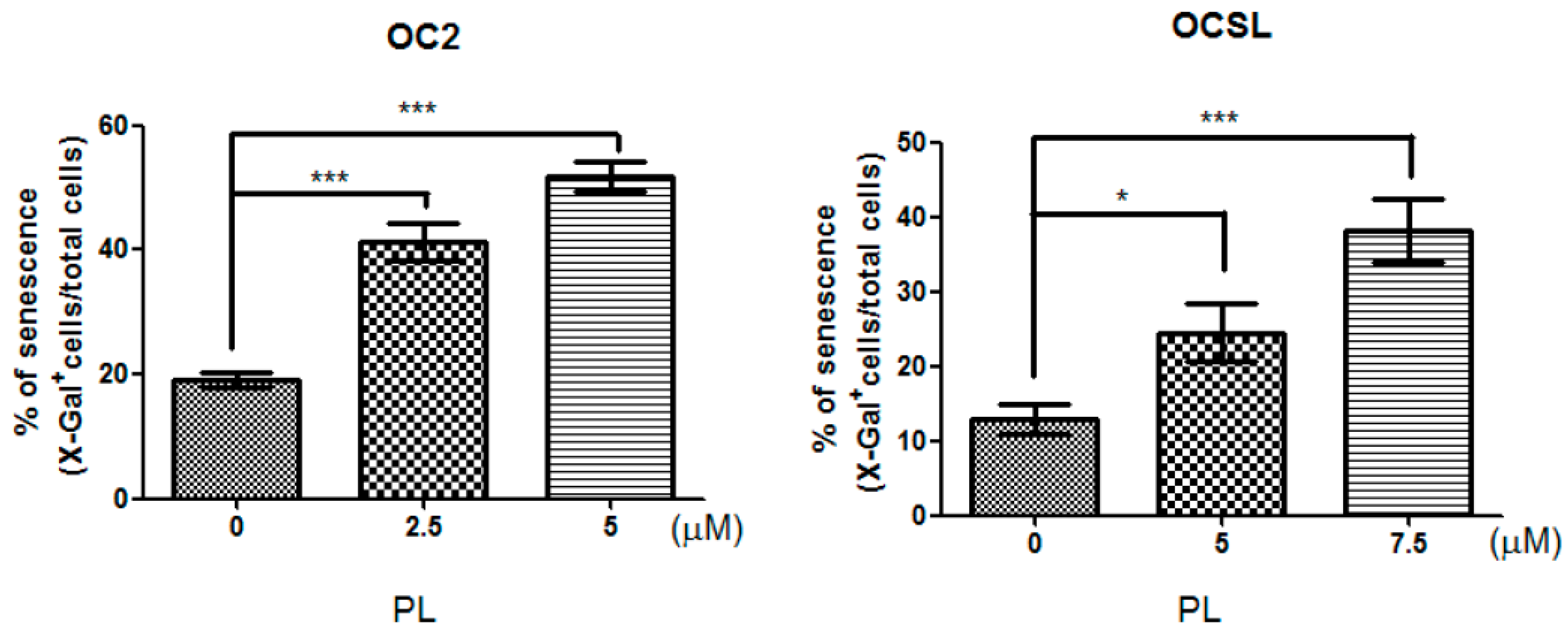
2.3. Senescence Induction in Piperlongumine-Treated Human Oral Squamous Cell Carcinoma
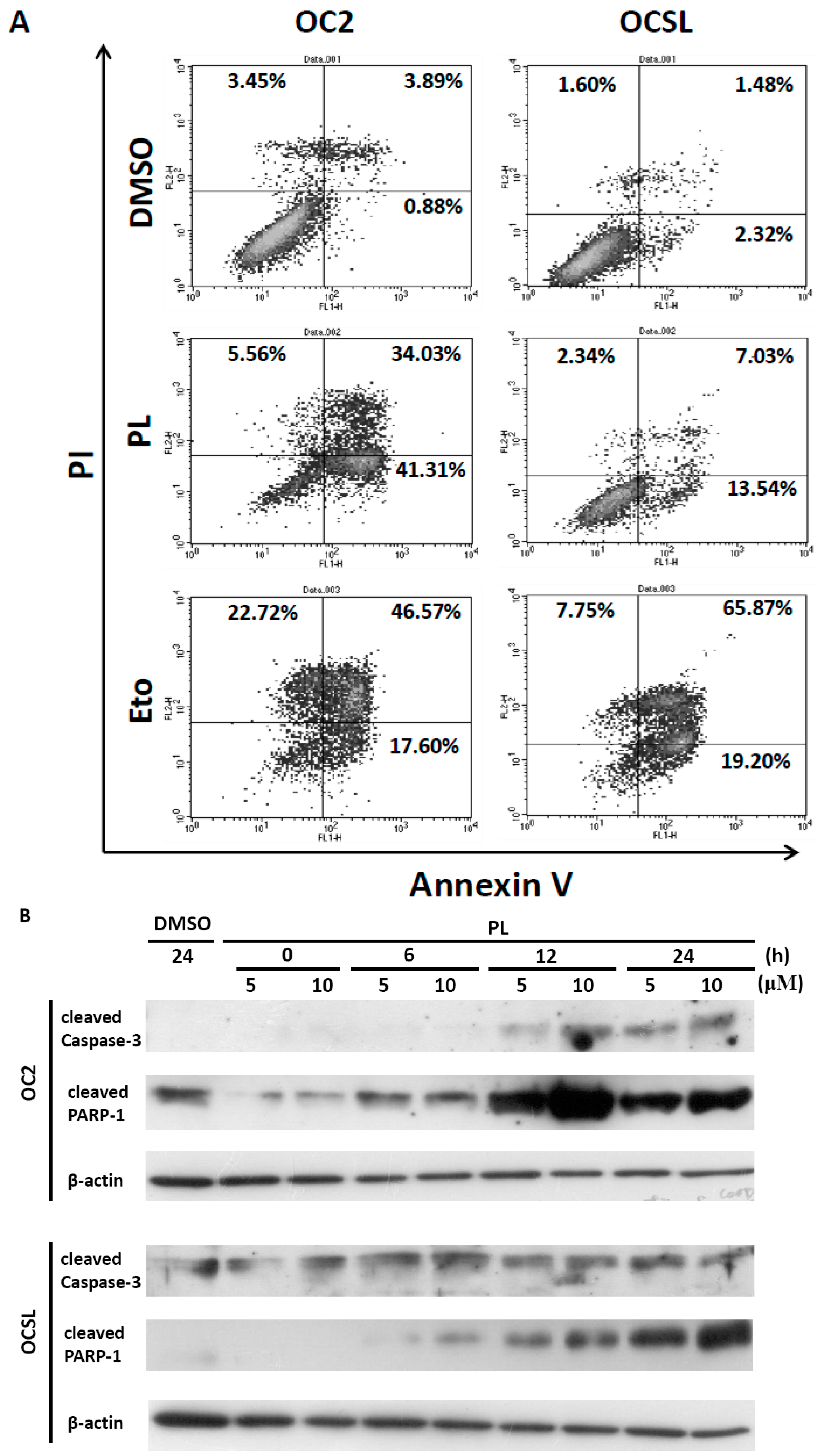
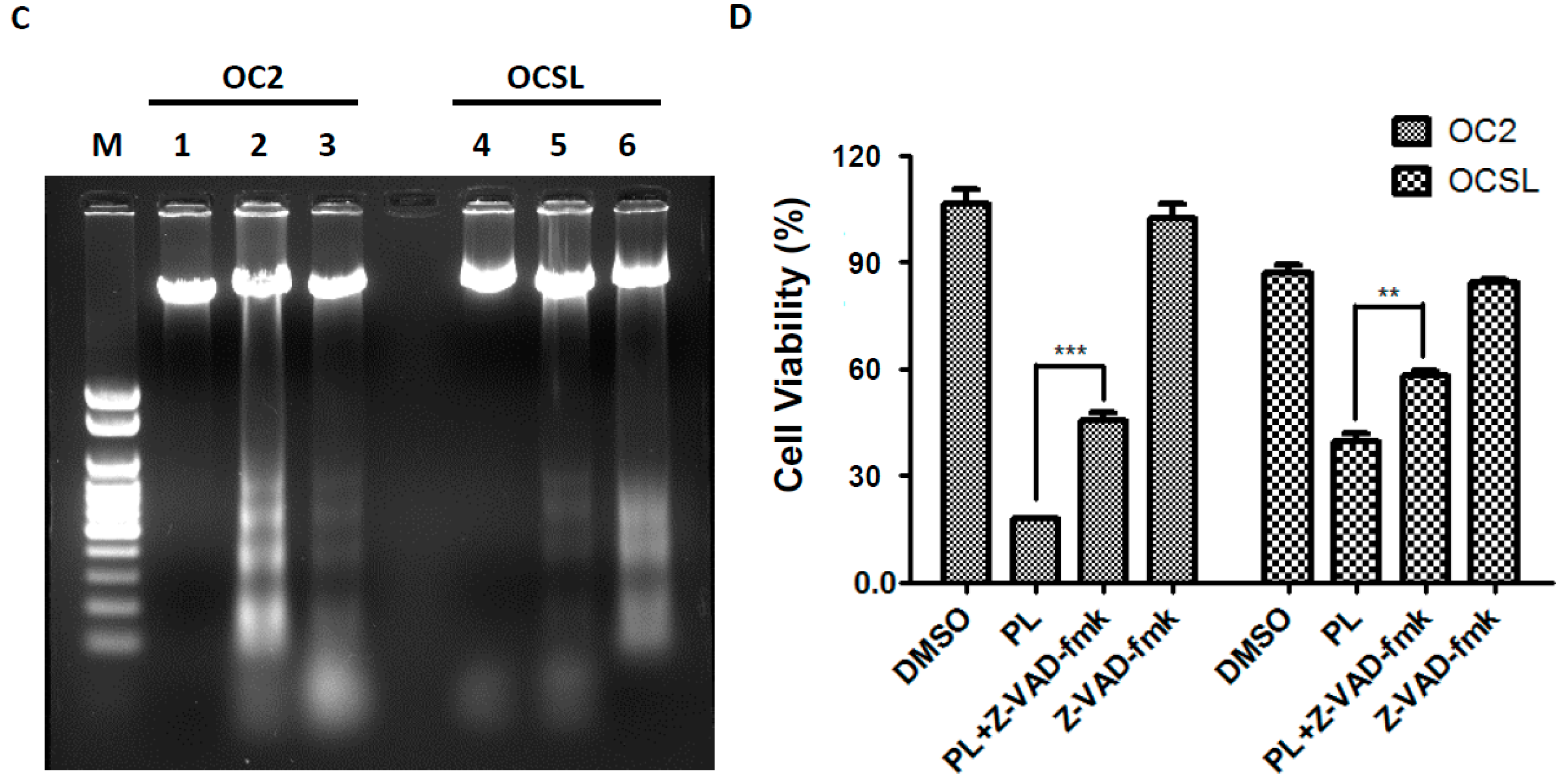
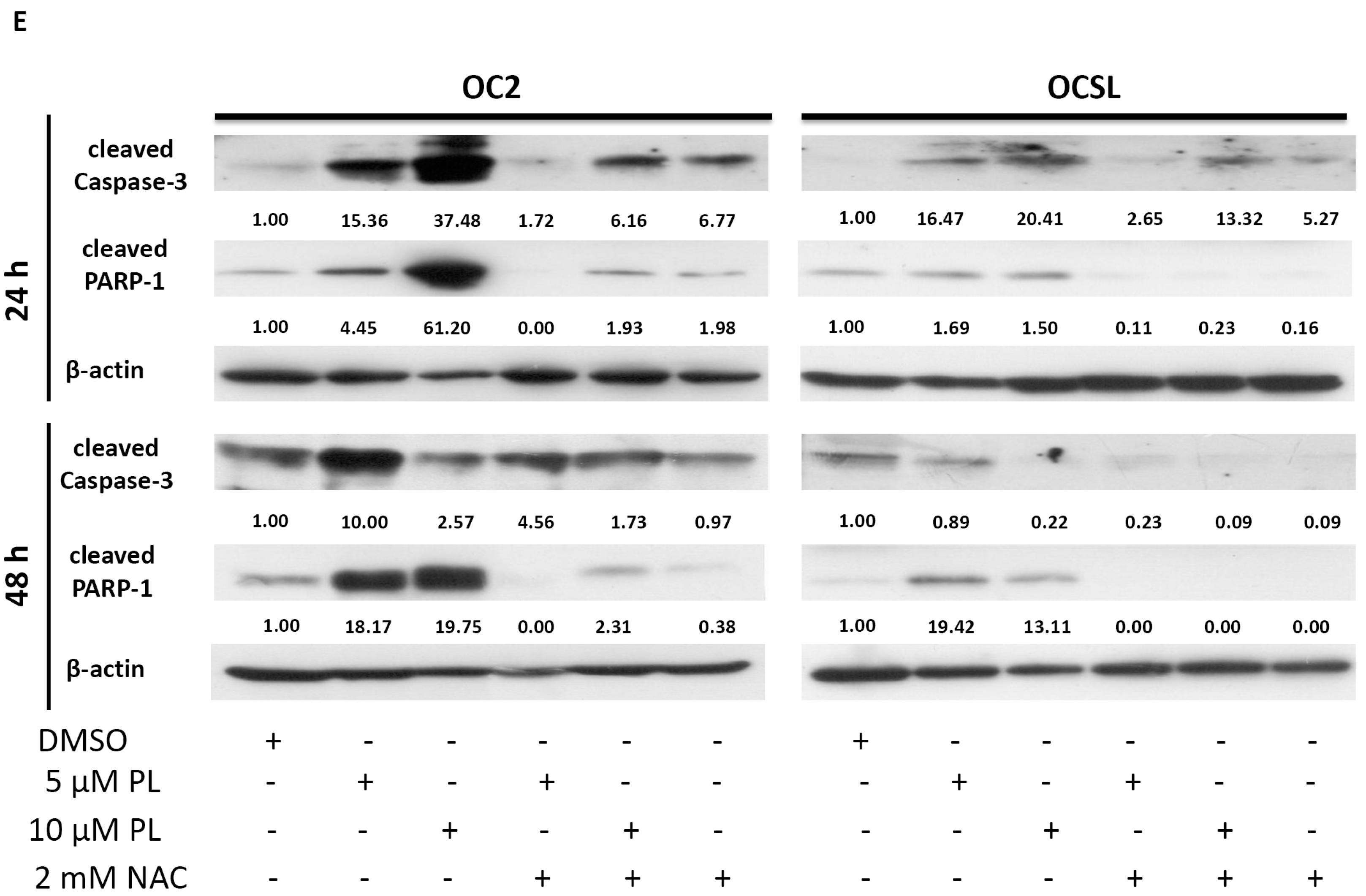
2.4. Piperlongumine Elevates Caspase-Dependent Apoptosis in Human Oral Squamous Cell Carcinoma
2.5. Piperlongumine Regulates ROS and Caspase-Dependent Apoptosis in Human Oral Squamous Cell Carcinoma
3. Discussion
4. Experimental Section
4.1. Cell Lines and Culture
4.2. Cell Viability Assay (CCK-8 Assay)
4.3. Cell Cycle Analysis
4.4. Apoptotic Cell Death Analysis
4.5. DNA Fragmentation Analysis
4.6. Senescent Cell Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ng, S.H.; Yen, T.C.; Liao, C.T.; Chang, J.T.; Chan, S.C.; Ko, S.F.; Wang, H.M.; Wong, H.F. 18F-FDG PET and CT/MRI in oral cavity squamous cell carcinoma: A prospective study of 124 patients with histologic correlation. J. Nucl. Med. 2005, 46, 1136–1143. [Google Scholar] [PubMed]
- Chung, T.T.; Pan, M.S.; Kuo, C.L.; Wong, R.H.; Lin, C.W.; Chen, M.K.; Yang, S.F. Impact of RECK gene polymorphisms and environmental factors on oral cancer susceptibility and clinicopathologic characteristics in Taiwan. Carcinogenesis 2011, 32, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Huang, H.C.; Lin, L.M.; Lin, C.C. Primary oral squamous cell carcinoma: An analysis of 703 cases in southern Taiwan. Oral Oncol. 1999, 35, 173–179. [Google Scholar] [CrossRef]
