Retrospective Study of Metastatic Melanoma and Renal Cell Carcinoma to the Brain with Multivariate Analysis of Prognostic Pre-Treatment Clinical Factors
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RCC | Renal cell carcinoma |
| SRS | Stereotactic radiosurgery |
| OS | Overall survival |
| WBRT | Whole brain radiation therapy |
| KPS | Karnofsky Performance Status |
References
- Posner, J.B. Management of brain metastases. Rev. Neurol. 1992, 148, 477–487. [Google Scholar] [PubMed]
- Patchell, R.A. The management of brain metastases. Cancer Treat. Rev. 2003, 29, 533–540. [Google Scholar] [CrossRef]
- Marko, N.F.; Angelov, L.; Toms, S.A.; Suh, J.H.; Chao, S.T.; Vogelbaum, M.A.; Barnett, G.H.; Weil, R.J. Stereotactic radiosurgery as single-modality treatment of incidentally identified renal cell carcinoma brain metastases. World Neurosurg. 2010, 73, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Samlowski, W.E.; Watson, G.A.; Wang, M.; Rao, G.; Klimo, P., Jr.; Boucher, K.; Shrieve, D.C.; Jensen, R.L. Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer 2007, 109, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, J.G.; Sarkissian, V.; Hou, L.C.; Veeravagu, A.; Tse, V. Molecular events of brain metastasis. Neurosurg. Focus 2007, 22, 1–5. [Google Scholar] [CrossRef]
- Mori, Y.; Kondziolka, D.; Flickinger, J.C.; Kirkwood, J.M.; Agarwala, S.; Lunsford, L.D. Stereotactic radiosurgery for cerebral metastatic melanoma: Factors affecting local disease control and survival. Int. J. Radiat. Oncol. Biol. Phys. 1998, 42, 581–589. [Google Scholar] [CrossRef]
- Rapp, S.R.; Case, L.D.; Peiffer, A.; Naughton, M.M.; Chan, M.D.; Stieber, V.W.; Moore, D.F., Jr.; Falchuk, S.C.; Piephoff, J.V.; Edenfield, W.J.; et al. Donepezil for irradiated brain tumor survivors: A phase III randomized placebo-controlled clinical trial. J. Clin. Oncol. 2015, 33, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Ruda, R.; Mutani, R. Management of brain metastases. J. Neurol. 2002, 249, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, J.W.; Chung, H.T.; Paek, S.H.; Kim, D.G.; Jung, H.W. Brain metastasis from renal cell carcinoma. Prog. Neurol. Surg. 2012, 25, 163–175. [Google Scholar] [PubMed]
- Nieder, C.; Berberich, W.; Schnabel, K. Tumor-related prognostic factors for remission of brain metastases after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 25–30. [Google Scholar] [CrossRef]
