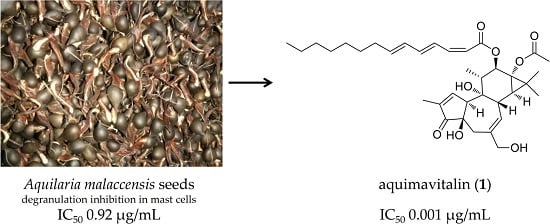
Antiallergic Phorbol Ester from the Seeds of Aquilaria malaccensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antiallergic, Antiinflammatory, Cytotoxic Effects of A. malaccensis Seeds (AMS)
2.2. Chemical Analysis and Bioactivity-Guided Fractionation
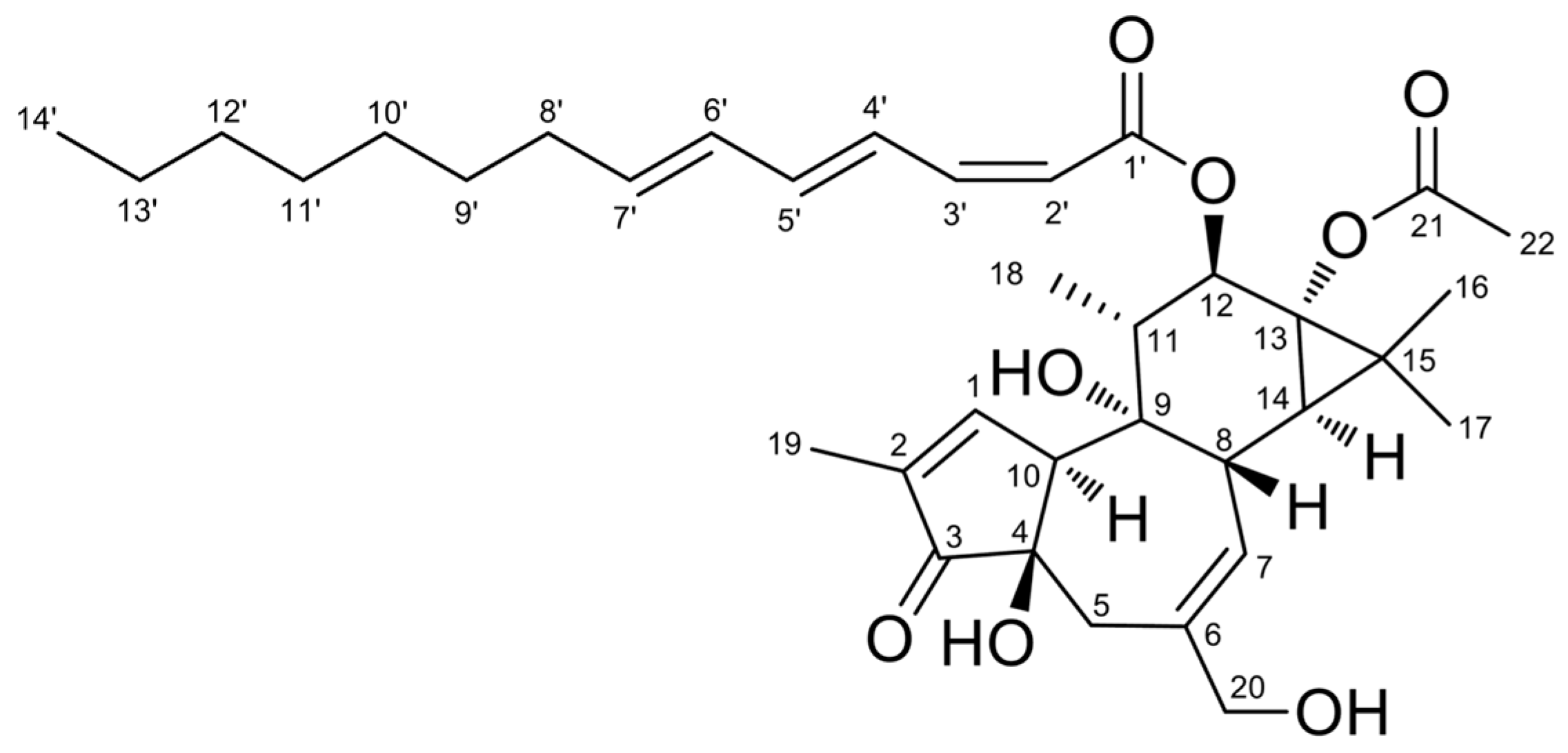
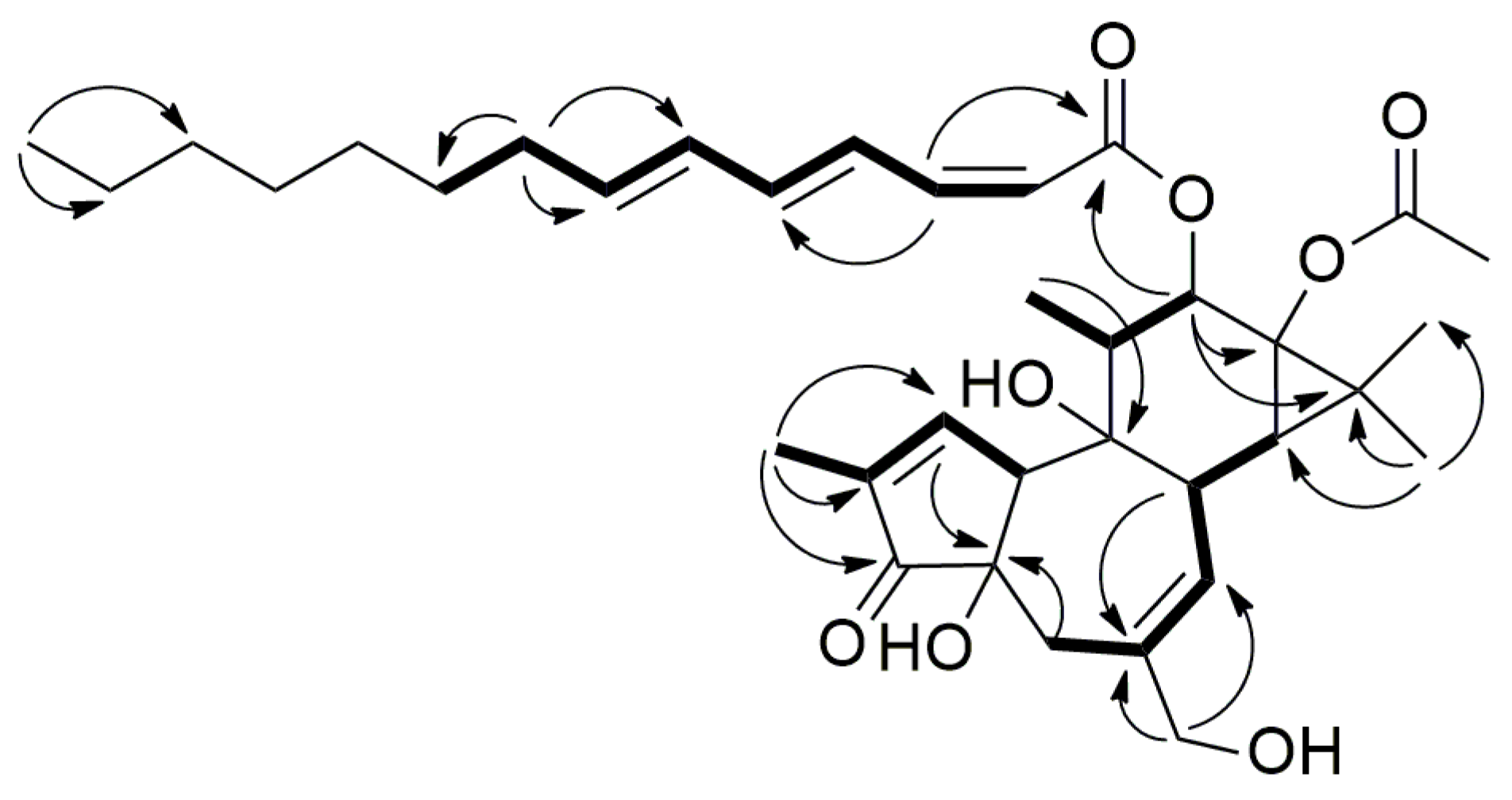
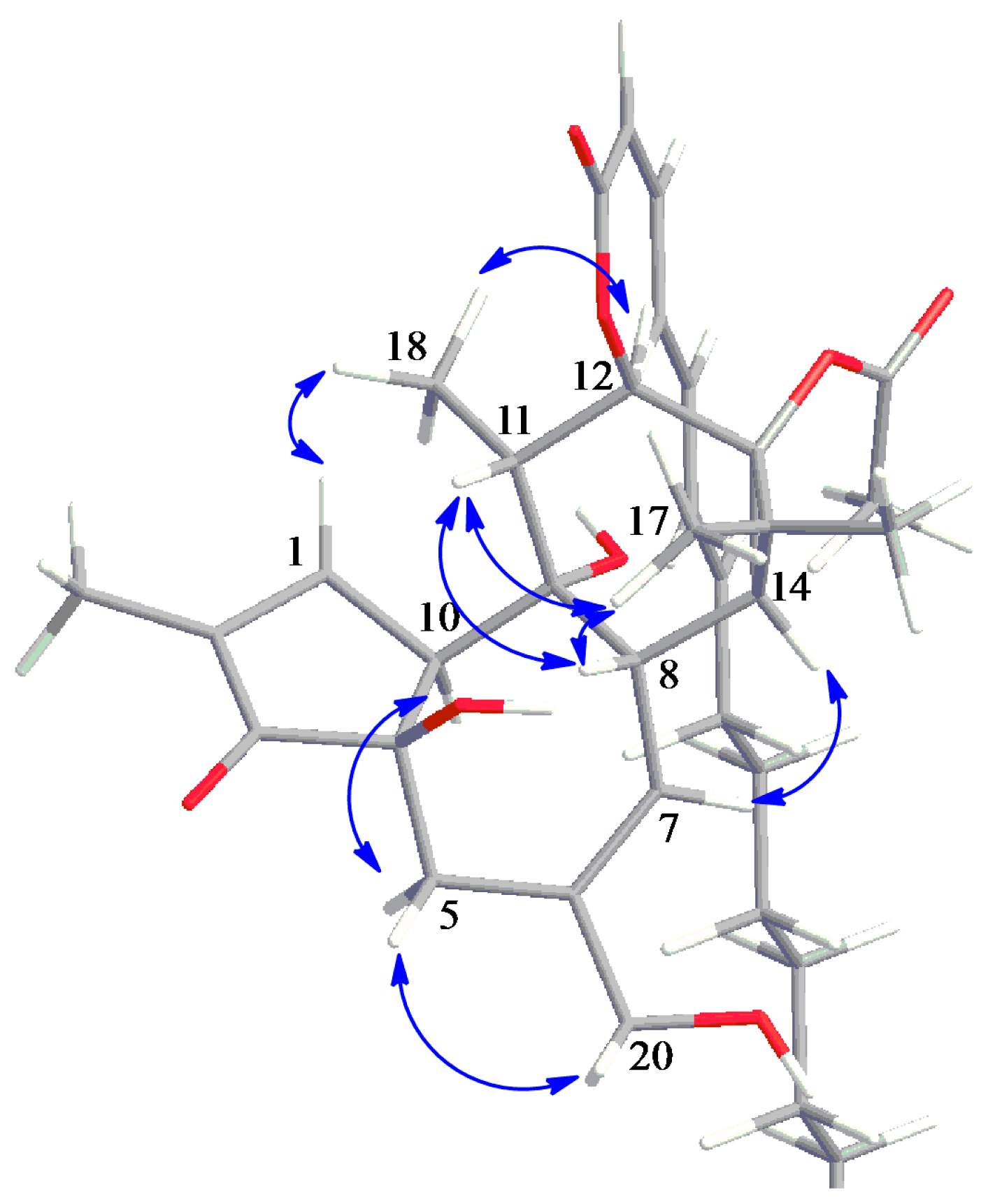
2.3. Structure Elucidation of Aquimavitalin (1)
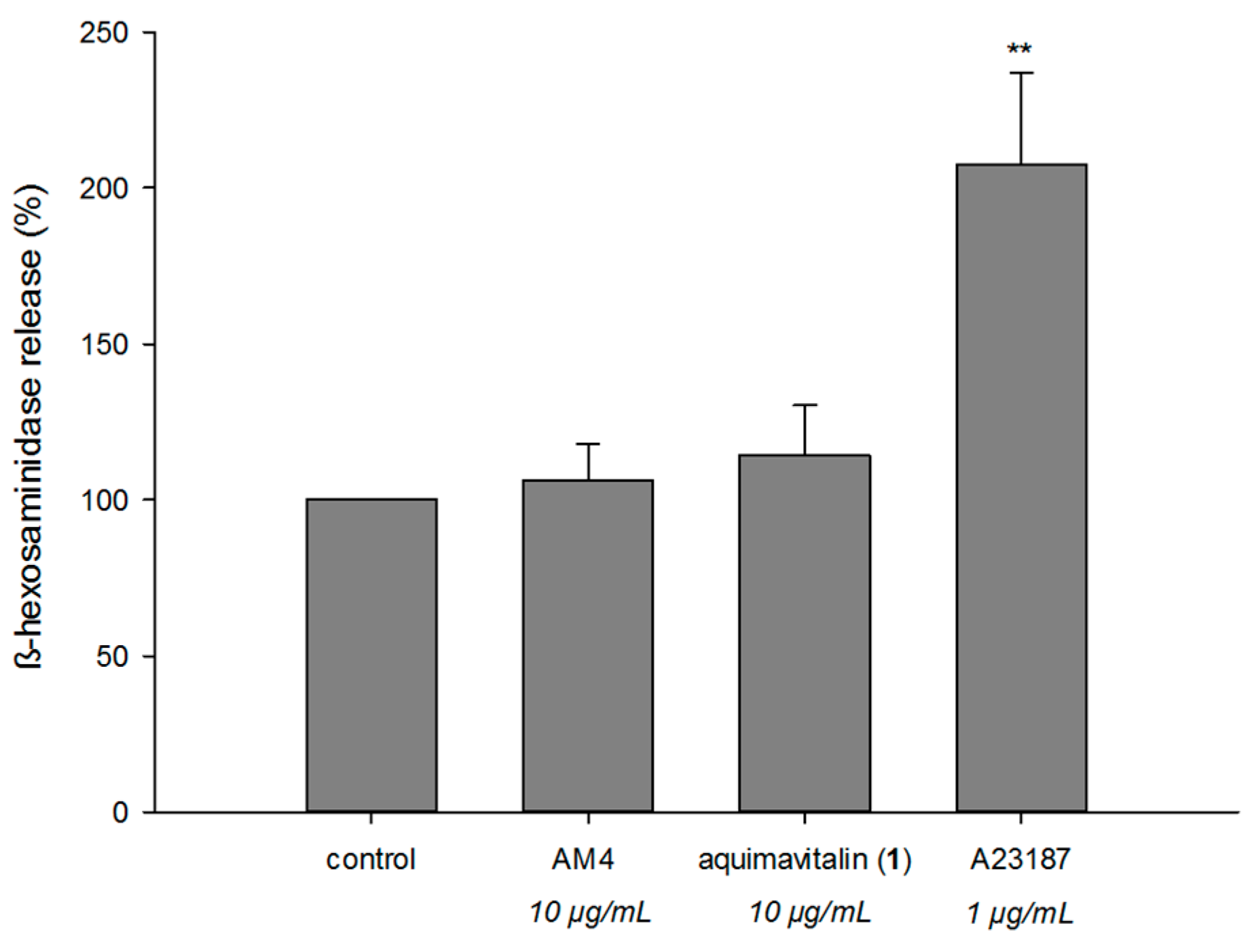
2.4. Antiallergic Activity of Aquimavitalin (1)
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Experimental Data of Aquimavitalin (1)
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. Degranulation β-Hexosaminidase Assay Induced by A23187 or Antigen
3.8. Effect on Enzymatic Activity of β-Hexosaminidase
3.9. Direct Degranulation β-Hexosaminidase Assay Induced by the Sample
3.10. Preparation of Human Neutrophils
3.11. Superoxide Anion Generation Assay and Elastase Release Inhibition Assay
3.12. Cytotoxic Assay
3.13. Statistics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMS | Aquilaria malaccensis seeds |
| A-EtOH | Crude ethanolic extract of Aquilaria malaccensis seeds |
| A-BuOH | n-Butanol layer from Aquilaria malaccensis seeds |
| A-Water | Water layer from Aquilaria malaccensis seeds |
| A-EtOAc | Ethyl acetate layer from Aquilaria malaccensis seeds |
| A-Hexane | n-Hexane layer from Aquilaria malaccensis seeds |
| A-MeOH | Methanol layer from Aquilaria malaccensis seeds |
| AM | Subfractions of methanol layer from Aquilaria malaccensis seeds |
| RBL-2H3 | Rat basophilic leukemia cells |
| HepG2 | Human hepatocellular carcinoma cells |
| A549 | Human breast adenocarcinoma cells |
| MDA-MB231 | Human lung adenocarcinoma cells |
| fMLP/CB | Formyl-methionyl-leucyl-phenylalanine/cytochalasin B |
References
