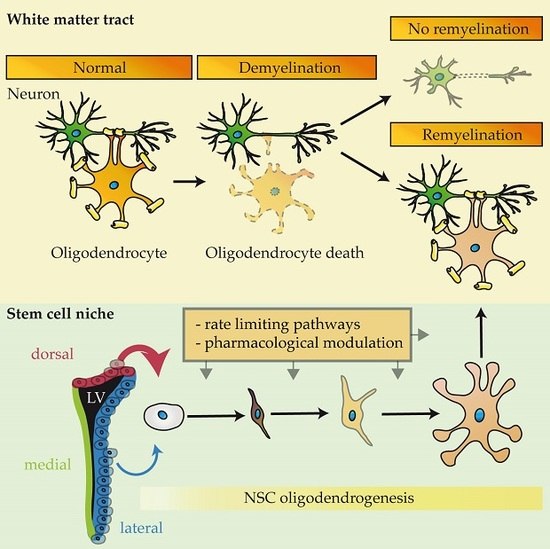
Taking Advantage of Nature’s Gift: Can Endogenous Neural Stem Cells Improve Myelin Regeneration?
Abstract
:1. Introduction
2. Endogenous versus Exogenous Stem Cell-Mediated Repair
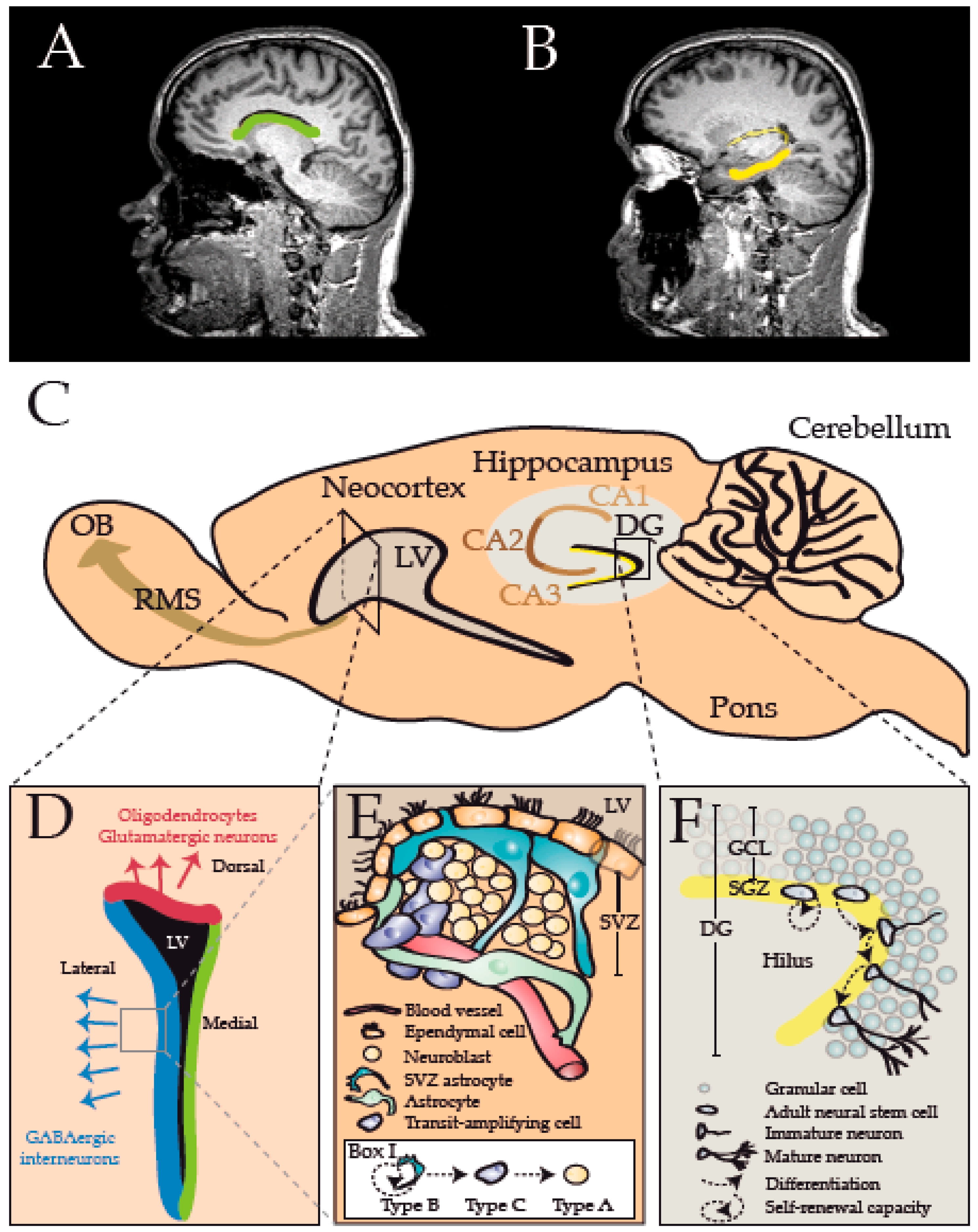
3. Neural Stem Cell Populations of the Central Nervous System
4. How Do NSCs Respond to Demyelination and Contribute to Myelin Regeneration?
5. How Is NSC-Oligodendrogenesis Regulated?
5.1. Extrinsic and Intrinsic Factors Regulating NSC-Oligodendrogenesis that Overlap with Parenchymal Oligodendrogenesis
5.2. Extrinsic and Intrinsic Factors Regulating NSC-Oligodendrogenesis
5.3. Inflammatory Factors Regulating NSC-Oligodendrogenesis
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fünfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Möbius, W.; et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Kornek, B.; Lassmann, H. Neuropathology of multiple sclerosis-new concepts. Brain Res. Bull. 2003, 61, 321–326. [Google Scholar] [CrossRef]
- Nave, K.-A. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010, 11, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2008, 372, 1502–1517. [Google Scholar] [CrossRef]
- Patani, R.; Balaratnam, M.; Vora, A.; Reynolds, R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 2007, 33, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Rivers, L.E.; Young, K.M.; Rizzi, M.; Jamen, F.; Psachoulia, K.; Wade, A.; Kessaris, N.; Richardson, W.D. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 2008, 11, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.R.; Sun, T.; Zhu, Z.; Ma, N.; Garcia, M.; Stiles, C.D.; Rowitch, D.H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 2002, 109, 75–86. [Google Scholar] [CrossRef]
- Takebayashi, H.; Nabeshima, Y.; Yoshida, S.; Chisaka, O.; Ikenaka, K.; Nabeshima, Y.I. The basic helix-loop-helix factor Olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 2002, 12, 1157–1163. [Google Scholar] [CrossRef]
- Redwine, J.M.; Armstrong, R.C. In vivo proliferation of oligodendrocyte progenitors expressing PDGFαR during early remyelination. J. Neurobiol. 1998, 37, 413–428. [Google Scholar] [CrossRef]
- Levine, J.M.; Reynolds, R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp. Neurol. 1999, 160, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Nishiyama, A.; Peterson, J.; Prineas, J.; Trapp, B.D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 2000, 20, 6404–6412. [Google Scholar] [PubMed]
- Kessaris, N.; Fogarty, M.; Iannarelli, P.; Grist, M.; Wegner, M.; Richardson, W.D. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 2006, 9, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.A.; Rudelson, J.J.; Tse, P.U. White matter structure changes as adults learn a second language. J. Cogn. Neurosci. 2012, 24, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, I.A.; Ohayon, D.; Li, H.; de Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Nedergaard, M.; Windrem, M.S. Glial progenitor cell-based treatment and modeling of neurological disease. Science 2012, 338, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002, 3, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Patrikios, P.; Stadelmann, C.; Kutzelnigg, A.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Bruck, W.; Lucchinetti, C.; Lassmann, H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 2006, 129, 3165–3172. [Google Scholar] [CrossRef] [PubMed]
- Piaton, G.; Williams, A.; Seilhean, D.; Lubetzki, C. Remyelination in multiple sclerosis. Prog. Brain Res. 2009, 175, 453–464. [Google Scholar] [PubMed]
