Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts
Abstract
:1. Introduction
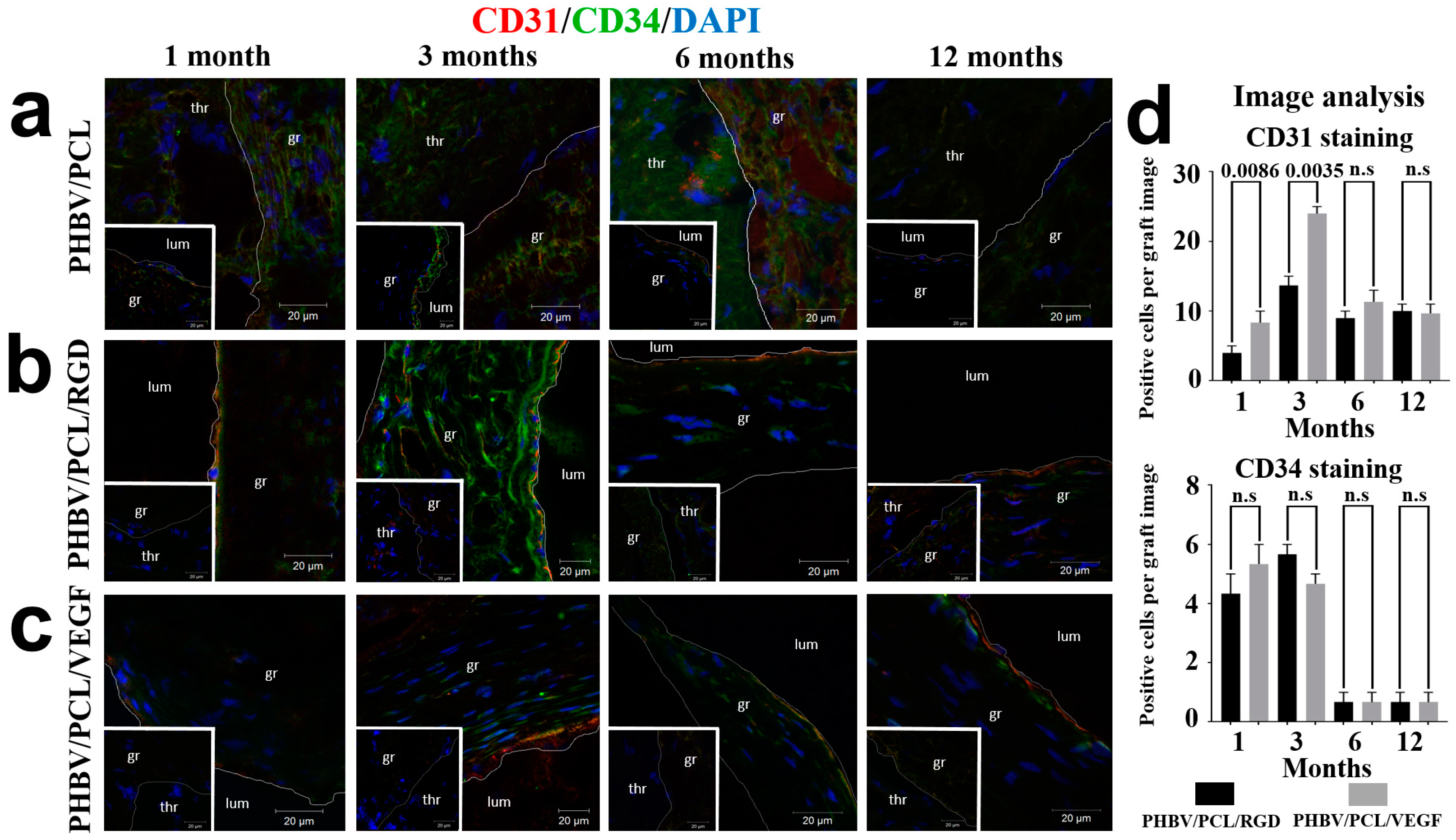
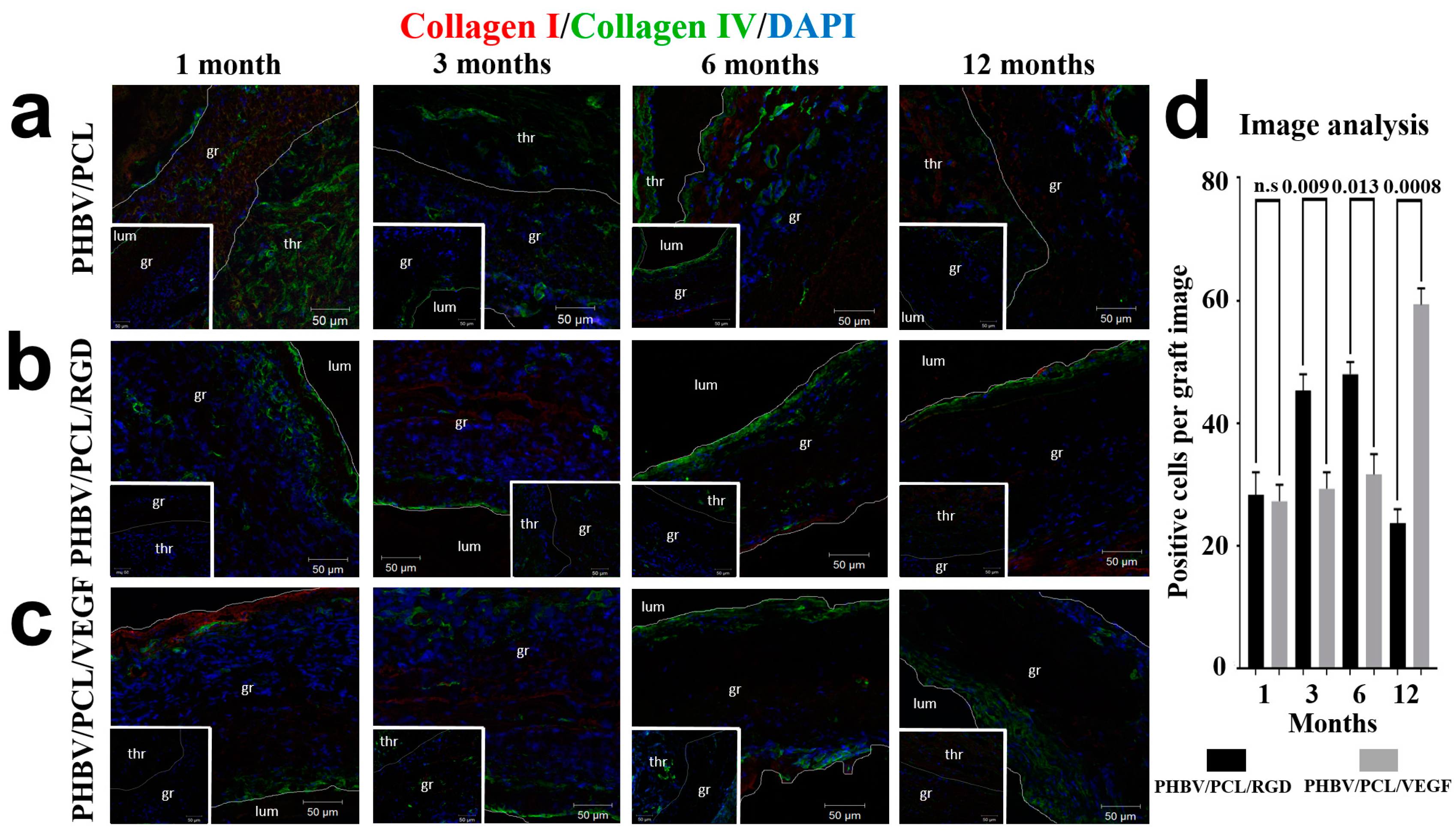
2. Results
3. Discussion
4. Materials and Methods
4.1. Graft Preparation
4.2. Polymer Amination–Activation
4.3. RGD Peptide Conjugation
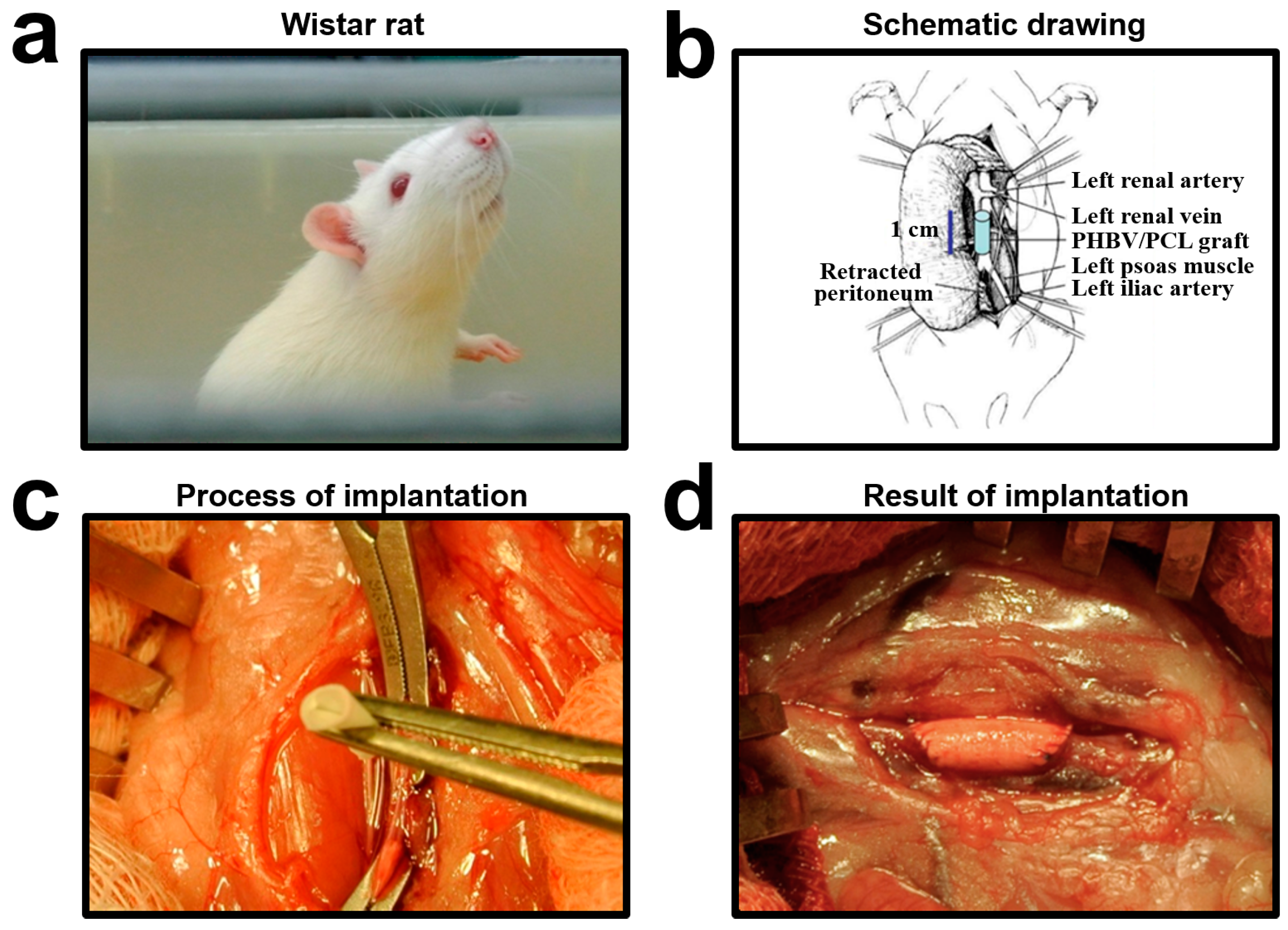
4.4. In Vivo Implantation
4.5. Histological Examination
4.6. Immunofluorescence Examination
4.7. Scanning Electron Microscopy
4.8. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Benrashid, E.; McCoy, C.C.; Youngwirth, L.M.; Kim, J.; Manson, R.J.; Otto, J.C.; Lawson, J.H. Tissue engineered vascular grafts: Origins, development, and current strategies for clinical application. Methods 2016, 99, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rocco, K.A.; Maxfield, M.W.; Best, C.A.; Dean, E.W.; Breuer, C.K. In vivo applications of electrospun tissue-engineered vascular grafts: A review. Tissue Eng. Part B Rev. 2014, 20, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Tara, S.; Rocco, K.A.; Hibino, N.; Sugiura, T.; Kurobe, H.; Breuer, C.K.; Shinoka, T. Vessel bioengineering. Circ. J. 2014, 78, 121–129. [Google Scholar] [CrossRef]
- Ren, X.; Feng, Y.; Guo, J.; Wang, H.; Li, Q.; Yang, J.; Hao, X.; Lv, J.; Ma, N.; Li, W. Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications. Chem. Soc. Rev. 2015, 44, 5680–5742. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.S.; Lindsey, B.; Dalby, M.J.; Gadegaard, N.; Seifalian, A.M.; Hamilton, G. Luminal surface engineering, “micro and nanopatterning”: Potential for self endothelialising vascular grafts? Eur. J. Vasc. Endovasc. Surg. 2014, 47, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, V.D.; Bruno, A.; Tomasello, G.; Damiano, G.; Lo Monte, A.I. Bioengineered vascular scaffolds: The state of the art. Int. J. Artif. Organs 2014, 37, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, K.K.; Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Nanoarchitecture of scaffolds and endothelial cells in engineering small diameter vascular