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Cheng, Y.; Zhong, C.; Wang, F. Inhibition of the IgE-mediated activation of RBL-2H3 cells by TIPP, a novel thymic immunosuppressive pentapeptide. Int. J. Mol. Sci. 2015, 16, 2252–2268. [Google Scholar] [CrossRef] [PubMed]
- Dearman, R.J.; Skinner, R.A.; Deakin, N.; Shaw, D.; Kimber, I. Evaluation of an in vitro method for the measurement of specific IgE antibody responses: The rat basophilic leukemia (RBL) cell assay. Toxicology 2005, 206, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wei, J.; Han, X.; Liang, L.; Yang, Y.; Meng, H.; Xu, Y.; Gao, Z. The sesquiterpene biosynthesis and vessel-occlusion formation in stems of Aquilaria sinensis (Lour.) Gilg trees induced by wounding treatments without variation of microbial communities. Int. J. Mol. Sci. 2014, 15, 23589–23603. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.C.-Y.; Chen, C.-H.; Chen, S.-H.; Lu, I.H.; Chu, M.-J.; Huang, L.-C.; Lin, C.-Y.; Chen, C.-Y.; Lo, H.-F.; Jeng, S.-T.; et al. The effect of red light and far-red light conditions on secondary metabolism in agarwood. BMC Plant Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Kamonwannasit, S.; Nantapong, N.; Kumkrai, P.; Luecha, P.; Kupittayanant, S.; Chudapongse, N. Antibacterial activity of Aquilaria crassna leaf extract against Staphylococcus epidermidis by disruption of cell wall. Ann. Clin. Microbiol. Antimicrob. 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Lee, E.H.; Lee, Y.M.; Kim, H.K.; Song, B.K.; Lee, E.J.; Kim, H.M. Effect of the aqueous extract of Aquilaria agallocha stems on the immediate hypersensitivity reactions. J. Ethnopharmacol. 1997, 58, 31–38. [Google Scholar] [CrossRef]
