Severe Cutaneous Adverse Reactions: The Pharmacogenomics from Research to Clinical Implementation
Abstract
:1. Introduction
2. Genetic Susceptibility to SCARs
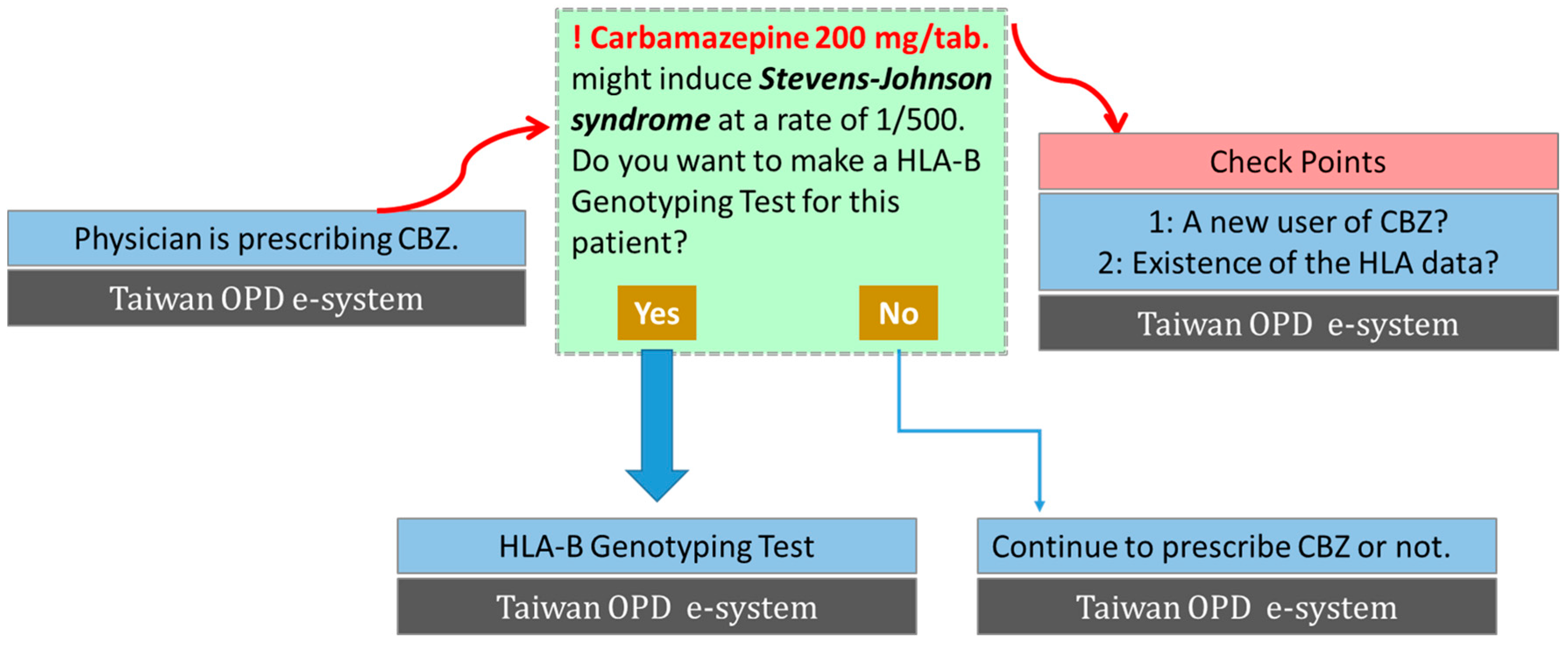
3. Clinical Implementation of Genetic Screening to Prevent SCARs
4. Causative Drug(s) Identification
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DRESS | drug reaction with eosinophilia and systemic symptoms |
| HLA | human leukocyte antigen |
| LTT | lymphocyte transformation test |
| SCAR | severe cutaneous adverse reaction |
| SJS | Stevens–Johnson syndrome |
| TEN | toxic epidermal necrolysis |
References
- Wolf, R.; Orion, E.; Marcos, B.; Matz, H. Life-threatening acute adverse cutaneous drug reactions. Clin. Dermatol. 2005, 23, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Stern, R.S.; Arndt, K.A.; Langlois, J.; Jick, S.S.; Jick, H.; Walker, A.M. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis. A population-based study with particular reference to reactions caused by drugs among outpatients. Arch. Dermatol. 1990, 126, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Di Pascuale, M.A.; Espana, E.M.; Liu, D.T.; Kawakita, T.; Li, W.; Gao, Y.Y.; Baradaran-Rafii, A.; Elizondo, A.; Raju, V.K.; Tseng, S.C. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology 2005, 112, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Hoetzenecker, W.; Nageli, M.; Mehra, E.T.; Jensen, A.N.; Saulite, I.; Schmid-Grendelmeier, P.; Guenova, E.; Cozzio, A.; French, L.E. Adverse cutaneous drug eruptions: Current understanding. Semin. Immunopathol. 2016, 38, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Chang, W.C.; Lee, Y.S.; Wu, Y.Y.; Yang, C.H.; Ho, H.C.; Chen, M.J.; Lin, J.Y.; Hui, R.C.; Ho, J.C.; et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA 2014, 312, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Chang, W.C.; Stocker, S.L.; Juo, C.G.; Graham, G.G.; Lee, M.H.; Williams, K.M.; Tian, Y.C.; Juan, K.C.; Wu, Y.J.; et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: The impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann. Rheum. Dis. 2015, 74, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, M.; Illing, P.; Theodossis, A.; Purcell, A.W.; Rossjohn, J.; McCluskey, J. Drug hypersensitivity and human leukocyte antigens of the major histocompatibility complex. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 401–431. [Google Scholar] [CrossRef] [PubMed]
- Camous, X.; Calbo, S.; Picard, D.; Musette, P. Drug reaction with eosinophilia and systemic symptoms: An update on pathogenesis. Curr. Opin. Immunol. 2012, 24, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Chung, W.H. Update on pathobiology in Stevens-Johnson syndrome and toxic epidermal necrolysis. Dermatol. Sin. 2013, 31, 175–180. [Google Scholar] [CrossRef]
- Yun, J.; Cai, F.; Lee, F.J.; Pichler, W.J. T-cell-mediated drug hypersensitivity: Immune mechanisms and their clinical relevance. Asia Pac. Allergy 2016, 6, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Wang, C.W.; Dao, R.L. Severe cutaneous adverse drug reactions. J. Dermatol. 2016, 43, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Pohl, L.R.; Satoh, H.; Christ, D.D.; Kenna, J.G. The immunologic and metabolic basis of drug hypersensitivities. Annu. Rev. Pharmacol. Toxicol. 1988, 28, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Padovan, E.; Bauer, T.; Tongio, M.M.; Kalbacher, H.; Weltzien, H.U. Penicilloyl peptides are recognized as T cell antigenic determinants in penicillin allergy. Eur. J. Immunol. 1997, 27, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J. Pharmacological interaction of drugs with antigen-specific immune receptors: The p-i concept. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Illing, P.T.; Vivian, J.P.; Dudek, N.L.; Kostenko, L.; Chen, Z.; Bharadwaj, M.; Miles, J.J.; Kjer-Nielsen, L.; Gras, S.; Williamson, N.A.; et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature 2012, 486, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Grant, B.J.; Pompeu, Y.A.; Sidney, J.; Harndahl, M.; Southwood, S.; Oseroff, C.; Lu, S.; Jakoncic, J.; de Oliveira, C.A.; et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc. Natl. Acad. Sci. USA 2012, 109, 9959–9964. [Google Scholar] [CrossRef] [PubMed]
- Norcross, M.A.; Luo, S.; Lu, L.; Boyne, M.T.; Gomarteli, M.; Rennels, A.D.; Woodcock, J.; Margulies, D.H.; McMurtrey, C.; Vernon, S.; et al. Abacavir induces loading of novel self-peptides into HLA-B*57:01: An autoimmune model for HLA-associated drug hypersensitivity. AIDS (London, England) 2012, 26, F21–F29. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Chung, W.H. Cytotoxic proteins and therapeutic targets in severe cutaneous adverse reactions. Toxins 2014, 6, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.C.; Huynh, T.N.; Bracq, C.; Guillaume, J.C.; Revuz, J.; Touraine, R. Genetic susceptibility to toxic epidermal necrolysis. Arch. Dermatol. 1987, 123, 1171–1173. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Hung, S.I.; Hong, H.S.; Hsih, M.S.; Yang, L.C.; Ho, H.C.; Wu, J.Y.; Chen, Y.T. Medical genetics: A marker for Stevens-Johnson syndrome. Nature 2004, 428, 486. [Google Scholar] [CrossRef] [PubMed]
- Man, C.B.; Kwan, P.; Baum, L.; Yu, E.; Lau, K.M.; Cheng, A.S.; Ng, M.H. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia 2007, 48, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Too, C.L.; Murad, S.; Hussein, S.H. Association of HLA-B*1502 allele with carbamazepine-induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int. J. Dermatol. 2011, 50, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Locharernkul, C.; Loplumlert, J.; Limotai, C.; Korkij, W.; Desudchit, T.; Tongkobpetch, S.; Kangwanshiratada, O.; Hirankarn, N.; Suphapeetiporn, K.; Shotelersuk, V. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia 2008, 49, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Mehta, T.Y.; Prajapati, L.M.; Mittal, B.; Joshi, C.G.; Sheth, J.J.; Patel, D.B.; Dave, D.M.; Goyal, R.K. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, K.W.; Song, W.J.; Jee, Y.K.; Lee, S.M.; Kang, H.R.; Park, H.W.; Cho, S.H.; Park, S.H.; Min, K.U.; et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011, 97, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, A.; Jorgensen, A.L.; Williamson, P.R.; Chadwick, D.W.; Park, B.K.; Pirmohamed, M. HLA-B locus in Caucasian patients with carbamazepine hypersensitivity. Pharmacogenomics 2006, 7, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Kaniwa, N.; Saito, Y.; Aihara, M.; Matsunaga, K.; Tohkin, M.; Kurose, K.; Sawada, J.; Furuya, H.; Takahashi, Y.; Muramatsu, M.; et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics 2008, 9, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Alfirevic, A.; Bourgeois, S.; Farrell, J.J.; Kasperaviciute, D.; Carrington, M.; Sills, G.J.; Marson, T.; Jia, X.; de Bakker, P.I.; et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N. Engl. J. Med. 2011, 364, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Mushiroda, T.; Yowang, A.; Takahashi, A.; Kubo, M.; Shirakata, Y.; Ikezawa, Z.; Iijima, M.; Shiohara, T.; Hashimoto, K.; et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum. Mol. Genet. 2011, 20, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Liou, L.B.; Chu, C.C.; Lin, M.; Huang, H.P.; Lin, Y.L.; Lan, J.L.; Yang, L.C.; Hong, H.S.; et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc. Natl. Acad. Sci. USA 2005, 102, 4134–4139. [Google Scholar] [CrossRef] [PubMed]