- Sargeran, K.; Murtomaa, H.; Safavi, S.M.; Vehkalahti, M.M.; Teronen, O. Survival after diagnosis of cancer of the oral cavity. Br. J. Oral Maxillofac. Surg. 2008, 46, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Hung, C.C.; Huang, K.L.; Chen, Y.T.; Liu, S.Y.; Chiang, W.F.; Chen, H.R.; Yen, C.Y.; Wu, Y.J.; Ko, J.Y.; et al. Src family kinases mediate betel quid-induced oral cancer cell motility and could be a biomarker for early invasion in oral squamous cell carcinoma. Neoplasia 2008, 10, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Chen, B.H.; Hour, T.C.; Chiang, W.F.; Wu, Y.J.; Chen, C.Y.; Chen, H.R.; Chan, P.T.; Liu, S.Y.; Chen, J.Y. Betel quid extract promotes oral cancer cell migration by activating a muscarinic M4 receptor-mediated signaling cascade involving SFKs and ERK1/2. Biochem. Biophys. Res. Commun. 2010, 399, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yin, P.H.; Yu, T.N.; Chang, Y.D.; Hsu, W.C.; Kao, S.Y.; Chi, C.W.; Liu, T.Y.; Wei, Y.H. Accumulation of mitochondrial DNA deletions in human oral tissues—Effects of betel quid chewing and oral cancer. Mutat. Res. 2001, 493, 67–74. [Google Scholar] [CrossRef]
- Chen, P.H.; Shieh, T.Y.; Ho, P.S.; Tsai, C.C.; Yang, Y.H.; Lin, Y.C.; Ko, M.S.; Tsai, P.C.; Chiang, S.L.; Tu, H.P.; et al. Prognostic factors associated with the survival of oral and pharyngeal carcinoma in Taiwan. BMC Cancer 2007, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Raj, L.; Ide, T.; Gurkar, A.U.; Foley, M.; Schenone, M.; Li, X.; Tolliday, N.J.; Golub, T.R.; Carr, S.A.; Shamji, A.F.; Stern, A.M.; et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011, 475, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Park, J.Y.; Kim, J.W.; Kwon, M.; Lee, B.H. Piperlongumine selectively kills cancer cells and increases cisplatin antitumor activity in head and neck cancer. Oncotarget 2014, 5, 9227–9238. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.H.; Chen, X.X.; Wang, H.; Jiang, Q.W.; Pan, S.S.; Qiu, J.G.; Mei, X.L.; Xue, Y.Q.; Qin, W.M.; Zheng, F.Y.; et al. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid. Med. Cell. Longev. 2014, 2014, 906804. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; Meyskens, F.L., Jr. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Wondrak, G.T. Redox-directed cancer therapeutics: Molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009, 11, 3013–3069. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Fenster, B.E.; Ito, H.; Takeda, K.; Bae, N.S.; Hirai, T.; Yu, Z.X.; Ferrans, V.J.; Howard, B.H.; Finkel, T. Ras proteins induce senescence by altering the intracellular levels of reactive oxygen species. J. Biol. Chem. 1999, 274, 7936–7940. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Bahar, G.; Feinmesser, R.; Shpitzer, T.; Popovtzer, A.; Nagler, R.M. Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer 2007, 109, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Korde, S.D.; Basak, A.; Chaudhary, M.; Goyal, M.; Vagga, A. Enhanced nitrosative and oxidative stress with decreased total antioxidant capacity in patients with oral precancer and oral squamous cell carcinoma. Oncology 2011, 80, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Wu, W.C.; Ji, W.T.; Chen, J.Y.; Cheng, Y.P.; Chiang, M.K.; Chen, H.R. Reversine suppresses oral squamous cell carcinoma via cell cycle arrest and concomitantly apoptosis and autophagy. J. Biomed. Sci. 2012, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Roberts, J.M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995, 9, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Boulaire, J.; Fotedar, A.; Fotedar, R. The functions of the CDK-cyclin kinase inhibitor p21WAF1. Pathol. Biol. 2000, 48, 190–202. [Google Scholar] [PubMed]
- Chen, Y.; Liu, J.M.; Xiong, X.X.; Qiu, X.Y.; Pan, F.; Liu, D.; Lan, S.J.; Jin, S.; Yu, S.B.; Chen, X.Q. Piperlongumine selectively kills hepatocellular carcinoma cells and preferentially inhibits their invasion via ROS-ER-MAPKs-CHOP. Oncotarget 2015, 6, 6406–6421. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Mao, Y.; You, Q.; Hua, D.; Cai, D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int. J. Immunopathol. Pharmacol. 2015, 28, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Son, D.J.; Yun, H.; Kamberos, N.L.; Janz, S. Piperlongumine inhibits proliferation and survival of Burkitt lymphoma in vitro. Leukemia Res. 2013, 37, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Kulkarni, P.; Thummuri, D.; Jeengar, M.K.; Naidu, V.G.; Alvala, M.; Redddy, G.B.; Ramakrishna, S. Piperlongumine, an alkaloid causes inhibition of PI3 K/Akt/mTOR signaling axis to induce caspase-dependent apoptosis in human triple-negative breast cancer cells. Apoptosis 2014, 19, 1148–1164. [Google Scholar] [CrossRef] [PubMed]
- Kahlem, P.; Dorken, B.; Schmitt, C.A. Cellular senescence in cancer treatment: Friend or foe? J. Clin. Investig. 2004, 113, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, S.; Hinds, P.W.; Munger, K. Re-expression of endogenous p16ink4a in oral squamous cell carcinoma lines by 5-AZA-2′-deoxycytidine treatment induces a senescence-like state. Oncogene 1998, 17, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.X.; Yao, Z.F.; Li, Z.F.; Liu, Y.B. Radio-sensitization by Piper longumine of human breast adenoma MDA-MB-231 cells in vitro. Asian Pac. J. Cancer Prev. 2014, 15, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Ginzburg, S.; Golovine, K.V.; Makhov, P.B.; Uzzo, R.G.; Kutikov, A.; Kolenko, V.M. Piperlongumine inhibits NF-κB activity and attenuates aggressive growth characteristics of prostate cancer cells. Prostate 2014, 74, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Golovine, K.; Teper, E.; Kutikov, A.; Mehrazin, R.; Corcoran, A.; Tulin, A.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. Br. J. Cancer 2014, 110, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.W.; Xiao, X.; Shan, Y.; Xue, B.; Jiang, G.; He, Q.; Chen, J.; Xu, H.G.; Zhao, R.X.; et al. Piperlongumine induces autophagy by targeting p38 signaling. Cell Death Dis. 2013, 4, e824. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Nicco, C.; Chereau, C.; Goulvestre, C.; Alexandre, J.; Alves, A.; Levy, E.; Goldwasser, F.; Panis, Y.; Soubrane, O.; et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005, 65, 948–956. [Google Scholar] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Cabello, C.M.; Bair, W.B., 3rd; Wondrak, G.T. Experimental therapeutics: Targeting the redox Achilles heel of cancer. Curr. Opin. Investig. Drugs 2007, 8, 1022–1037. [Google Scholar] [PubMed]