- Hashim, Y.Z.H.-Y.; Phirdaous, A.; Azura, A. Screening of anticancer activity from agarwood essential oil. Pharmacogn. Res. 2014, 6, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A. Gas chromatography–mass spectrometric analysis of the essential oil of eaglewood (Aquilaria agallocha Roxb). Int. J. Pharm. Pharm. Sci. 2014, 6, 629–631. [Google Scholar]
- Yang, L.; Qiao, L.; Ji, C.; Xie, D.; Gong, N.-B.; Lu, Y.; Zhang, J.; Dai, J.; Guo, S. Antidepressant abietane diterpenoids from Chinese eaglewood. J. Nat. Prod. 2013, 76, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.T.L.; Dat, N.T.; Minh, C.V.; Kang, J.S.; Kim, Y.H. Monoamine oxidase inhibitors from Aquilaria Agallocha. Nat. Prod. Sci. USA 2002, 8, 30–33. [Google Scholar]
- Huo, H.-X.; Zhu, Z.-X.; Pang, D.-R.; Li, Y.-T.; Huang, Z.; Shi, S.-P.; Zheng, J.; Zhang, Q.; Zhao, Y.-F.; Tu, P.-F.; et al. Anti-neuroinflammatory sesquiterpenes from Chinese eaglewood. Fitoterapia 2015, 106, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.; Suolangjiba; Kou, J.; Yu, B. Antinociceptive and anti-inflammatory activities of Aquilaria sinensis (Lour.) Gilg. leaves extract. J. Ethnopharmacol. 2008, 117, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Pranakhon, R.; Aromdee, C.; Pannangpetch, P. Effects of iriflophenone 3-C-β-glucoside on fasting blood glucose level and glucose uptake. Pharmacogn. Mag. 2015, 11, 82–89. [Google Scholar] [PubMed]
- Hara, H.; Ise, Y.; Morimoto, N.; Shimazawa, M.; Ichihashi, K.; Ohyama, M.; Iinuma, M. Laxative effect of agarwood leaves and its mechanism. Biosci. Biotechnol. Biochem. 2008, 72, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.; Daud, N.; Halis, R.; Mohamed, R. Effects of plant growth regulators, carbon sources and pH values on callus induction in Aquilaria malaccensis leaf explants and characteristics of the resultant calli. J. For. Res. 2014, 25, 535–540. [Google Scholar] [CrossRef]
