Immunohistochemical Analysis of Cerebral Thrombi Retrieved by Mechanical Thrombectomy from Patients with Acute Ischemic Stroke
Abstract
:1. Introduction
2. Results
2.1. Demographic and Clinical Characterization of Patients
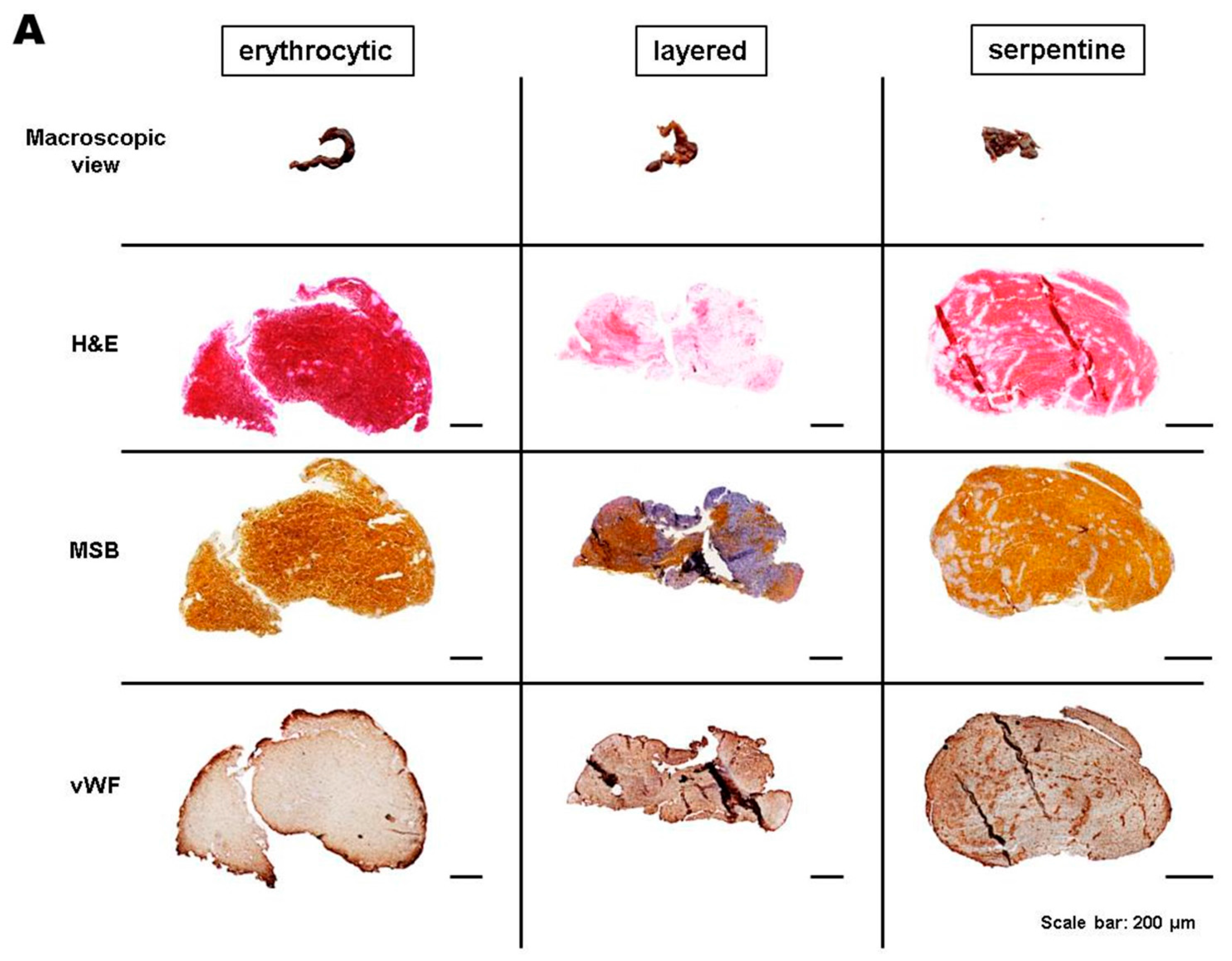
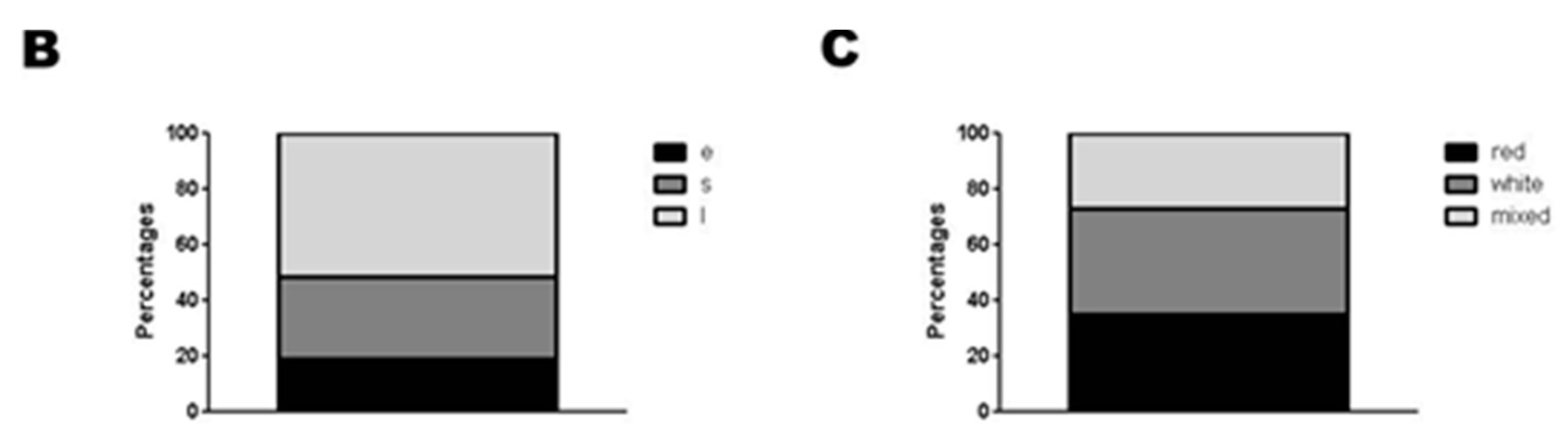
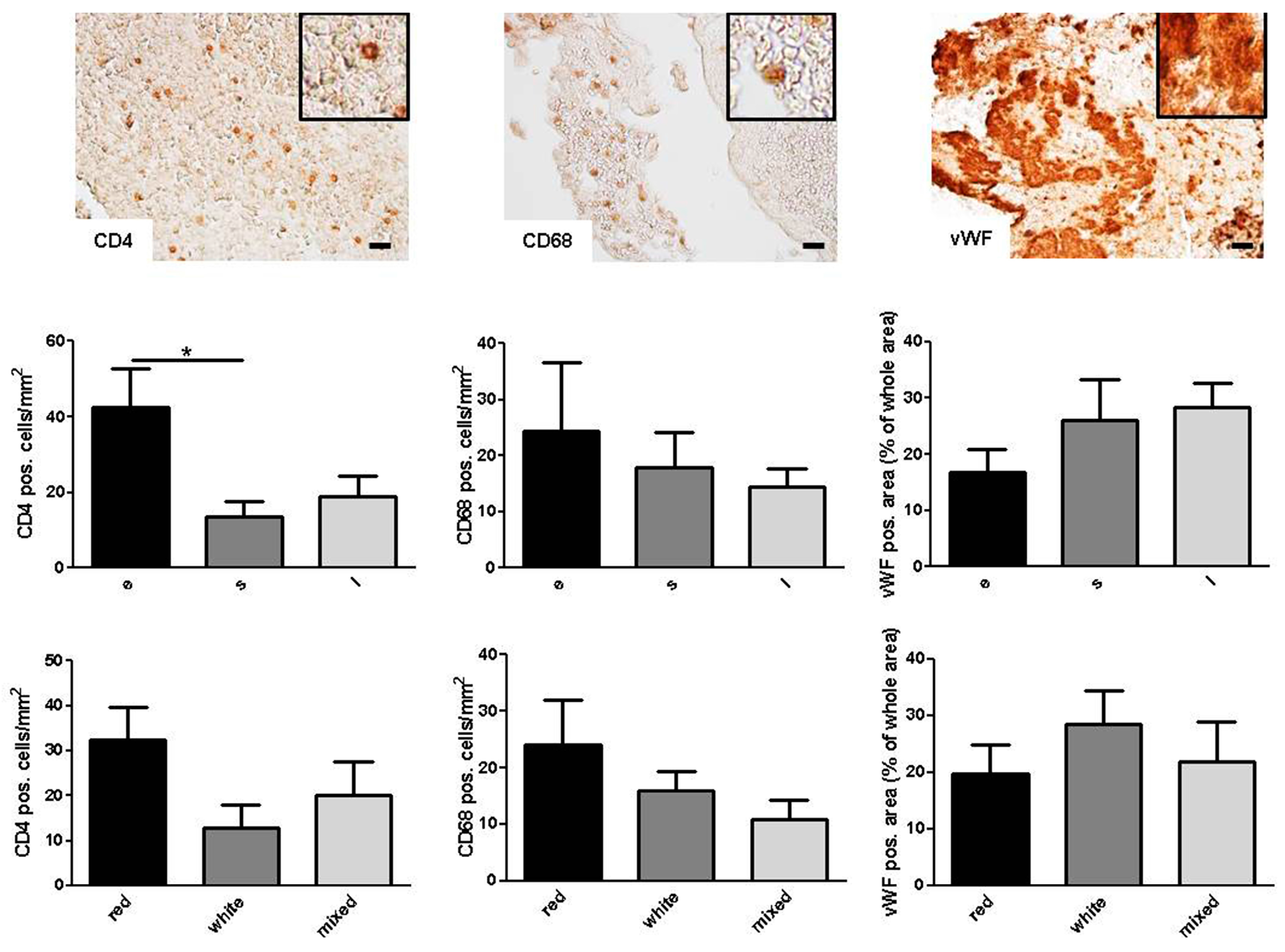
2.2. Categorization of Retrieved Clots
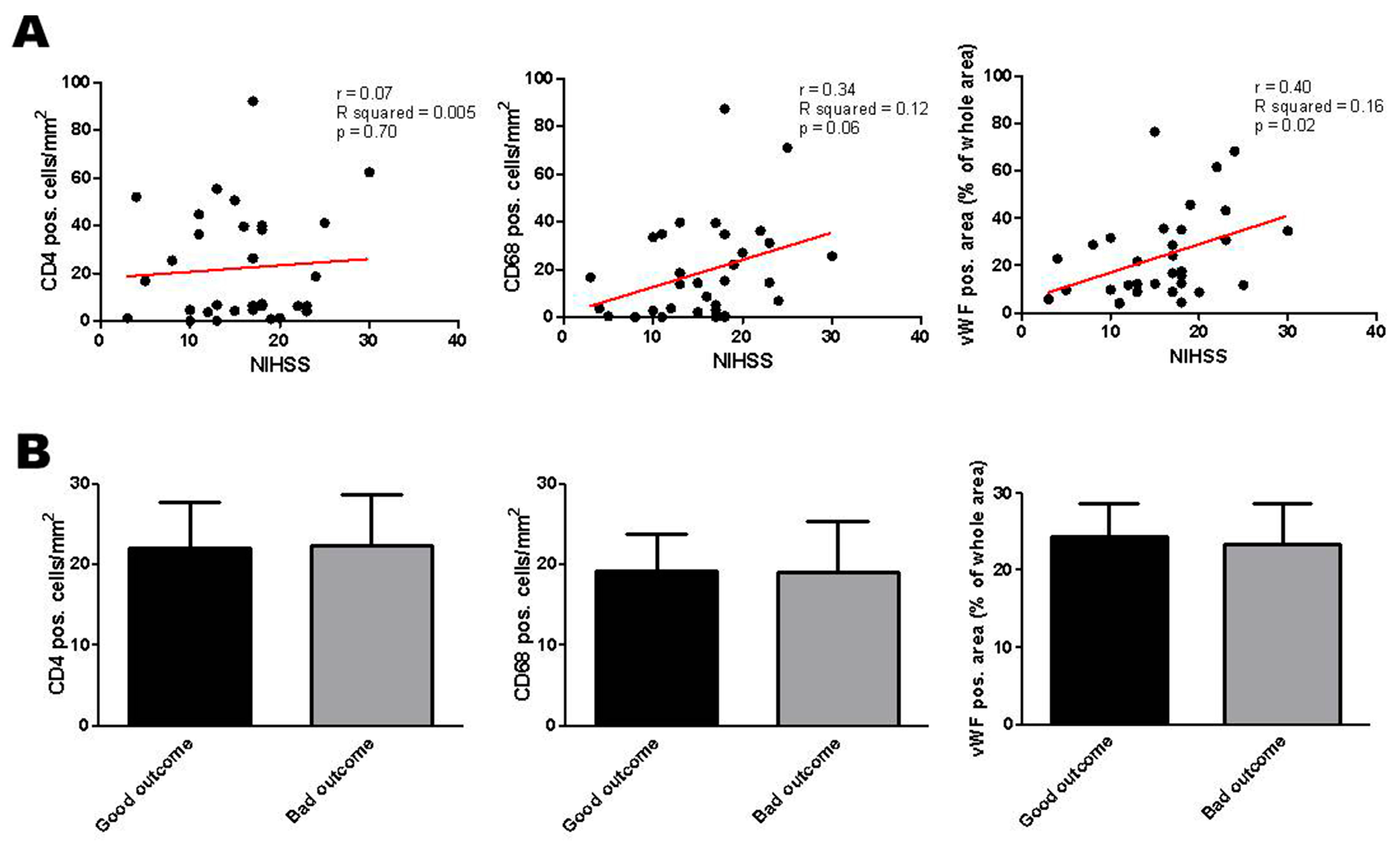
2.3. Correlation of Histologic Results with Clinical Parameters
3. Discussion
4. Materials and Methods
4.1. Patient Population and Study Design
4.2. Thrombectomy Procedure
4.3. Processing of Thrombi and Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AIS | acute ischemic stroke |
| BA | basilar artery |
| CD | cluster of differentiation |
| C-T | intracranial part of the internal carotid artery |
| CT | computed tomography |
| H&E | hematoxylin and eosin |
| IVT | intravenous thrombolysis |
| MCA | middle cerebral artery |
| MRI | magnetic resonance imaging |
| MSB | Martius scarlet blue |
| MT | mechanical thrombectomy |
| NIHSS | National Institutes of Health Stroke Scale |
| RBC | red blood cell |
| TOAST | Trial of Org 10172 in Acute Stroke Treatment |
| TICI | thrombolysis in cerebral infarction |
| vWF | von Willebrand factor |
References
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Mitchell, P.J.; Investigators, E.I. Endovascular therapy for ischemic stroke. N. Engl. J. Med. 2015, 372, 2365–2366. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Roman, L.; Serena, J.; Abilleira, S.; Ribo, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Jauch, E.C.; Saver, J.L.; Adams, H.P., Jr.; Bruno, A.; Connors, J.J.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W., Jr.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef] [PubMed]