grafts. Biotechnol. J. 2015, 10, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Ingavle, G.C.; Leach, J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Rocco, K.; Kurobe, H.; Maxfield, M.; Breuer, C.; Shinoka, T. Tissue engineering in the vasculature. Anat. Rec. 2014, 297, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Quillaguamán, J.; Guzmán, H.; van-Thuoc, D.; Hatti-Kaul, R. Synthesis and production of polyhydroxyalkanoates by halophiles: Current potential and future prospects. Appl. Microbiol. Biotechnol. 2010, 85, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Giavaresi, G.; Tschon, M.; Borsari, V.; Daly, J.H.; Liggat, J.J.; Fini, M.; Bonazzi, V.; Nicolini, A.; Carpi, A.; Morra, M.; et al. New polymers for drug delivery systems in orthopaedics: In vivo biocompatibility evaluation. Biomed. Pharmacother. 2004, 58, 411–417. [Google Scholar] [CrossRef]
- Chen, G.Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Mukhamadiyarov, R.A.; Mironov, A.V.; Burago, A.Y.; Velikanova, E.A.; Sidorova, O.D.; Kudryavtseva, Y.A.; Barbarash, O.L.; Barbarash, L.S. A morphological investigation of the polyhydroxybutyrate/valerate and polycaprolactone biodegradable small-diameter vascular graft biocompatibility. Genes Cells 2015, 10, 71–77. [Google Scholar]
- Woods, I.; Flanagan, T.C. Electrospinning of biomimetic scaffolds for tissue-engineered vascular grafts: Threading the path. Expert Rev. Cardiovasc. Ther. 2014, 12, 815–832. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Shen, Y.; Wang, A.; Wang, S.; Xie, T. The functions and applications of RGD in tumor therapy and tissue engineering. Int. J. Mol. Sci. 2013, 14, 13447–13462. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Azimi-Nezhad, M. Vascular endothelial growth factor from embryonic status to cardiovascular pathology. Rep. Biochem. Mol. Biol. 2014, 2, 59–69. [Google Scholar] [PubMed]