- Pant, P.; Rastogi, R.P. Agarol, a new sesquiterpene from Aquilaria agallocha. Phytochemistry 1980, 19, 1869–1870. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Kinghorn, A.D.; Cordell, G.A.; Farnsworth, N.R. Plant anticancer agents. XIX. Constituents of Aquilaria malaccensis. J. Nat. Prod. 1981, 44, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Dyary, H.O.; Arifah, A.K.; Sharma, R.S.; Rasedee, A.; Mohd-Aspollah, M.S.; Zakaria, Z.A.; Zuraini, A.; Somchit, M.N. Antitrypanosomal screening and cytotoxic effects of selected medicinal plants. Trop. Biomed. 2014, 31, 89–96. [Google Scholar] [PubMed]
- Dash, M.; Patra, J.K.; Panda, P.P. Phytochemical and antimicrobial screening of extracts of Aquilaria agallocha Roxb. Afr. J. Biotechnol. 2008, 7, 3531–3534. [Google Scholar]
- Jong, P.L.; Tsan, P.; Mohamed, R. Gas chromatography-mass spectrometry analysis of agarwood extracts from mature and juvenile Aquilaria malaccensis. Int. J. Agr. Biol. 2014, 16, 644–648. [Google Scholar]
- Wang, Q.; Matsuda, H.; Matsuhira, K.; Nakamura, S.; Yuan, D.; Yoshikawa, M. Inhibitory effects of thunberginols A, B, and F on degranulations and releases of TNF-alpha; and IL-4 in RBL-2H3 cells. Biol. Pharm. Bull. 2007, 30, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Wang, L.; Li, F.; Yu, K.; Wang, M.-K. Cytotoxic phorbol esters of Croton tiglium. J. Nat. Prod. 2013, 76, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-F.; Yang, S.-H.; Liu, Y.-Q.; Li, D.-X.; He, W.-J.; Zhang, X.-X.; Liu, Y.-H.; Zhou, X.-J. Five new phorbol esters with cytotoxic and selective anti-inflammatory activities from Croton tiglium. Bioorg. Med. Chem. Lett. 2015, 25, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mohan, S.; Negishi, E.-I. Highly selective synthesis of conjugated dienoic and trienoic esters via alkyne elementometalation–Pd-catalyzed cross-coupling. Proc. Natl. Acad. Sci. USA 2011, 108, 11344–11349. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Wang, X.-Y.; Liu, L.-P.; Qin, G.-W.; Kang, T.-G. Tigliane diterpenoids from the Euphorbiaceae and Thymelaeaceae families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberger, G.; Hecker, E. On the active principles of the Euphorbiaceae, XII. Highly unsaturated irritant diterpene esters from Euphorbia tirucalli originating from Madagascar. J. Nat. Prod. 1986, 49, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Wu, P.Y.; Chen, K.M.; Fu, T.F.; Wang, H.M.; Chen, C.Y. Antiallergic potential on RBL-2H3 cells of some phenolic constituents of Zingiber officinale (Ginger). J. Nat. Prod. 2009, 72, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Tewtrakul, S.; Morikawa, T.; Nakamura, A.; Yoshikawa, M. Anti-allergic principles from Thai zedoary: Structural requirements of curcuminoids for inhibition of degranulation and effect on the release of TNF-α and IL-4 in RBL-2H3 cells. Bioorg. Med. Chem. 2004, 12, 5891–5898. [Google Scholar] [CrossRef] [PubMed]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 1968, 97, 77–89. [Google Scholar]
- Jauregui, H.O.; Hayner, N.T.; Driscoll, J.L.; Williams-Holland, R.; Lipsky, M.H.; Galletti, P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro 1981, 17, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Invest. 1973, 52, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Leu, Y.L.; Kao, S.H.; Tang, M.C.; Chang, H.L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radic. Biol. Med. 2006, 41, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-C.; Tsui, Y.-T.; Singab, A.N.B.; El-Shazly, M.; Du, Y.-C.; Hwang, T.-L.; Wu, C.-C.; Yen, M.-H.; Lee, C.-K.; Hou, M.-F.; et al. Phyto-SERM Constitutes from Flemingia macrophylla. Int. J. Mol. Sci. 2013, 14, 15578–15594. [Google Scholar] [CrossRef] [PubMed]