- Goldschmidt, T.; Antel, J.; König, F.B.; Brück, W.; Kuhlmann, T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology 2009, 72, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Tourtellotte, W.W.; Rudick, R.; Trapp, B.D. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N. Engl. J. Med. 2002, 346, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Aktas, O.; Hartung, H.-P.; Küry, P. The complex world of oligodendroglial differentiation inhibitors. Ann. Neurol. 2011, 69, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Miron, V.; Cui, Q.; Cuo, Q.; Wegner, C.; Antel, J.; Brück, W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 2008, 131, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Scolding, N.; Franklin, R.; Stevens, S.; Heldin, C.H.; Compston, A.; Newcombe, J. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain 1998, 121, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Wolswijk, G. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 1998, 18, 601–609. [Google Scholar] [PubMed]
- Rivera, F.J.; Steffenhagen, C.; Kremer, D.; Kandasamy, M.; Sandner, B.; Couillard-Despres, S.; Weidner, N.; Küry, P.; Aigner, L. Deciphering the oligodendrogenic program of neural progenitors: Cell intrinsic and extrinsic regulators. Stem Cells Dev. 2010, 19, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Buchholtz, A. On the occurrence of karyokinesis in cells of the central nervous system of neonatal and adolescent dogs and rabbits. Neur. Zbl. 1890, 9, 140–142. [Google Scholar]
- Allen, E. The cessation of mitosis in the central nervous system of the albino rat. J. Comp. Neurol. 1912, 22, 547–568. [Google Scholar]
- Doetsch, F.; García-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 1997, 17, 5046–5061. [Google Scholar] [PubMed]
- Gage, F.H.; Kempermann, G.; Palmer, T.D.; Peterson, D.A.; Ray, J. Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 1998, 36, 249–266. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef]
- Quiñones-Hinojosa, A.; Sanai, N.; Soriano-Navarro, M.; Gonzalez-Perez, O.; Mirzadeh, Z.; Gil-Perotin, S.; Romero-Rodriguez, R.; Berger, M.S.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cellular composition and cytoarchitecture of the adult human subventricular zone: A niche of neural stem cells. J. Comp. Neurol. 2006, 494, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Gould, E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 1994, 61, 203–209. [Google Scholar] [CrossRef]
- Doetsch, F.; Caillé, I.; Lim, D.A.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Graham, V.; Khudyakov, J.; Ellis, P.; Pevny, L. SOX2 functions to maintain neural progenitor identity. Neuron 2003, 39, 749–765. [Google Scholar] [CrossRef]
- Platel, J.C.; Gordon, V.; Heintz, T.; Bordey, A. GFAP-GFP neural progenitors are antigenically homogeneous and anchored in their enclosed mosaic niche. Glia 2009, 57, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Giachino, C.; Basak, O.; Lugert, S.; Knuckles, P.; Obernier, K.; Fiorelli, R.; Frank, S.; Raineteau, O.; Alvarez-Buylla, A.; Taylor, V. Molecular diversity subdivides the adult forebrain neural stem cell population. Stem Cells 2014, 32, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Temple, S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature 1994, 372, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Picard-Riera, N.; Decker, L.; Delarasse, C.; Goude, K.; Nait-Oumesmar, B.; Liblau, R.; Pham-Dinh, D.; Baron-van Evercooren, A. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. USA 2002, 99, 13211–13216. [Google Scholar] [CrossRef] [PubMed]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.L.; Röth, P.T.; Stratton, J.A.S.; Chuang, B.H.A.; Danne, J.; Ellis, S.L.; Ng, S.W.; Kilpatrick, T.J.; Merson, T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 2014, 34, 14128–14146. [Google Scholar] [CrossRef] [PubMed]