- Riedel, C.H.; Zimmermann, P.; Jensen-Kondering, U.; Stingele, R.; Deuschl, G.; Jansen, O. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011, 42, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Jindal, G.; Miller, T.; Shivashankar, R.; Mitchell, J.; Stern, B.J.; Yarbrough, K.; Gandhi, D. Relationship of thrombus length to number of stent retrievals, revascularization, and outcomes in acute ischemic stroke. J. Vasc. Interv. Radiol. 2014, 25, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Pedraza, S.; Demchuk, A.; Daunis, I.E.J.; Termes, H.; Blasco, G.; Soria, G.; Boada, I.; Remollo, S.; Banos, J.; et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. Am. J. Neuroradiol. 2012, 33, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, P.; English, J.D.; Cooke, D.L.; Kim, W.T.; Stout, C.; Smith, W.S.; Dowd, C.F.; Higashida, R.T.; Halbach, V.V.; Hetts, S.W. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013, 44, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Mokin, M.; Morr, S.; Natarajan, S.K.; Lin, N.; Snyder, K.V.; Hopkins, L.N.; Siddiqui, A.H.; Levy, E.I. Thrombus density predicts successful recanalization with Solitaire stent retriever thrombectomy in acute ischemic stroke. J. Neurointerv. Surg. 2015, 7, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L.; Torvik, A. Ischaemic cerebrovascular diseases in an autopsy series: Part 1. Prevalence, location and predisposing factors in verified thrombo-embolic occlusions, and their significance in the pathogenesis of cerebral infarction. J. Neurol. Sci. 1966, 3, 490–509. [Google Scholar] [CrossRef]
- Marder, V.J.; Chute, D.J.; Starkman, S.; Abolian, A.M.; Kidwell, C.; Liebeskind, D.; Ovbiagele, B.; Vinuela, F.; Duckwiler, G.; Jahan, R.; et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006, 37, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Niesten, J.M.; van der Schaaf, I.C.; van Dam, L.; Vink, A.; Vos, J.A.; Schonewille, W.J.; de Bruin, P.C.; Mali, W.P.; Velthuis, B.K. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE 2014, 9, e88882. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S.; Sanossian, N.; Yong, W.H.; Starkman, S.; Tsang, M.P.; Moya, A.L.; Zheng, D.D.; Abolian, A.M.; Kim, D.; Ali, L.K.; et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011, 42, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Yoon, W.; Kim, T.S.; Kim, H.S.; Heo, T.W.; Park, M.S. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. Am. J. Neuroradiol. 2015, 36, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Boeckh-Behrens, T.; Schubert, M.; Forschler, A.; Prothmann, S.; Kreiser, K.; Zimmer, C.; Riegger, J.; Bauer, J.; Neff, F.; Kehl, V.; et al. The Impact of Histological Clot Composition in Embolic Stroke. Clin. Neuroradiol. 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liebeskind, D.S.; Jahan, R.; Nogueira, R.G.; Jovin, T.G.; Lutsep, H.L.; Saver, J.L.; Investigators, S. Serial Alberta Stroke Program early CT score from baseline to 24 hours in Solitaire Flow Restoration with the Intention for Thrombectomy study: A novel surrogate end point for revascularization in acute stroke. Stroke 2014, 45, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Niesten, J.M.; van der Schaaf, I.C.; Biessels, G.J.; van Otterloo, A.E.; van Seeters, T.; Horsch, A.D.; Luitse, M.J.; van der Graaf, Y.; Kappelle, L.J.; Mali, W.P.; et al. Relationship between thrombus attenuation and different stroke subtypes. Neuroradiology 2013, 55, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Simons, N.; Mitchell, P.; Dowling, R.; Gonzales, M.; Yan, B. Thrombus composition in acute ischemic stroke: A histopathological study of thrombus extracted by endovascular retrieval. J. Neuroradiol. 