- Muacevic, A.; Kreth, F.W.; Mack, A.; Tonn, J.C.; Wowra, B. Stereotactic radiosurgery without radiation therapy providing high local tumor control of multiple brain metastases from renal cell carcinoma. Minim. Invasive Neurosurg. 2004, 47, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Brown, C.A.; Pollock, B.E.; Gorman, D.A.; Foote, R.L. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery 2008, 62, 790–801. [Google Scholar] [CrossRef] [PubMed]
- El Gantery, M.M.; Abd El Baky, H.M.; El Hossieny, H.A.; Mahmoud, M.; Youssef, O. Management of brain metastases with stereotactic radiosurgery alone versus whole brain irradiation alone versus both. Radiat. Oncol. 2014, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.H.; Al-Sarraf, M.; Baker, L.H.; Vaitkevicius, V.K. Malignant melanoma and central nervous system metastases: Incidence, diagnosis, treatment and survival. Cancer 1978, 42, 660–668. [Google Scholar] [CrossRef]
- Barth, A.; Wanek, L.A.; Morton, D.L. Prognostic factors in 1521 melanoma patients with distant metastases. J. Am. Coll. Surg. 1995, 181, 193–201. [Google Scholar] [PubMed]
- Yu, C.; Chen, J.C.; Apuzzo, M.L.; O’Day, S.; Giannotta, S.L.; Weber, J.S.; Petrovich, Z. Metastatic melanoma to the brain: Prognostic factors after gamma knife radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1277–1287. [Google Scholar] [CrossRef]
- Lavine, S.D.; Petrovich, Z.; Cohen-Gadol, A.A.; Masri, L.S.; Morton, D.L.; O’Day, S.J.; Essner, R.; Zelman, V.; Yu, C.; Luxton, G.; et al. Gamma knife radiosurgery for metastatic melanoma: An analysis of survival, outcome, and complications. Neurosurgery 1999, 44, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Carter, J.H., Jr.; Friedman, A.H.; Seigler, H.F. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998, 88, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Petrovich, Z.; O’Day, S.; Morton, D.; Essner, R.; Giannotta, S.L.; Yu, C.; Apuzzo, M.L. Stereotactic radiosurgery in the treatment of metastatic disease to the brain. Neurosurgery 2000, 47, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Huttenlocher, S.; Gebauer, N.; Hornung, D.; Trang, N.T.; Khoa, M.T.; Schild, S.E. Impact of stereotactic radiosurgery dose on control of cerebral metastases from renal cell carcinoma. Anticancer Res. 2015, 35, 3571–3574. [Google Scholar]