- Brousse, B.; Magalon, K.; Durbec, P.; Cayre, M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol. Open 2015, 4, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Feliú, A.; Carrillo-Salinas, F.J.; Mestre, L.; Guaza, C. Mobilization of progenitors in the subventricular zone to undergo oligodendrogenesis in the Theiler’s virus model of multiple sclerosis: Implications for remyelination at lesions sites. Exp. Neurol. 2013, 250, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Nait-Oumesmar, B.; Picard-Riera, N.; Kerninon, C.; Decker, L.; Seilhean, D.; Höglinger, G.U.; Hirsch, E.C.; Reynolds, R.; Baron-van Evercooren, A. Activation of the subventricular zone in multiple sclerosis: Evidence for early glial progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 4694–4699. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Sospedra, M.; Rosito, M.; Engelhardt, B. Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur. J. Immunol. 2016, 9, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Groves, A.K.; Barnett, S.C.; Franklin, R.J.; Crang, A.J.; Mayer, M.; Blakemore, W.F.; Noble, M. Repair of demyelinated lesions by transplantation of purified O-2A progenitor cells. Nature 1993, 362, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Jadasz, J.J.; Aigner, L.; Rivera, F.J.; Küry, P. The remyelination Philosopher’s Stone: Stem and progenitor cell therapies for multiple sclerosis. Cell Tissue Res. 2012, 349, 331–347. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, W.F.; Gilson, J.M.; Crang, A.J. Transplanted glial cells migrate over a greater distance and remyelinate demyelinated lesions more rapidly than endogenous remyelinating cells. J. Neurosci. Res. 2000, 61, 288–294. [Google Scholar] [CrossRef]
- Sher, F.; Amor, S.; Gerritsen, W.; Baker, D.; Jackson, S.L.; Boddeke, E.; Copray, S. Intraventricularly injected Olig2-NSCs attenuate established relapsing-remitting EAE in mice. Cell Transplant. 2012, 21, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Salewski, R.P.; Mitchell, R.A.; Li, L.; Shen, C.; Milekovskaia, M.; Nagy, A.; Fehlings, M.G. Transplantation of induced pluripotent stem cell-derived neural stem cells mediate functional recovery following thoracic spinal cord injury through remyelination of axons. Stem Cells Transl. Med. 2015, 4, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Salewski, R.P.; Mitchell, R.A.; Shen, C.; Fehlings, M.G. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015, 24, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Quattrini, A.; Brambilla, E.; Gritti, A.; Salani, G.; Dina, G.; Galli, R.; del Carro, U.; Amadio, S.; Bergami, A.; et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 2003, 422, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Jadasz, J.J.; Rivera, F.J.; Taubert, A.; Kandasamy, M.; Sandner, B.; Weidner, N.; Aktas, O.; Hartung, H.-P.; Aigner, L.; Küry, P. p57kip2 regulates glial fate decision in adult neural stem cells. Development 2012, 139, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Jadasz, J.J.; Lubetzki, C.; Zalc, B.; Stankoff, B.; Hartung, H.-P.; Küry, P. Recent achievements in stem cell-mediated myelin repair. Curr. Opin. Neurol. 2016, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Meamar, R.; Nematollahi, S.; Dehghani, L.; Mirmosayyeb, O.; Shayegannejad, V.; Basiri, K.; Tanhaei, A.P. The role of stem cell therapy in multiple sclerosis: An overview of the current status of the clinical studies. Adv. Biomed. Res. 2016, 5. [Google Scholar] [CrossRef]
- Atkins, H.L.; Bowman, M.; Allan, D.; Anstee, G.; Arnold, D.L.; Bence-Bruckler, I.; Birch, P.; Bredeson, C.; Chen, J.; Fergusson, D.; et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: A multicentre single-group phase 2 trial. Funding Mult. Scler. Sci. Res. Found. 2016, 388, 576–585. [Google Scholar] [CrossRef]