2015, 42, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Minnerup, J.; Kleinschnitz, C. Visualization of clot composition in ischemic stroke: Do we get what we see? Stroke 2011, 42, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Von zur Muhlen, C.; von Elverfeldt, D.; Moeller, J.A.; Choudhury, R.P.; Paul, D.; Hagemeyer, C.E.; Olschewski, M.; Becker, A.; Neudorfer, I.; Bassler, N.; et al. Magnetic resonance imaging contrast agent targeted toward activated platelets allows in vivo detection of thrombosis and monitoring of thrombolysis. Circulation 2008, 118, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Overoye-Chan, K.; Koerner, S.; Looby, R.J.; Kolodziej, A.F.; Zech, S.G.; Deng, Q.; Chasse, J.M.; McMurry, T.J.; Caravan, P. EP-2104R: A fibrin-specific gadolinium-Based MRI contrast agent for detection of thrombus. J. Am. Chem. Soc. 2008, 130, 6025–6039. [Google Scholar] [CrossRef] [PubMed]
- Spuentrup, E.; Botnar, R.M.; Wiethoff, A.J.; Ibrahim, T.; Kelle, S.; Katoh, M.; Ozgun, M.; Nagel, E.; Vymazal, J.; Graham, P.B.; et al. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: Initial results in patients. Eur. Radiol. 2008, 18, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Arumugam, T.V.; Stokes, K.Y.; Granger, D.N. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 2006, 113, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Kraft, P.; Dreykluft, A.; Hagedorn, I.; Gobel, K.; Schuhmann, M.K.; Langhauser, F.; Helluy, X.; Schwarz, T.; Bittner, S.; et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013, 121, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Schwab, N.; Kraft, P.; Hagedorn, I.; Dreykluft, A.; Schwarz, T.; Austinat, M.; Nieswandt, B.; Wiendl, H.; Stoll, G. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 2010, 115, 3835–3842. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Gob, E.; Schuhmann, M.K.; Gobel, K.; Deppermann, C.; Thielmann, I.; Herrmann, A.M.; Lorenz, K.; Brede, M.; Stoll, G.; et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 2013, 44, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Nieswandt, B.; Kleinschnitz, C.; Stoll, G. Ischaemic stroke: A thrombo-inflammatory disease? J. Physiol. 2011, 589, 4115–4123. [Google Scholar] [CrossRef] [PubMed]