- Bennani, O.; Derrey, S.; Langlois, O.; Castel, H.; Laquerriere, A.; Freger, P.; Proust, F. Brain metastasis from renal cell carcinoma. Neurochirurgie 2014, 60, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.P.; Sun, M.H.; Kondziolka, D.; Flickinger, J.; Lunsford, L.D. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: Long-term outcomes and prognostic factors influencing survival and local tumor control. J. Neurosurg. 2003, 98, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Dyer, M.A.; Arvold, N.D.; Chen, Y.H.; Pinnell, N.E.; Mitin, T.; Lee, E.Q.; Hodi, F.S.; Ibrahim, N.; Weiss, S.E.; Kelly, P.J.; et al. The role of whole brain radiation therapy in the management of melanoma brain metastases. Radiat. Oncol. 2014, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Dye, N.B.; Gondi, V.; Mehta, M.P. Strategies for preservation of memory function in patients with brain metastases. Chin. Clin. Oncol. 2015, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Asher, A.L.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G.; Deming, R.L.; Burri, S.; et al. NCCTG N0574 (alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. J. Clin. Oncol. 2015, 33, LBA4. [Google Scholar]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Hanson, P.W.; Elaimy, A.L.; Lamoreaux, W.T.; Demakas, J.J.; Fairbanks, R.K.; Mackay, A.R.; Taylor, B.; Cooke, B.S.; Thumma, S.R.; Lee, C.M. A concise review of the efficacy of stereotactic radiosurgery in the management of melanoma and renal cell carcinoma brain metastases. World J. Surg. Oncol. 2012, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Lwu, S.; Goetz, P.; Monsalves, E.; Aryaee, M.; Ebinu, J.; Laperriere, N.; Menard, C.; Chung, C.; Millar, B.A.; Kulkarni, A.V.; et al. Stereotactic radiosurgery for the treatment of melanoma and renal cell carcinoma brain metastases. Oncol. Rep. 2013, 29, 407–412. [Google Scholar] [PubMed]
- Frakes, J.M.; Figura, N.D.; Ahmed, K.A.; Juan, T.H.; Patel, N.; Latifi, K.; Sarangkasiri, S.; Strom, T.J.; Chinnaiyan, P.; Rao, N.G.; et al. Potential role for linac-based stereotactic radiosurgery for the treatment of 5 or more radioresistant melanoma brain metastases. J. Neurosurg. 2015, 123, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Mikoshiba, A.; Uhara, H.; Murata, H.; Okuyama, R. Clinical effects of stereotactic radiation surgery in patients with metastatic melanoma. J. Dermatol. 2013, 40, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Henzel, M.; Hamm, K.; Surber, G.; Kleinert, G.; Engenhart-Cabillic, R. Radiotherapy for brain metastases from renal cell cancer: Should whole-brain radiotherapy be added to stereotactic radiosurgery? Strahlenther. Onkol. 2010, 186, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.W.; Chung, C.T.; Shah, H.R.; Canute, G.W.; Hodge, C.J.; Bassano, D.A.; Liu, L.; Mitchell, L.; Hahn, S.S. Gamma knife surgery in the management of radioresistant brain metastases in high-risk patients with melanoma, renal cell carcinoma, and sarcoma. J. Neurosurg. 2008, 109, 122–128. [Google Scholar] [PubMed]