- Merkle, F.T.; Tramontin, A.D.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 2004, 101, 17528–17532. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, L.C.; Rompani, S.B.; Parraguez, J.I.; Obernier, K.; Romero, R.; Cepko, C.L.; Alvarez-Buylla, A. Embryonic origin of postnatal neural stem cells. Cell 2015, 161, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Codega, P.; Silva-Vargas, V.; Paul, A.; Maldonado-Soto, A.R.; de Leo, A.M.; Pastrana, E.; Doetsch, F. Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 2014, 82, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, F.; Michel, J.; Baumgart, E.V.; Theis, F.; Götz, M.; Ninkovic, J. Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 2015, 18, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Carleton, A.; Petreanu, L.T.; Lansford, R.; Alvarez-Buylla, A.; Lledo, P.-M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003, 6, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Liebl, J.; Bernard, S.; Alkass, K.; Yeung, M.S.Y.; Steier, P.; Kutschera, W.; Johnson, L.; Landén, M.; Druid, H.; et al. The age of olfactory bulb neurons in humans. Neuron 2012, 74, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisén, J. Neurogenesis in the striatum of the adult human brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Laywell, E.D.; Rakic, P.; Kukekov, V.G.; Holland, E.C.; Steindler, D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. USA 2000, 97, 13883–13888. [Google Scholar] [CrossRef] [PubMed]
- Brill, M.S.; Ninkovic, J.; Winpenny, E.; Hodge, R.D.; Ozen, I.; Yang, R.; Lepier, A.; Gascón, S.; Erdelyi, F.; Szabo, G.; et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 2009, 12, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Winpenny, E.; Lebel-Potter, M.; Fernandez, M.E.; Brill, M.S.; Götz, M.; Guillemot, F.; Raineteau, O. Sequential generation of olfactory bulb glutamatergic neurons by Neurog2-expressing precursor cells. Neural Dev. 2011, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Fuentealba, L.C.; Sanders, T.A.; Magno, L.; Kessaris, N.; Alvarez-Buylla, A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 2014, 17, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, R.; Azim, K.; Fischer, B.; Raineteau, O. Adding a spatial dimension to postnatal ventricular-subventricular zone neurogenesis. Development 2015, 142, 2109–2120. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, S.; Bovetti, S.; Carletti, B.; Hsieh, Y.-C.; Garzotto, D.; Peretto, P.; Fasolo, A.; Puche, A.C.; Rossi, F. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: Implication for intrinsic properties of the subventricular zone progenitor population. J. Neurosci. 2007, 27, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Merkle, F.T.; Mirzadeh, Z.; Alvarez-Buylla, A. Mosaic organization of neural stem cells in the adult brain. Science 2007, 317, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Young, K.M.; Merson, T.D.; Sotthibundhu, A.; Coulson, E.J.; Bartlett, P.F. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J. Neurosci. 2007, 27, 5146–5155. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell 2015, 17, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Hurtado-Chong, A.; Fischer, B.; Kumar, N.; Zweifel, S.; Taylor, V.; Raineteau, O. Transcriptional hallmarks of heterogeneous neural stem cell niches of the subventricular zone. Stem Cells 2015, 33, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Fischer, B.; Hurtado-Chong, A.; Draganova, K.; Cantù, C.; Zemke, M.; Sommer, L.; Butt, A.; Raineteau, O. Persistent Wnt/β-catenin signaling determines dorsalization of the postnatal subventricular zone and neural stem cell specification into oligodendrocytes and glutamatergic neurons. Stem Cells 2014, 32, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Rivera, A.; Raineteau, O.; Butt, A.M. GSK3β regulates oligodendrogenesis in the dorsal microdomain of the subventricular zone via Wnt-β-catenin signaling. Glia 2014, 62, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Gascón, S.; Masserdotti, G.; Deshpande, A.; Simon, C.; Fischer, J.; Dimou, L.; Chichung Lie, D.; Schroeder, T.; Berninger, B. Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat. Cell Biol. 2013, 15, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Nait-Oumesmar, B.; Decker, L.; Lachapelle, F.; Avellana-Adalid, V.; Bachelin, C.; Evercooren, V.; Baron, A. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999, 11, 4357–4366. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, H.; Martin, E.; Hassani, H.; Clavairoly, A.; Maire, C.L.; Viadieu, A.; Kerninon, C.; Delmasure, A.; Frah, M.; Weber, M.; et al. Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J. Neurosci. 2013, 33, 9752–9768. [Google Scholar] [CrossRef] [PubMed]
- Rafalski, V.A.; Ho, P.P.; Brett, J.O.; Ucar, D.; Dugas, J.C.; Pollina, E.A.; Chow, L.M.L.; Ibrahim, A.; Baker, S.J.; Barres, B.A.; et al. Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain. Nat. Cell Biol. 2013, 15, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Rivera, F.J.; Couillard-Despres, S.; Pedre, X.; Ploetz, S.; Caioni, M.; Lois, C.; Bogdahn, U.; Aigner, L. Mesenchymal stem cells instruct oligodendrogenic fate decision on adult neural stem cells. Stem Cells 2006, 24, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Steffenhagen, C.; Dechant, F.-X.; Oberbauer, E.; Furtner, T.; Weidner, N.; Küry, P.; Aigner, L.; Rivera, F.J. Mesenchymal stem cells prime proliferating adult neural progenitors toward an oligodendrocyte fate. Stem Cells Dev. 2012, 21, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Toni, N.; Clemenson, G.D.; Ray, J.; Gage, F.H. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat. Neurosci. 2008, 11, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.M.G.; Pilz, G.-A.; Machado, R.A.C.; Becher, B.; Toni, N.; Jessberger, S.; Moss, J. Programming hippocampal neural stem/progenitor cells into oligodendrocytes enhances remyelination in the adult brain after injury. Cell Rep. 2015, 11, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.J.; Zhou, Y.; Ito, S.; Bonaguidi, M.A.; Stein-O’Brien, G.; Kawasaki, N.K.; Modak, N.; Zhu, Y.; Ming, G.L.; Song, H. Latent tri-lineage potential of adult hippocampal neural stem cells revealed by Nf1 inactivation. Nat. Neurosci. 2015, 18, 1722–1724. [Google Scholar] [CrossRef] [PubMed]
- Rolando, C.; Erni, A.; Grison, A.; Beattie, R.; Engler, A.; Gokhale, P.J.; Milo, M.; Wegleiter, T.; Jessberger, S.; Taylor, V. Multipotency of adult hippocampal NSCs in vivo is restricted by Drosha/NFIB. Cell Stem Cell 2016, 19, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Chetty, S.; Friedman, A.R.; Taravosh-Lahn, K.; Kirby, E.D.; Mirescu, C.; Guo, F.; Krupik, D.; Nicholas, A.; Geraghty, A.C.; Krishnamurthy, A.; et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol. Psychiatry 2014, 19, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Ffrench-Constant, C. Remyelination in the CNS: From biology to therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Küry, P.; Dutta, R. Promoting remyelination in multiple sclerosis: Current drugs and future prospects. Mult. Scler. J. 2015, 21, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kremer, D.; Göttle, P.; Hartung, H.P.; Küry, P. Pushing forward: Remyelination as the new frontier in CNS diseases. Trends Neurosci. 2016, 39, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.H.; Tripathi, R.B.; Richardson, W.D.; Franklin, R.J.M. Developmental origin of oligodendrocyte lineage cells determines response to demyelination and susceptibility to age-associated functional decline. Cell Rep. 2016, 15, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, B.; Aguirre, A.; Raymond, M.; Szabo, G.; Kitabatake, Y.; Sailor, K.A.; Ming, G.