| Sample | Viability, RBL-2H3 | Inhibition of β-Hexosaminidase Release, Degranulation Assay, RBL-2H3 Cells a | Inhibitory Effect on Enzyme | |||
|---|---|---|---|---|---|---|
| IC50 (μg/mL) b (% Viability at 100 μg/mL) | A23187-Induced IC50 (μg/mL) b | Therapeutical Index c | Antigen-Induced IC50 (μg/mL) b | Therapeutical Index c | β-Hexosaminidase (%) d | |
| A-EtOH | >100 (86.0%) | 0.92 | >109.0 | 3.9 | >25.7 | 12.7 ± 4.2 (100 μg/mL) |
| A-BuOH | >100 (93.3%) | 1.1 | >92.1 | 6.0 | >16.7 | 7.3 ± 5.5 (100 μg/mL) |
| A-Water | >100 (94.0%) | – | – | – | – | N/A e |
| A-EtOAc | >100 (90.3%) | 0.56 | >177.9 | 0.86 | >116.8 | 13.3 ± 2.1 (100 μg/mL) |
| A-Hexane | >100 (95.3%) | 0.83 | >120.1 | 5.1 | >19.5 | 13.7 ± 2.5 (100 μg/mL) |
| A-MeOH | 96.8 | 0.0089 | 10,910.9 | 0.069 | 1405.2 | 5.3 ± 3.2 (10 μg/mL) |
| AM4 | 98.0 | 0.0034 | 28,677.6 | 0.0065 | 15,098.4 | 4.7 ± 4.0 (10 μg/mL) |
| AM4-4 | 70.6 | 4.8 × 10−5 | 1,477,328.2 | 6.8 × 10−4 | 103,776.5 | N/A e |
| AM4-4-7 | 73.8 | 7.4 × 10−4 | 99,680.2 | 0.0065 | 11,309.9 | N/A e |
| AM4-4-8 | 73.4 | 7.6 × 10−6 | 9,645,374.3 | 8.0 × 10−5 | 917,440.9 | N/A e |
| Aquimavitalin (1) | 71.5 | 0.0010 (0.0017 μM) | 71,538.5 | 0.0068 (0.011 μM) | 10,550.2 | 4.3 ± 4.5 (10 μg/mL) |
| Sample | Superoxide Anion Generation (Inh %) | Elastase Release (Inh %) | ||
|---|---|---|---|---|
| A-EtOH | 90.1 ± 5.3 | ** | 85.3 ± 0.8 | ** |
| A-BuOH | 93.9 ± 8.3 | ** | 77.6 ± 2.4 | ** |
| A-Water | 11.4 ± 1.6 | * | 2.7 ± 4.1 | – |
| A-EtOAc | 94.8 ± 5.6 | ** | 85.4 ± 1.8 | ** |
| A-Hexane | 103.4 ± 1.8 | ** | 80.2 ± 4.0 | ** |
| A-MeOH | 96.5 ± 8.0 | ** | 90.4 ± 6.0 | ** |
| AM1 | 54.5 ± 5.7 | ** | 99.2 ± 2.3 | ** |
| AM2 | 68.7 ± 5.0 | ** | 47.5 ± 5.3 | ** |
| AM3 | 105.9 ± 3.4 | ** | 86.8 ± 2.0 | ** |
| AM4 | 100.7 ± 8.1 | ** | 70.9 ± 1.0 | ** |
| AM5 | 102.6 ± 1.5 | ** | 93.5 ± 3.7 | ** |
| AM6 | 102.4 ± 2.0 | ** | 99.3 ± 2.3 | ** |
| Sample | HepG2 b | MDA-MB231 c | A549 d |