- Kano, H.; Iyer, A.; Kondziolka, D.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D. Outcome predictors of gamma knife radiosurgery for renal cell carcinoma metastases. Neurosurgery 2011, 69, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Elaimy, A.L.; Mackay, A.R.; Lamoreaux, W.T.; Fairbanks, R.K.; Demakas, J.J.; Cooke, B.S.; Lee, C.M. Clinical outcomes of stereotactic radiosurgery in the treatment of patients with metastatic brain tumors. World Neurosurg. 2011, 75, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Shuto, T.; Matsunaga, S.; Suenaga, J.; Inomori, S.; Fujino, H. Treatment strategy for metastatic brain tumors from renal cell carcinoma: Selection of gamma knife surgery or craniotomy for control of growth and peritumoral edema. J. Neurooncol. 2010, 98, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.M.; Lowe, M.; Khan, M.K.; Lawson, D.H.; Crocker, I.R.; Shelton, J.W.; Melton, A.; Maynard, N.; Delman, K.A.; Carlson, G.W.; et al. Prognostic factors for overall survival after radiosurgery for brain metastases from melanoma. Am. J. Clin. Oncol. 2014, 37, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Knisely, J.P.; Yu, J.B.; Flanigan, J.; Sznol, M.; Kluger, H.M.; Chiang, V.L. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 2012, 117, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Mahadevan, A.; Floyd, S.R.; Dyer, M.A.; Catalano, P.J.; Alexander, B.M.; McDermott, D.F.; Kaplan, I.D. Ipilimumab and cranial radiation in metastatic melanoma patients: A case series and review. J. Immunother. Cancer 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.B.; Stenehjem, D.D.; Gill, D.M.; Tantravahi, S.K.; Agarwal, A.M.; Hsu, J.; Vuong, W.; Pal, S.K.; Agarwal, N. Everolimus versus temsirolimus in metastatic renal cell carcinoma after progression with previous systemic therapies. Clin. Genitourin. Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Santoni, M.; Verzoni, E.; Grassi, P.; Testa, I.; de Braud, F.; Cascinu, S.; Procopio, G. Everolimus and temsirolimus are not the same second-line in metastatic renal cell carcinoma: A systematic review and meta-analysis of literature data. Clin Genitourin Cancer 2015, 13, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.K.; Yang, H.; Signorovitch, J.E.; Wang, X.; Liu, Z.; Liu, N.S.; Qi, C.Z.; George, D.J. Comparative outcomes of everolimus, temsirolimus, and sorafenib as second targeted therapies for metastatic renal cell carcinoma: A US medical record review. Curr. Med. Res. Opin. 2014, 30, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]