- Liu, J.M.; Pan, F.; Li, L.; Liu, Q.R.; Chen, Y.; Xiong, X.X.; Cheng, K.; Yu, S.B.; Shi, Z.; Yu, A.C.; et al. Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem. Biophys. Res. Commun. 2013, 437, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Abdollahi, M. Antioxidants: Friends or foe in prevention or treatment of cancer: The debate of the century. Toxicol. Appl. Pharmacol. 2013, 271, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.R.; Liu, J.M.; Chen, Y.; Xie, X.Q.; Xiong, X.X.; Qiu, X.Y.; Pan, F.; Liu, D.; Yu, S.B.; Chen, X.Q. Piperlongumine inhibits migration of glioblastoma cells via activation of ROS-dependent p38 and JNK signaling pathways. Oxid. Med. Cell. Longev. 2014, 2014, 653732. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-Y.; Liu, G.-H.; Chao, W.-Y.; Shi, C.-S.; Lin, C.-Y.; Lim, Y.-P.; Lu, C.-H.; Lai, P.-Y.; Chen, H.-R.; Lee, Y.-R. Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence. Int. J. Mol. Sci. 2016, 17, 616. https://doi.org/10.3390/ijms17040616
Chen S-Y, Liu G-H, Chao W-Y, Shi C-S, Lin C-Y, Lim Y-P, Lu C-H, Lai P-Y, Chen H-R, Lee Y-R. Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence. International Journal of Molecular Sciences. 2016; 17(4):616. https://doi.org/10.3390/ijms17040616
Chicago/Turabian StyleChen, San-Yuan, Geng-Hung Liu, Wen-Ying Chao, Chung-Sheng Shi, Ching-Yen Lin, Yun-Ping Lim, Chieh-Hsiang Lu, Peng-Yeh Lai, Hau-Ren Chen, and Ying-Ray Lee. 2016. "Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence" International Journal of Molecular Sciences 17, no. 4: 616. https://doi.org/10.3390/ijms17040616
APA StyleChen, S.-Y., Liu, G.-H., Chao, W.-Y., Shi, C.-S., Lin, C.-Y., Lim, Y.-P., Lu, C.-H., Lai, P.-Y., Chen, H.-R., & Lee, Y.-R. (2016). Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence. International Journal of Molecular Sciences, 17(4), 616. https://doi.org/10.3390/ijms17040616