|---|---|---|---|
| A-EtOH | 16.0 | 37.2 | 29.7 |
| A-BuOH | 4.2 | 34.4 | 57.1 |
| A-Water | −9.3 | 6.8 | 13.3 |
| A-EtOAc | 1.5 | 41.2 | 23.5 |
| A-Hexane | 25.1 | 42.5 | 16.8 |
| A-MeOH | −0.8 | 30.3 | 32.7 |
| AM1 | 8.1 | 1.7 | −12.6 |
| AM2 | 2.4 | 11.5 | 19.8 |
| AM3 | 25.5 | 46.3 | 39.5 |
| AM4 | 23.4 | 56.5 | 79.3 |
| AM5 | 7.9 | 39.9 | 29.2 |
| AM6 | 5.3 | 56.0 | 39.5 |
| doxorubicin e | 91.3 | 97.7 | 98.0 |
| Position | δH, Multiplicity (J in Hz) | δC, Type | COSY (1H–1H) | HMBC (1H–13C) | NOESY (1H–1H) |
|---|---|---|---|---|---|
| 1 | 7.57 (s) | 160.8 CH | 10, 19 | 4, 10 | 18 |
| 2 | – | 132.8 C | – | – | – |
| 3 | – | 209.3 C | – | – | – |
| 4 | – | 73.6 C | – | – | – |
| 5α | 2.48 (d, J = 18.8) | 38.3 CH2 | 7 | 4, 6, 7 | 5, 20 |
| 5β | 2.58 (d, J = 18.8) | – | – | – | – |
| 6 | – | 140.6 C | – | – | – |
| 7 | 5.68 (brs) | 129.1 CH | 5, 8 | 14, 20 | 14, 20 |
| 8 | 3.26 (t, J = 5.2) | 38.8 CH | 7, 14 | 6, 14, 15 | 11, 17 |
| 9 | – | 78.4 C | – | – | – |
| 10 | 3.22 (brs) | 55.9 CH | 1, 19 | – | – |
| 11 | 2.13 (m) | 43.0 CH | 12, 18 | – | 17, 18 |
| 12 | 5.43 (d, J = 10.4) | 75.9 CH | 11 | 11, 13, 15, 18, 1′ | 18 |
| 13 | – | 65.7 C | – | – | – |
| 14 | 1.08 (d, J = 5.2) | 36.1 CH | 8 | 7, 13, 15, 16 | – |
| 15 | – | 25.6 C | – | – | – |
| 16 | 1.19 (s) | 23.8 CH3 | – | 13, 14, 15, 17 | – |
| 17 | 1.24 (s) | 16.7 CH3 | – | 13, 14, 15, 16 | – |
| 18 | 0.88 (d, overlap) | 14.0 CH3 | 11 | 9, 11, 12 | – |
| 19 | 1.73 (brs) | 10.0 CH3 | 1, 10 | 1, 2, 3 | – |
| 20a | 4.02 (d, J = 12.8) | 67.9 CH2 | – | 5, 6, 7 | – |
| 20b | 3.95 (d, J = 12.8) | – | – | – | – |
| 21 | – | 173.9 C | – | 22 | – |
| 22 | 2.10 (s) | 21.1 CH3 | – | – | – |
| 1′ | – | 166.3 C | – | – | – |
| 2′ | 5.57 (d, J = 11.2) | 115.6 CH | 3′ | – | 3′ |
| 3′ | 6.59 (t, J = 11.6) | 145.6 CH | 2′, 4′ | 1′, 5′ | – |