| Treatment Group | Primary Tumor | ||
|---|---|---|---|
| RCC | Melanoma | Total | |
| (n = 28) | (n = 94) | (n = 122) | |
| Age at Diagnosis | |||
| <65 | 18 | 71 | 89 |
| ≥65 | 9 | 22 | 31 |
| Unknown | 1 | 1 | 2 |
| KPS | |||
| 90–100 | 4 | 8 | 12 |
| 70–80 | 3 | 14 | 17 |
| ≤60 | 1 | 6 | 7 |
| Unknown | 20 | 66 | 86 |
| # Brain Mets | |||
| 1 | 9 | 38 | 47 |
| 2–5 | 13 | 33 | 46 |
| KPS > 5 | 5 | 22 | 27 |
| Unknown | 1 | 1 | 2 |
| Received WBRT | |||
| No | 23 | 77 | 100 |
| Yes | 5 | 17 | 22 |
| Underwent Resection | |||
| No | 23 | 68 | 91 |
| Yes | 5 | 25 | 30 |
| Unknown | 0 | 1 | 1 |
| Treatment Group | Median Survival | Hazard Ratio | |||
|---|---|---|---|---|---|
| n | 95% CI | Estimate | 95% CI | p Value ** | |
| Age at Diagnosis | |||||
| <65 * | 89 | 10.30 ± 3.49 | reference | ||
| ≥65 | 31 | 8.60 ± 6.27 | 1.09 | 0.61–1.88 | 0.784 |
| Unknown | 2 | 1.30 ± unknown | 7.99 | 0.18–63.46 | 0.133 |
| KPS | |||||
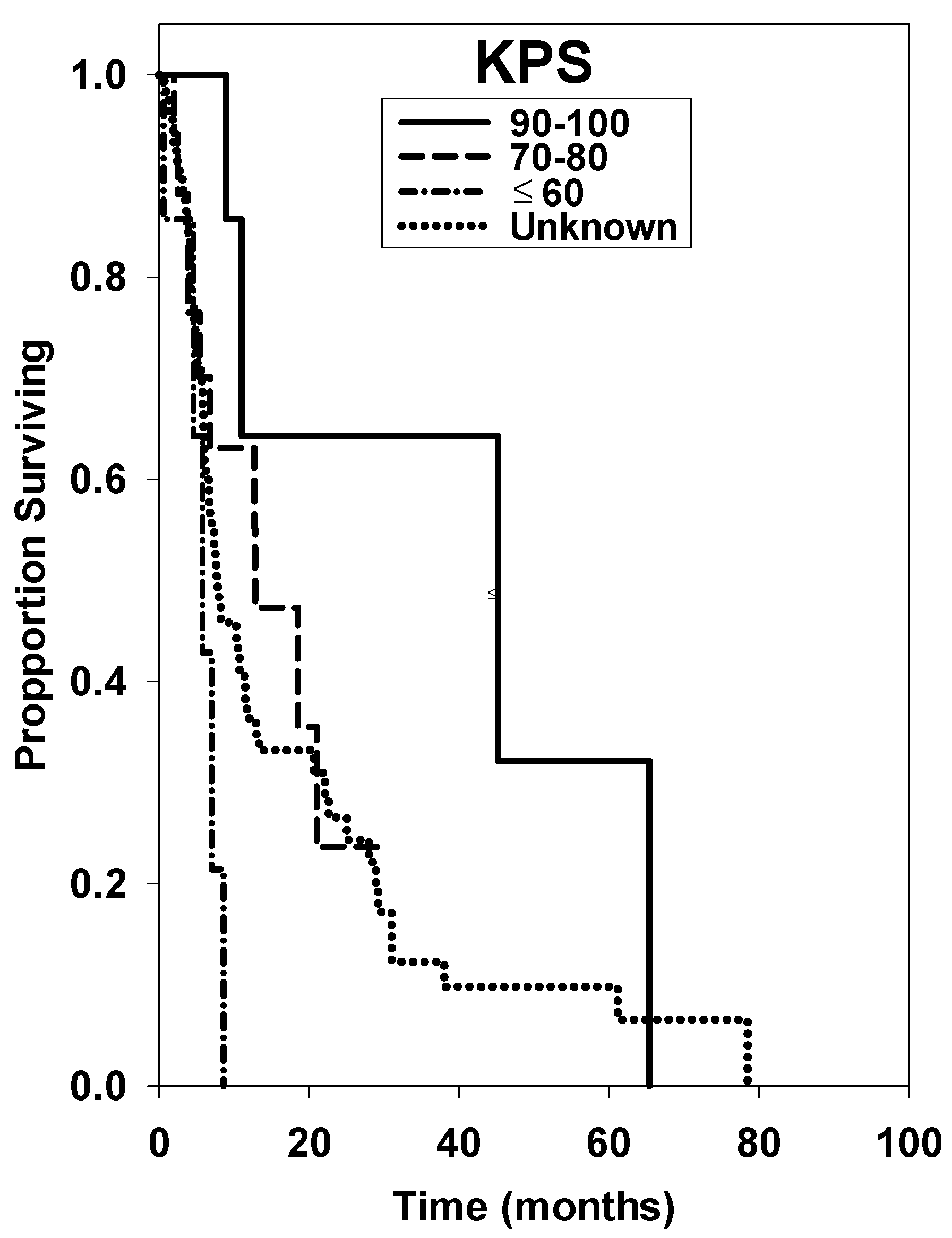
| 90–100 * | 12 | 45.20 ± 43.52 | reference | ||
| 70–80 | 17 | 12.80 ± 7.64 | 3.28 | 0.69–31.14 | 0.139 |
| ≤60 | 7 | 5.80 ± 2.46 | 7.90 | 1.18–52.97 | <0.001 |
| Unknown | 86 | 7.80 ± 3.24 | 2.87 | 1.05–10.95 | 0.035 |
| # Brain Mets | |||||
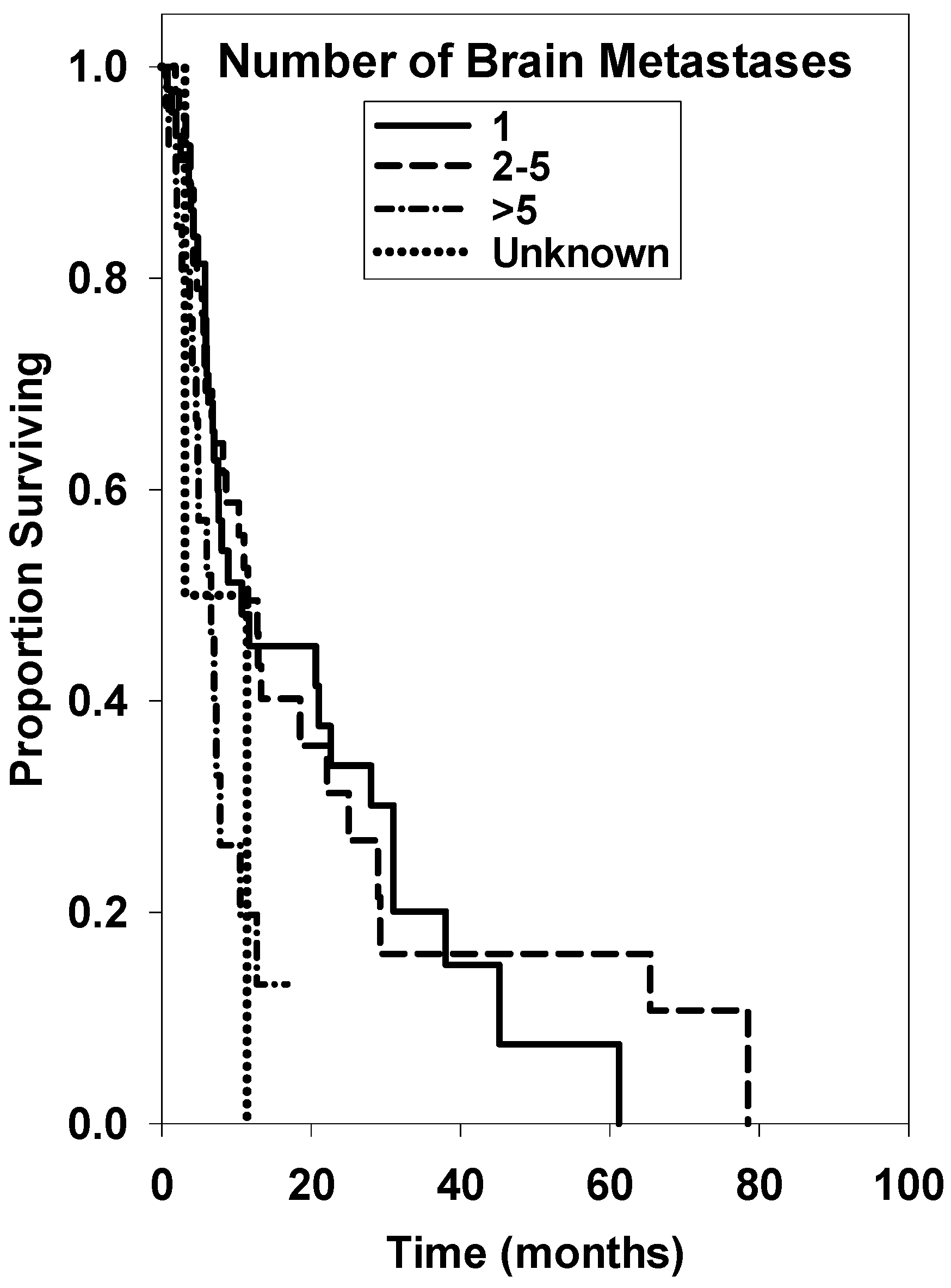
| 1 * | 47 | 10.70 ± 13.40 | reference | ||
| 2–5 | 46 | 11.50 ± 3.36 | 0.95 | 0.55–1.67 | 0.895 |
| >5 | 27 | 6.60 ± 2.45 | 2.24 | 1.09–4.51 | 0.024 |
| Unknown | 2 | 3.10 ± unknown | 2.42 | 0.27–10.09 | 0.219 |
| WBRT Received | |||||
| No * | 100 | 10.70 ± 3.34 | reference | ||
| Yes | 22 | 7.00 ± 1.89 | 3.10 | 0.80–2.57 | 0.161 |
| Resection Undergone | |||||
| No * | 91 | 8.60 ± 3.16 | reference | ||
| Yes | 30 | 11.40 ± 18.13 | 0.74 | 0.42–1.25 | 0.275 |
| Unknown | 1 | ||||
| Primary Tumor | |||||
| RCC * | 28 | 12.70 ± 2.63 | reference | ||
| Melanoma | 94 | 8.20 ± 3.06 | 1.21 | 0.70–2.19 | 0.519 |
| Treatment Group | n | Hazard Ratio | ||
|---|---|---|---|---|
| Estimate | 95% CI | p Value ** | ||
| Age at Diagnosis | ||||
| <65 * | 89 | reference | ||
| ≥65 | 31 | 1.03 | 0.60–1.77 | 0.906 |
| Unknown | 2 | 57.16 | 5.41–603.98 | <0.001 |
| KPS | ||||
| 90–100 * | 12 | reference | ||