-L.; Song, H.; Gallo, V. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci. 2010, 13, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Hack, M.A.; Saghatelyan, A.; de Chevigny, A.; Pfeifer, A.; Ashery-Padan, R.; Lledo, P.-M.; Götz, M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat. Neurosci. 2005, 8, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Maire, C.L.; Wegener, A.; Kerninon, C.; Oumesmar, B.N. Gain-of-function of olig transcription factors enhances oligodendrogenesis and myelination. Stem Cells 2010, 28, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Butt, A.M. GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 2011, 59, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Soundarapandian, M.M.; Selvaraj, V.; Lo, U.-G.; Golub, M.S.; Feldman, D.H.; Pleasure, D.E.; Deng, W. Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination. Sci. Rep. 2011, 1. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.K.; Joppé, S.E.; Cochard, L.M.; Fernandes, K.J.L. Aging and neurogenesis in the adult forebrain: What we have learned and where we should go from here. Eur. J. Neurosci. 2013, 37, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Bouab, M.; Paliouras, G.N.; Aumont, A.; Forest-Bérard, K.; Fernandes, K.J.L. Aging of the subventricular zone neural stem cell niche: Evidence for quiescence-associated changes between early and mid-adulthood. Neuroscience 2011, 173, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Capilla-Gonzalez, V.; Cebrian-Silla, A.; Guerrero-Cazares, H.; Garcia-Verdugo, J.M.; Quiñones-Hinojosa, A. The generation of oligodendroglial cells is preserved in the rostral migratory stream during aging. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [PubMed]
- Jaskelioff, M.; Muller, F.L.; Paik, J.-H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden, P.; Franklin, R.J.M. Ageing stem and progenitor cells: Implications for rejuvenation of the central nervous system. Development 2013, 140, 2562–2575. [Google Scholar] [CrossRef] [PubMed]
- Vernerey, J.; Macchi, M.; Magalon, K.; Cayre, M.; Durbec, P. Ciliary neurotrophic factor controls progenitor migration during remyelination in the adult rodent brain. J. Neurosci. 2013, 33, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Casarosa, S.; Fode, C.; Guillemot, F. Mash1 regulates neurogenesis in the ventral telencephalon. Development 1999, 126, 525–534. [Google Scholar] [PubMed]
- Cate, H.S.; Sabo, J.K.; Merlo, D.; Kemper, D.; Aumann, T.D.; Robinson, J.; Merson, T.D.; Emery, B.; Perreau, V.M.; Kilpatrick, T.J. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J. Neurochem. 2010, 115, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Sabo, J.K.; Aumann, T.D.; Merlo, D.; Kilpatrick, T.J.; Cate, H.S. Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions. J. Neurosci. 2011, 31, 4504–4510. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Dupree, J.L.; Mangin, J.M.; Gallo, V. A functional role for EGFR signaling in myelination and remyelination. Nat. Neurosci. 2007, 10, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, O.; Romero-Rodriguez, R.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells 2009, 27, 2032–2043. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Raineteau, O.; Butt, A.M. Intraventricular injection of FGF-2 promotes generation of oligodendrocyte-lineage cells in the postnatal and adult forebrain. Glia 2012, 60, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Göttle, P.; Sabo, J.K.; Heinen, A.; Venables, G.; Torres, K.; Tzekova, N.; Parras, C.M.; Kremer, D.; Hartung, H.-P.; Cate, H.S.; et al. Oligodendroglial maturation is dependent on intracellular protein shuttling. J. Neurosci. 2015, 35, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Choi, G.; Anderson, D.J. The bHLH transcription factor Olig2 Promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 2001, 31, 791–807. [Google Scholar] [CrossRef]