- Thanigaimani, S.; Kichenadasse, G.; Mangoni, A.A. The emerging role of vascular endothelial growth factor (VEGF) in vascular homeostasis: Lessons from recent trials with anti-VEGF drugs. Curr. Vasc. Pharmacol. 2011, 9, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Krivkina, E.O.; Mironov, A.V.; Burago, A.Y.; Velikanova, E.A.; Matveeva, V.G.; Glushkova, T.V.; et al. Bioabsorbable bypass grafts biofunctionalised with RGD have enhanced biophysical properties and endothelialisation tested in vivo. Front. Pharmacol. 2016, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Antonova, L.V.; Sevostyanova, V.V.; Kutikhin, A.G.; Mironov, A.V.; Krivkina, E.O.; Shabaev, A.R.; Matveeva, V.G.; Velikanova, E.A.; Sergeeva, E.A.; Burago, A.Y.; et al. Vascular endothelial growth factor improves physico-mechanical properties and enhances endothelialization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(ε-caprolactone) small-diameter vascular grafts in vivo. Front. Pharmacol. 2016, 7, 230. [Google Scholar] [CrossRef] [PubMed]
- Talacua, H.; Smits, A.I.; Muylaert, D.E.; van Rijswijk, J.W.; Vink, A.; Verhaar, M.C.; Driessen-Mol, A.; van Herwerden, L.A.; Bouten, C.V.; Kluin, J.; et al. In situ tissue engineering of functional small-diameter blood vessels by host circulating cells only. Tissue Eng. Part A 2015, 21, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yamamoto, K.; Ando, J.; Matsumoto, K.; Matsuda, T. Arterial shear stress augments the differentiation of endothelial progenitor cells adhered to VEGF-bound surfaces. Biochem. Biophys. Res. Commun. 2012, 423, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Inoue, O.; Suzuki-Inoue, K.; Ozaki, Y. Redundant mechanism of platelet adhesion to laminin and collagen under flow: Involvement of von Willebrand factor and glycoprotein Ib-IX-V. J. Biol. Chem. 2008, 283, 16279–16282. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. Basement membranes: Structure; assembly and role in tumour angiogenesis. Nat. Rev. Cancer. 2003, 3, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Bondar, B.; Fuchs, S.