- Smout, J.; Dyker, A.; Cleanthis, M.; Ford, G.; Kesteven, P.; Stansby, G. Platelet function following acute cerebral ischemia. Angiology 2009, 60, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Htun, P.; Fateh-Moghadam, S.; Tomandl, B.; Handschu, R.; Klinger, K.; Stellos, K.; Garlichs, C.; Daniel, W.; Gawaz, M. Course of platelet activation and platelet-leukocyte interaction in cerebrovascular ischemia. Stroke 2006, 37, 2283–2287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Fu, Y.; Tian, D.; Sun, N.; Han, W.; Chang, G.; Dong, Y.; Xu, X.; Liu, Q.; Huang, D.; et al. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation 2015, 132, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Weir, C.J.; Murray, G.D.; Povey, C.; Lees, K.R. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke 1996, 27, 1817–1820. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Higashida, R.T.; Furlan, A.J.; Roberts, H.; Tomsick, T.; Connors, B.; Barr, J.; Dillon, W.; Warach, S.; Broderick, J.; Tilley, B.; et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003, 34, e109–e137. [Google Scholar] [CrossRef] [PubMed]
- Benakis, C.; Garcia-Bonilla, L.; Iadecola, C.; Anrather, J. The role of microglia and myeloid immune cells in acute cerebral ischemia. Front. Cell. Neurosci. 2014, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, E.T.; Bulte, J.W. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 2013, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Jacobin-Valat, M.J.; Laroche-Traineau, J.; Lariviere, M.; Mornet, S.; Sanchez, S.; Biran, M.; Lebaron, C.; Boudon, J.; Lacomme, S.; Cerutti, M.; et al. Nanoparticles functionalised with an anti-platelet human antibody for in vivo detection of atherosclerotic plaque by magnetic resonance imaging. Nanomedicine 2015, 11, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Kraft, P.; Drechsler, C.; Schuhmann, M.K.; Gunreben, I.; Kleinschnitz, C. Characterization of Peripheral Immune Cell Subsets in Patients with Acute and Chronic Cerebrovascular Disease: A Case-Control Study. Int. J. Mol. Sci. 2015, 16, 25433–25449. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, Q.; Anrather, J.; Shi, F.D. Immune interventions in stroke. Nat. Rev. Immunol. 2015, 11, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Rinder, H.M.; Bonan, J.L.; Rinder, C.S.; Ault, K.A.; Smith, B.R. Dynamics of leukocyte-platelet adhesion in whole blood. Blood 1991, 78, 1730–1737. [Google Scholar] [PubMed]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: Role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Pende, A.; Artom, N.; Bertolotto, M.; Montecucco, F.; Dallegri, F. Role of Neutrophils in Atherogenesis: An Update. Eur. J. Clin. Investig. 2016, 46, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Idzkowska, E.; Eljaszewicz, A.; Miklasz, P.; Musial, W.J.; Tycinska, A.M.; Moniuszko, M. The Role of Different Monocyte Subsets in the Pathogenesis of Atherosclerosis and Acute Coronary Syndromes. Scand. J. Immunol. 2015, 82, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zernecke, A. Dendritic cells in atherosclerosis: Evidence in mice and humans. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Makris, G.C.; Teng, Z.; Patterson, A.J.; Lin, J.M.; Young, V.; Graves, M.J.; Gillard, J.H. Advances in MRI for the evaluation of carotid atherosclerosis. Br. J Radiol. 2015, 88, 20140282. [Google Scholar] [CrossRef] [PubMed]
- Alie, N.; Eldib, M.; Fayad, Z.A.; Mani, V. Inflammation, Atherosclerosis, and Coronary Artery Disease: PET/CT for the Evaluation of Atherosclerosis and Inflammation. Clin. Med. Insights Cardiol. 2014, 8 (Suppl. 3), S13–S21. [Google Scholar]
- Singh, P.; Doostkam, S.; Reinhard, M.; Ivanovas, V.; Taschner, C.A. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: Does stent-retriever cause intimal damage? Stroke 2013, 44, 1720–1722. [Google Scholar] [CrossRef] [PubMed]