- Tassaneeyakul, W.; Jantararoungtong, T.; Chen, P.; Lin, P.Y.; Tiamkao, S.; Khunarkornsiri, U.; Chucherd, P.; Konyoung, P.; Vannaprasaht, S.; Choonhakarn, C.; et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet. Genom. 2009, 19, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Lonjou, C.; Borot, N.; Sekula, P.; Ledger, N.; Thomas, L.; Halevy, S.; Naldi, L.; Bouwes-Bavinck, J.N.; Sidoroff, A.; de Toma, C.; et al. A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet. Genom. 2008, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.R.; Liu, H.; Irwanto, A.; Fu, X.A.; Li, Y.; Yu, G.Q.; Yu, Y.X.; Chen, M.F.; Low, H.Q.; Li, J.H.; et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N. Engl. J. Med. 2013, 369, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Kaniwa, N.; Saito, Y.; Aihara, M.; Matsunaga, K.; Tohkin, M.; Kurose, K.; Furuya, H.; Takahashi, Y.; Muramatsu, M.; Kinoshita, S.; et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia 2010, 51, 2461–2465. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Jee, S.H.; Chen, W.C.; Chang, Y.T.; Lee, W.R.; Hu, S.L.; Wu, M.T.; Chen, G.S.; Wong, T.W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet. Genom. 2006, 16, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.I.; Chung, W.H.; Liu, Z.S.; Chen, C.H.; Hsih, M.S.; Hui, R.C.; Chu, C.Y.; Chen, Y.T. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics 2010, 11, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Mallal, S.; Nolan, D.; Witt, C.; Masel, G.; Martin, A.M.; Moore, C.; Sayer, D.; Castley, A.; Mamotte, C.; Maxwell, D.; et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 2002, 359, 727–732. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.; Lee, K.W.; Kim, S.H.; Kang, H.R.; Park, H.W.; Jee, Y.K. HLA-B*5901 is strongly associated with methazolamide-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. Pharmacogenomics 2010, 11, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Van Booven, D.; Marsh, S.; McLeod, H.; Carrillo, M.W.; Sangkuhl, K.; Klein, T.E.; Altman, R.B. Cytochrome P450 2C9-CYP2C9. Pharmacogenet. Genom. 2010, 20, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Tan, T. HLA and allopurinol drug eruption. Dermatologica 1989, 179, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Lonjou, C.; Thomas, L.; Borot, N.; Ledger, N.; de Toma, C.; LeLouet, H.; Graf, E.; Schumacher, M.; Hovnanian, A.; Mockenhaupt, M.; et al. A marker for Stevens-Johnson syndrome ...: Ethnicity matters. Pharmacogenom. J. 2006, 6, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.; Chen, D.P.; Hung, S.I.; Sekula, P.; Schumacher, M.; Chang, P.Y.; Tsai, S.H.; Wu, T.L.; Bellon, T.; Tamouza, R.; et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: An international study and meta-analysis. Pharmacogenom. J. 2014, 14, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, K.; Sumikawa, Y.; Niihara, H.; Morita, E. Case of carbamazepine-induced hypersensitivity syndrome associated with human leukocyte antigen-A*3101. J. Dermatol. 2012, 39, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.A.; Seshadri, S.; Bis, J.C.; Fornage, M.; DeStefano, A.L.; Aulchenko, Y.S.; Debette, S.; Lumley, T.; Folsom, A.R.; van den Herik, E.G.; et al. Genomewide association studies of stroke. N. Engl. J. Med. 2009, 360, 1718–1728. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, S.; Hughes, A.R.; Mosteller, M.; Shortino, D.; Baker, K.L.; Spreen, W.; Lai, E.; Davies, K.; Handley, A.; Dow, D.J.; et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet 2002, 359, 1121–1122. [Google Scholar] [CrossRef]