| 4′ | 7.39 (dd, J = 15.2 and 11.6) | 126.5 CH | 3′, 5′ | – | 6′ |
| 5′ | 6.46 (dd, J = 14.8 and 10.4) | 142.4 CH | 4′, 6′ | – | 7′ |
| 6′ | 6.20 (dd, J = 15.2 and 10.8) | 130.1 CH | 5′, 7′ | – | – |
| 7′ | 5.92 (dt, J = 15.2 and 7.2) | 141.0 CH | 6′, 8′ | – | – |
| 8′ | 2.13 (m) | 33.0 CH2 | 7′, 9′ | 6′, 7′, 9′ | 9′ |
| 9′ | 1.38 (m) | 28.9 CH2 | 8′ | 10′ | – |
| 10′ | 1.26–1.28 (m, overlap) | 29.1 CH2 | – | – | – |
| 11′ | 1.26–1.28 (m, overlap) | 29.1 CH2 | – | – | – |
| 12′ | 1.26–1.28 (m, overlap) | 31.7 CH2 | – | – | – |
| 13′ | 1.26–1.28 (m, overlap) | 22.6 CH2 | – | – | – |
| 14′ | 0.86 (t, J = 7.2) | 14.4 CH3 | – | 12′, 13′ | – |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korinek, M.; Wagh, V.D.; Lo, I.-W.; Hsu, Y.-M.; Hsu, H.-Y.; Hwang, T.-L.; Wu, Y.-C.; Cheng, Y.-B.; Chen, B.-H.; Chang, F.-R. Antiallergic Phorbol Ester from the Seeds of Aquilaria malaccensis. Int. J. Mol. Sci. 2016, 17, 398. https://doi.org/10.3390/ijms17030398
Korinek M, Wagh VD, Lo I-W, Hsu Y-M, Hsu H-Y, Hwang T-L, Wu Y-C, Cheng Y-B, Chen B-H, Chang F-R. Antiallergic Phorbol Ester from the Seeds of Aquilaria malaccensis. International Journal of Molecular Sciences. 2016; 17(3):398. https://doi.org/10.3390/ijms17030398
Chicago/Turabian StyleKorinek, Michal, Vitthal D. Wagh, I-Wen Lo, Yu-Ming Hsu, Hsue-Yin Hsu, Tsong-Long Hwang, Yang-Chang Wu, Yuan-Bin Cheng, Bing-Hung Chen, and Fang-Rong Chang. 2016. "Antiallergic Phorbol Ester from the Seeds of Aquilaria malaccensis" International Journal of Molecular Sciences 17, no. 3: 398. https://doi.org/10.3390/ijms17030398
APA StyleKorinek, M., Wagh, V. D., Lo, I.-W., Hsu, Y.-M., Hsu, H.-Y., Hwang, T.-L., Wu, Y.-C., Cheng, Y.-B., Chen, B.-H., & Chang, F.-R. (2016). Antiallergic Phorbol Ester from the Seeds of Aquilaria malaccensis. International Journal of Molecular Sciences, 17(3), 398. https://doi.org/10.3390/ijms17030398