- Mohlenbruch, M.; Stampfl, S.; Behrens, L.; Herweh, C.; Rohde, S.; Bendszus, M.; Hametner, C.; Nagel, S.; Ringleb, P.A.; Pham, M. Mechanical thrombectomy with stent retrievers in acute basilar artery occlusion. Am. J. Neuroradiol. 2014, 35, 959–964. [Google Scholar] [CrossRef] [PubMed]
| No. | Sex | Age, Years | Smoker | Vascular Site | Lysis | NIHSS Admission | NIHSS Discharge | Thrombus Histology |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 54 | Yes | Right MCA | Yes | 15 | 8 | e/red |
| 2 | F | 72 | No | Right C-T | No | 4 | 8 | e/red |
| 3 | F | 83 | No | Right C-T | Yes | 17 | 7 | e/red |
| 4 | F | 57 | No | Left MCA | Yes | 18 | 2 | s/red |
| 5 | M | 61 | Yes | Left MCA | Yes | 16 | 4 | l/mixed |
| 6 | M | 42 | No | Left C-T | Yes | 18 | 10 | l/white |
| 7 | M | 50 | Yes | Right MCA | Yes | 24 | 3 | s/mixed |
| 8 | F | 72 | No | BA | No | 23 | 9 | l/red |
| 9 | F | 74 | No | BA | Yes | 28 | 10 | l/red |
| 10 | F | 59 | No | Left MCA | No | 23 | 7 | s/white |
| 11 | F | 80 | No | Left C-T | Yes | 23 | 14 | l/white |
| 12 | M | 56 | Yes | Right MCA | Yes | 8 | 8 | e/red |
| 13 | M | 54 | No | Right MCA | Yes | 30 | 11 | l/white |
| 14 | F | 83 | Yes | BA | Yes | 31 | Deceased | s/mixed |
| 15 | F | 56 | No | Left MCA | Yes | 17 | 10 | l/mixed |
| 16 | M | 58 | No | Left MCA | Yes | 18 | 8 | s/red |
| 17 | M | 57 | No | Left MCA | Yes | 10 | 4 | l/white |
| 18 | F | 84 | No | Left MCA | Yes | 15 | 9 | s/white |
| 19 | F | 75 | No | Left C-T | Yes | 19 | 7 | l/mixed |
| 20 | M | 61 | Yes | BA | Yes | 4 | 0 | l/white |
| 21 | M | 67 | No | Left C-T | No | 13 | 15 | l/white |
| 22 | M | 75 | No | Left MCA | Yes | 13 | 3 | l/white |
| 23 | F | 82 | No | Right MCA | Yes | 22 | 5 | l/white |
| 24 | F | 83 | No | Right MCA | No | 17 | 7 | e/red |
| 25 | F | 80 | No | Right MCA | Yes | 5 | 2 | s/mixed |