- Martin, A.M.; Nolan, D.; Gaudieri, S.; Almeida, C.A.; Nolan, R.; James, I.; Carvalho, F.; Phillips, E.; Christiansen, F.T.; Purcell, A.W.; et al. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proc. Natl. Acad. Sci. USA 2004, 101, 4180–4185. [Google Scholar] [CrossRef] [PubMed]
- Mallal, S.; Phillips, E.; Carosi, G.; Molina, J.M.; Workman, C.; Tomazic, J.; Jagel-Guedes, E.; Rugina, S.; Kozyrev, O.; Cid, J.F.; et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008, 358, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Nolan, D.; James, I.; Cameron, P.; Keller, J.; Moore, C.; Phillips, E.; Christiansen, F.T.; Mallal, S. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. Aids 2005, 19, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Littera, R.; Carcassi, C.; Masala, A.; Piano, P.; Serra, P.; Ortu, F.; Corso, N.; Casula, B.; La Nasa, G.; Contu, L.; et al. HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. Aids 2006, 20, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lin, J.J.; Lu, C.S.; Ong, C.T.; Hsieh, P.F.; Yang, C.C.; Tai, C.T.; Wu, S.L.; Lu, C.H.; Hsu, Y.C.; et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N. Engl. J. Med. 2011, 364, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liew, D.; Kwan, P. Effects of a HLA-B*15:02 screening policy on antiepileptic drug use and severe skin reactions. Neurology 2014, 83, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chen, C.H.; Deng, S.T.; Huang, C.S.; Lin, Y.J.; Chen, Y.J.; Wu, C.Y.; Hung, S.I.; Chung, W.H. Allopurinol use and risk of fatal hypersensitivity reactions: A nationwide population-based study in Taiwan. JAMA Intern. Med. 2015, 175, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Fitzgerald, J.D.; Khanna, P.P.; Bae, S.; Singh, M.K.; Neogi, T.; Pillinger, M.H.; Merill, J.; Lee, S.; Prakash, S.; et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012, 64, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Ko, T.M.; Tsai, C.Y.; Chen, S.Y.; Chen, K.S.; Yu, K.H.; Chu, C.S.; Huang, C.M.; Wang, C.R.; Weng, C.T.; Yu, C.L.; et al. Use of HLA-B*58:01 genotyping to prevent allopurinol induced severe cutaneous adverse reactions in Taiwan: National prospective cohort study. BMJ 2015, 351, h4848. [Google Scholar] [CrossRef] [PubMed]
- Karlin, E.; Phillips, E. Genotyping for severe drug hypersensitivity. Curr. Allergy Asthma Rep. 2014, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.C.; Stern, R.S. Severe adverse cutaneous reactions to drugs. N. Engl. J. Med. 1994, 331, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Mockenhaupt, M.; Viboud, C.; Dunant, A.; Naldi, L.; Halevy, S.; Bouwes Bavinck, J.N.; Sidoroff, A.; Schneck, J.; Roujeau, J.C.; Flahault, A. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J. Investig. Dermatol. 2008, 128, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.C.; Kelly, J.P.; Naldi, L.; Rzany, B.; Stern, R.S.; Anderson, T.; Auquier, A.; Bastuji-Garin, S.; Correia, O.; Locati, F.; et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N. Engl. J. Med. 1995, 333, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Rzany, B.; Correia, O.; Kelly, J.P.; Naldi, L.; Auquier, A.; Stern, R. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: A case-control study. Study Group of the International Case Control Study on Severe Cutaneous Adverse Reactions. Lancet 1999, 353, 2190–2194. [Google Scholar] [CrossRef]