- Parras, C.M.; Hunt, C.; Sugimori, M.; Nakafuku, M.; Rowitch, D.; Guillemot, F. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J. Neurosci. 2007, 27, 4233–4242. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Grund, E.M.; Silva, H.M.; Lafaille, J.J.; Fishell, G.; Salzer, J.L. Inhibition of Gli1 mobilizes endogenous neural stem cells for remyelination. Nature 2015, 526, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Osinski, J.M.; Mateo, J.L.; Martynoga, B.; Sim, F.J.; Campbell, C.E.; Guillemot, F.; Piper, M.; Gronostajski, R.M. Loss of NFIX transcription factor biases postnatal neural stem/progenitor cells toward oligodendrogenesis. Stem Cells Dev. 2015, 24, 2114–2126. [Google Scholar] [CrossRef] [PubMed]
- Grebbin, B.M.; Hau, A.-C.; Groß, A.; Anders-Maurer, M.; Schramm, J.; Koss, M.; Wille, C.; Mittelbronn, M.; Selleri, L.; Schulte, D. Pbx1 is required for adult SVZ neurogenesis. Development 2016. [Google Scholar] [CrossRef] [PubMed]
- Bunk, E.C.; Ertaylan, G.; Ortega, F.; Pavlou, M.A.; Gonzalez-Cano, L.; Stergiopoulos, A.; Safaiyan, S.; Völs, S.; van Cann, M.; Politis, P.K.; et al. Prox1 is required for oligodendrocyte cell identity in adult neural stem cells of the subventricular zone. Stem Cells 2016. [Google Scholar] [CrossRef] [PubMed]
- Karalay, O.; Doberauer, K.; Vadodaria, K.C.; Knobloch, M.; Berti, L.; Miquelajauregui, A.; Schwark, M.; Jagasia, R.; Taketo, M.M.; Tarabykin, V.; et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sage, J.C.; Miller, M.R.; Verhaak, R.G.W.; Hippenmeyer, S.; Vogel, H.; Foreman, O.; Bronson, R.T.; Nishiyama, A.; Luo, L.; et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 2011, 146, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Jadasz, J.J.; Kremer, D.; Göttle, P.; Tzekova, N.; Domke, J.; Rivera, F.J.; Adjaye, J.; Hartung, H.P.; Aigner, L.; Küry, P. Mesenchymal stem cell conditioning promotes rat oligodendroglial cell maturation. PLoS ONE 2013, 8, e71814. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Muzio, L.; Imitola, J.; Deleidi, M.; Alfaro-Cervello, C.; Salani, G.; Porcheri, C.; Brambilla, E.; Cavasinni, F.; Bergamaschi, A.; et al. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain 2008, 131, 2564–2578. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Nemirovsky, A.; Harpaz, I.; Cohen, H.; Owens, T.; Monsonego, A. IFN-γ enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 2008, 22, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Tepavcevic, V.; Lazarini, F.; Alfaro-Cervello, C.; Kerninon, C.; Yoshikawa, K.; Garcia-Verdugo, J.M.; Lledo, P.M.; Nait-Oumesmar, B.; van Evercooren, A.B. Inflammation-induced subventricular zone dysfunction leads to olfactory deficits in a targeted mouse model of multiple sclerosis. J. Clin. Investig. 2011, 121, 4722–4734. [Google Scholar] [CrossRef] [PubMed]
- Covacu, R.; Danilov, A.I.; Rasmussen, B.S.; Hallen, K.; Moe, M.C.; Lobell, A.; Johansson, C.B.; Svensson, M.A.; Tomas, O.; Brundin, L. Nitric oxide exposure diverts neural stem cell fate from neurogenesis towards astrogliogenesis. Stem Cells 2006, 24, 2792–2800. [Google Scholar] [CrossRef] [PubMed]
- Bergsland, M.; Covacu, R.; Perez Estrada, C.; Svensson, M.; Brundin, L. Nitric oxide-induced neuronal to glial lineage fate-change depends on NRSF/REST function in neural progenitor cells. Stem Cells 2014, 32, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Perez Estrada, C.; Covacu, R.; Sankavaram, S.R.; Svensson, M.; Brundin, L. Oxidative stress increases neurogenesis and oligodendrogenesis in adult neural progenitor cells. Stem Cells Dev. 2014, 23, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Imitola, J.; Ayuso-Sacido, A.; Wang, Y.; Starossom, S.C.; Kivisäkk, P.; Zhu, B.; Meyer, M.; Bronson, R.T.; Garcia-Verdugo, J.M.; et al. Reversible neural stem cell niche dysfunction in a model of multiple sclerosis. Ann. Neurol. 2011, 69, 878–891. [Google Scholar] [CrossRef] [PubMed]