| 26 | F | 69 | No | Left MCA | Yes | 18 | 13 | e/red |
| 27 | M | 43 | No | Left MCA | Yes | 18 | 6 | s/red |
| 28 | F | 93 | No | Right MCA | Yes | 11 | 4 | l/white |
| 29 | M | 40 | No | Right MCA | Yes | 10 | 1 | l/white |
| 30 | M | 77 | No | Left C-T | Yes | 20 | 13 | s/white |
| 31 | M | 28 | No | Right C-T | Yes | 12 | 0 | s/mixed |
| 32 | F | 63 | No | Left MCA | No | 25 | 10 | s/red |
| 33 | F | 84 | No | Right MCA | No | 17 | 9 | l/white |
| 34 | M | 47 | No | Right C-T | No | 11 | 1 | e/red |
| 35 | M | 95 | n.d. | Left MCA | No | 13 | Deceased | l/red |
| 36 | M | 58 | Yes | Left C-T | No | 3 | 12 | l/mixed |
| 37 | M | 60 | Yes | BA | No | 23 | 3 | l/mixed |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuhmann, M.K.; Gunreben, I.; Kleinschnitz, C.; Kraft, P. Immunohistochemical Analysis of Cerebral Thrombi Retrieved by Mechanical Thrombectomy from Patients with Acute Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 298. https://doi.org/10.3390/ijms17030298
Schuhmann MK, Gunreben I, Kleinschnitz C, Kraft P. Immunohistochemical Analysis of Cerebral Thrombi Retrieved by Mechanical Thrombectomy from Patients with Acute Ischemic Stroke. International Journal of Molecular Sciences. 2016; 17(3):298. https://doi.org/10.3390/ijms17030298
Chicago/Turabian StyleSchuhmann, Michael K., Ignaz Gunreben, Christoph Kleinschnitz, and Peter Kraft. 2016. "Immunohistochemical Analysis of Cerebral Thrombi Retrieved by Mechanical Thrombectomy from Patients with Acute Ischemic Stroke" International Journal of Molecular Sciences 17, no. 3: 298. https://doi.org/10.3390/ijms17030298
APA StyleSchuhmann, M. K., Gunreben, I., Kleinschnitz, C., & Kraft, P. (2016). Immunohistochemical Analysis of Cerebral Thrombi Retrieved by Mechanical Thrombectomy from Patients with Acute Ischemic Stroke. International Journal of Molecular Sciences, 17(3), 298. https://doi.org/10.3390/ijms17030298