| 70–80 | 17 | 1.67 | 1.20–2.34 | <0.001 |
| ≤60 | 7 | 5.56 | 5.17–5.97 | <0.001 |
| Unknown | 86 | 2.43 | 0.85–6.90 | 0.100 |
| # Brain Mets | ||||
| 1 * | 47 | reference | ||
| 2–5 | 46 | 1.04 | 0.61–1.79 | 0.880 |
| >5 | 27 | 2.30 | 2.09–2.53 | <0.001 |
| Unknown | 2 | 2.98 | 1.16–7.68 | 0.020 |
| WBRT Received | ||||
| No * | 100 | reference | ||
| Yes | 22 | 1.14 | 0.63–2.06 | 0.669 |
| Resection Undergone | ||||
| No * | 91 | reference | ||
| Yes | 30 | 0.72 | 0.41–1.28 | 0.267 |
| Unknown | 1 | - | - | - |
| Primary Tumor | ||||
| Kidney * | 28 | reference | ||
| Melanoma | 94 | 1.21 | 0.70-2.17 | 0.496 |
| Year | Survival Rate | 95% CI |
|---|---|---|
| 0.5 | 65.7 | 55.9–73.9 |
| 1 | 40.0 | 30.0–49.7 |
| 2 | 26.0 | 16.7–36.3 |
| 5 | 8.1 | 2.4–18.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrel, E.A.; Roehrig, A.T.; Kaya, E.A.; Carlson, J.D.; Ling, B.C.; Wagner, A.; MacKay, A.R.; Call, J.A.; Demakas, J.J.; Lamoreaux, W.T.; et al. Retrospective Study of Metastatic Melanoma and Renal Cell Carcinoma to the Brain with Multivariate Analysis of Prognostic Pre-Treatment Clinical Factors. Int. J. Mol. Sci. 2016, 17, 400. https://doi.org/10.3390/ijms17030400
Ferrel EA, Roehrig AT, Kaya EA, Carlson JD, Ling BC, Wagner A, MacKay AR, Call JA, Demakas JJ, Lamoreaux WT, et al. Retrospective Study of Metastatic Melanoma and Renal Cell Carcinoma to the Brain with Multivariate Analysis of Prognostic Pre-Treatment Clinical Factors. International Journal of Molecular Sciences. 2016; 17(3):400. https://doi.org/10.3390/ijms17030400
Chicago/Turabian StyleFerrel, Ethan A., Andrew T. Roehrig, Erin A. Kaya, Jonathan D. Carlson, Benjamin C. Ling, Aaron Wagner, Alexander R. MacKay, Jason A. Call, John J. Demakas, Wayne T. Lamoreaux, and et al. 2016. "Retrospective Study of Metastatic Melanoma and Renal Cell Carcinoma to the Brain with Multivariate Analysis of Prognostic Pre-Treatment Clinical Factors" International Journal of Molecular Sciences 17, no. 3: 400. https://doi.org/10.3390/ijms17030400
APA StyleFerrel, E. A., Roehrig, A. T., Kaya, E. A., Carlson, J. D., Ling, B. C., Wagner, A., MacKay, A. R., Call, J. A., Demakas, J. J., Lamoreaux, W. T., Fairbanks, R. K., Cooke, B. S., Peressini, B., & Lee, C. M. (2016). Retrospective Study of Metastatic Melanoma and Renal Cell Carcinoma to the Brain with Multivariate Analysis of Prognostic Pre-Treatment Clinical Factors. International Journal of Molecular Sciences, 17(3), 400. https://doi.org/10.3390/ijms17030400