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Functionality of endothelial cells on silk fibroin nets: Comparative study of micro- and nanometric fibre size. Biomaterials 2008, 29, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Patra, P.K.; Warner, S.B.; Bhowmick, S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007, 13, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Rim, N.G.; Shin, C.S.; Shin, H. Current approaches to electrospun nanofibers for tissue engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tsukada, M.; Morikawa, H.; Aojima, K.; Zhang, G.; Miura, M. Production of silk sericin/silk fibroin blend nanofibers. Nanoscale Res. Lett. 2011, 6, 510. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, D.E.; van Almen, G.C.; Talacua, H.; Fledderus, J.O.; Kluin, J.; Hendrikse, S.I.; van Dongen, J.L.; Sijbesma, E.; Bosman, A.W.; Mes, T.; et al. Early in-situ cellularization of a supramolecular vascular graft is modified by synthetic stromal cell-derived factor-1α derived peptides. Biomaterials 2016, 76, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.V.; Lekshmi, V.; Nair, P.D. Tissue engineered vascular grafts—Preclinical aspects. Int. J. Cardiol. 2013, 167, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Swartz, D.D.; Andreadis, S.T. Animal models for vascular tissue-engineering. Curr. Opin. Biotechnol. 2013, 24, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Hamilton, G.; Seifalian, A.M. The performance of a small-calibre graft for vascular reconstructions in a senescent sheep model. Biomaterials 2014, 35, 9033–9040. [Google Scholar] [CrossRef] [PubMed]
- Causa, F.; Battista, E.; Della Moglie, R.; Guarnieri, D.; Iannone, M.; Netti, P.A. Surface investigation on biomimetic materials to control cell adhesion: The case of RGD conjugation on PCL. Langmuir 2010, 26, 9875–9884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hollister, S. Comparison of bone marrow stromal cell behaviors on poly(caprolactone) with or without surface modification: Studies on cell adhesion; survival and proliferation. J. Biomater. Sci. Polym. Ed. 2009, 20, 1975–1993. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; van Nieuw Amerongen, G.P.; van Hinsbergh, V.W.; Amerongen, A.V.; Zentner, A. Direct grafting of RGD-motif-containing peptide on the surface of polycaprolactone films. J. Biomater. Sci. Polym. Ed. 2006, 17, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Sedaghati, T.; Jell, G.; Seifalian, A. Investigation of Schwann cell behaviour on RGD-functionalised bioabsorbable nanocomposite for peripheral nerve regeneration. New Biotechnol. 2014, 31, 203–213. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonova, L.V.; Seifalian, A.M.; Kutikhin, A.G.; Sevostyanova, V.V.; Matveeva, V.G.; Velikanova, E.A.; Mironov, A.V.; Shabaev, A.R.; Glushkova, T.V.; Senokosova, E.A.; et al. Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts. Int. J. Mol. Sci. 2016, 17, 1920. https://doi.org/10.3390/ijms17111920
Antonova LV, Seifalian AM, Kutikhin AG, Sevostyanova VV, Matveeva VG, Velikanova EA, Mironov AV, Shabaev AR, Glushkova TV, Senokosova EA, et al. Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts. International Journal of Molecular Sciences. 2016; 17(11):1920. https://doi.org/10.3390/ijms17111920
Chicago/Turabian StyleAntonova, Larisa V., Alexander M. Seifalian, Anton G. Kutikhin, Victoria V. Sevostyanova, Vera G. Matveeva, Elena A. Velikanova, Andrey V. Mironov, Amin R. Shabaev, Tatiana V. Glushkova, Evgeniya A. Senokosova, and et al. 2016. "Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts" International Journal of Molecular Sciences 17, no. 11: 1920. https://doi.org/10.3390/ijms17111920
APA StyleAntonova, L. V., Seifalian, A. M., Kutikhin, A. G., Sevostyanova, V. V., Matveeva, V. G., Velikanova, E. A., Mironov, A. V., Shabaev, A. R., Glushkova, T. V., Senokosova, E. A., Vasyukov, G. Y., Krivkina, E. O., Burago, A. Y., Kudryavtseva, Y. A., Barbarash, O. L., & Barbarash, L. S. (2016). Conjugation with RGD Peptides and Incorporation of Vascular Endothelial Growth Factor Are Equally Efficient for Biofunctionalization of Tissue-Engineered Vascular Grafts. International Journal of Molecular Sciences, 17(11), 1920. https://doi.org/10.3390/ijms17111920