| Factor | Effect on NSCs | Same Effect on pOPCs? | References |
|---|---|---|---|
| Oligodendrocyte transcription factor 2 (Olig2) | ↑ oligodendrogenesis; increased myelination and remyelination | yes | [84,85,94,95] |
| Nk2 homeobox 2 (Nkx2.2) | ↑ oligodendrogenesis; increased myelination and remyelination | yes | [112] |
| SRY-Box 10 (Sox10) | ↑ oligodendrogenesis and remyelination | yes | [84,85] |
| Achaete-scute homolog 1 (Ascl1)/Mash1 | ↑ oligodendrogenesis and remyelination | yes | [80,84,85,113] |
| Zinc finger protein 488 (Zfp488) | ↑ oligodendrogenesis | yes | [97] |
| p57kip2 (Cdkn1c) (inhibitory) | ↑ oligodendrogenesis upon p57kip2 suppression | yes | [54,111] |
| Gli1 (inhibitory) | ↑ recruitment and oligodendrogenesis upon Gli1 inhibition | no | [114] |
| Sirtuin 1 (Sirt1) (inhibitory) | ↑ oligodendrogenesis and remyelination upon Sirt1 inactivation | no | [81] |
| Nuclear factor I X (NFIX) (inhibitory) | ↑ oligodendrogenesis upon NFIX deletion | no | [115] |
| B-cell leukemia homeodomain 1 (Pbx1) (inhibitory; SVZ specific) | ↑ oligodendrogenesis upon Pbx1 deletion | no | [116] |
| Prospero-related homeobox 1 gene (Prox1) | ↑ oligodendrogenesis in SVZ (but ↓ oligodendrogenesis in SGZ) | no | [117,118] |
| Drosha (inhibitory)/ Nuclear factor IB (NFIB) | ↑ oligodendrogenesis upon Drosha deletion, via relieve of NFIB repression | no | [87] |
| Neurofibromin 1 (Nf1) (inhibitory) | ↑ oligodendrogenesis upon Nf1 deletion | to some extent | [86,119] |
| Factor | Effect on NSCs | Same Effect on pOPCs? | References |
|---|---|---|---|
| Ciliary neurotrophic factor (CNTF) | ↑ migration toward demyelinated lesions | yes | [104] |
| Epidermal growth factor receptor (EGFR) | ↑ oligodendrogenesis, migration toward demyelinated lesions and increased remyelination | yes | [108,109] |
| Fibroblast growth factor receptor (FGFR) | ↑ oligodendrogenesis (differential expression of FGFR1/2 and FGFR3 in dorsal and lateral SVZ, respectively) | yes | [110] |
| Wnt/β-catenin | ↑ oligodendrogenesis in dorsal SVZ | yes | [76,77,78] |
| Bone morphogenic proteins (BMPs) (inhibitory) | ↑ oligodendrogenesis and remyelination upon BMP inhibition | yes | [93,107] |
| Mesenchymal stem cell (MSC) conditioned medium | ↑ oligodendrogenesis | yes | [82,120] |
| Corticosterone | ↑ oligodendrogenesis | yes | [88] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkermann, R.; Jadasz, J.J.; Azim, K.; Küry, P. Taking Advantage of Nature’s Gift: Can Endogenous Neural Stem Cells Improve Myelin Regeneration? Int. J. Mol. Sci. 2016, 17, 1895. https://doi.org/10.3390/ijms17111895
Akkermann R, Jadasz JJ, Azim K, Küry P. Taking Advantage of Nature’s Gift: Can Endogenous Neural Stem Cells Improve Myelin Regeneration? International Journal of Molecular Sciences. 2016; 17(11):1895. https://doi.org/10.3390/ijms17111895
Chicago/Turabian StyleAkkermann, Rainer, Janusz Joachim Jadasz, Kasum Azim, and Patrick Küry. 2016. "Taking Advantage of Nature’s Gift: Can Endogenous Neural Stem Cells Improve Myelin Regeneration?" International Journal of Molecular Sciences 17, no. 11: 1895. https://doi.org/10.3390/ijms17111895
APA StyleAkkermann, R., Jadasz, J. J., Azim, K., & Küry, P. (2016). Taking Advantage of Nature’s Gift: Can Endogenous Neural Stem Cells Improve Myelin Regeneration? International Journal of Molecular Sciences, 17(11), 1895. https://doi.org/10.3390/ijms17111895