- Letko, E.; Papaliodis, D.N.; Papaliodis, G.N.; Daoud, Y.J.; Ahmed, A.R.; Foster, C.S. Stevens-Johnson syndrome and toxic epidermal necrolysis: A review of the literature. Ann. Allergy Asthma Immunol. 2005, 94, 419–436. [Google Scholar] [CrossRef]
- Lin, Y.F.; Yang, C.H.; Sindy, H.; Lin, J.Y.; Rosaline Hui, C.Y.; Tsai, Y.C.; Wu, T.S.; Huang, C.T.; Kao, K.C.; Hu, H.C.; et al. Severe cutaneous adverse reactions related to systemic antibiotics. Clin. Infect. Dis. 2014, 58, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Rive, C.M.; Bourke, J.; Phillips, E.J. Testing for drug hypersensitivity syndromes. Clin. Biochem. Rev. 2013, 34, 15–38. [Google Scholar] [PubMed]
- Elzagallaai, A.A.; Rieder, M.J. In vitro testing for diagnosis of idiosyncratic adverse drug reactions: Implications for pathophysiology. Br. J. Clin. Pharmacol. 2015, 80, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Hirahara, K.; Mitsuyama, Y.; Takahashi, R.; Shiohara, T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: Dependence on its timing and the type of drug eruption. Allergy 2007, 62, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Nagao-Dias, A.T.; Teixeira, F.M.; Coelho, H.L. Diagnosing immune-mediated reactions to drugs. Allergol. Immunopathol. 2009, 37, 98–104. [Google Scholar] [CrossRef]
- Chung, W.H.; Hung, S.I.; Yang, J.Y.; Su, S.C.; Huang, S.P.; Wei, C.Y.; Chin, S.W.; Chiou, C.C.; Chu, S.C.; Ho, H.C.; et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat. Med. 2008, 14, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Abe, R.; Yoshioka, N.; Murata, J.; Fujita, Y.; Shimizu, H. Prolonged elevation of serum granulysin in drug-induced hypersensitivity syndrome. Br. J. Dermatol. 2012, 167, 452–453. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Pan, R.Y.; Chu, M.T.; Chin, S.W.; Huang, Y.L.; Wang, W.C.; Chang, J.Y.; Hung, S.I. Oxypurinol-specific T cells possess preferential TCR clonotypes and express granulysin in allopurinol-induced severe cutaneous adverse reactions. J. Investig. Dermatol. 2015, 135, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, M.R.; Berti, A.; Campochiaro, C.; Tombetti, E.; Ramirez, G.A.; Nico, A.; Di Leo, E.; Fantini, P.; Sabbadini, M.G.; Nettis, E.; et al. Drug induced exfoliative dermatitis: State of the art. Clin. Mol. Allergy 2016, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Viard, I.; Wehrli, P.; Bullani, R.; Schneider, P.; Holler, N.; Salomon, D.; Hunziker, T.; Saurat, J.H.; Tschopp, J.; French, L.E. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998, 282, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Sanclemente, G.; de la Roche, C.A.; Escobar, C.E.; Falabella, R. Pentoxyfylline in toxic epidermal necrolysis and Stevens-Johnson syndrome. Int. J. Dermatol. 1999, 38, 878–879. [Google Scholar] [PubMed]
- Wolkenstein, P.; Latarjet, J.; Roujeau, J.C.; Duguet, C.; Boudeau, S.; Vaillant, L.; Maignan, M.; Schuhmacher, M.H.; Milpied, B.; Pilorget, A.; et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998, 352, 1586–1589. [Google Scholar] [CrossRef]
- Fischer, M.; Fiedler, E.; Marsch, W.C.; Wohlrab, J. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br. J. Dermatol. 2002, 146, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.I. HLA-B*5801: Utility and cost-effectiveness in the Asia-Pacific Region. Int. J. Rheum. Dis. 2013, 16, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Vilar, F.J.; Ward, C.C.; Alfirevic, A.; Park, B.K.; Pirmohamed, M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogenetics 2004, 14, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Sung, C.; Finkelstein, E.A. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 2012, 79, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
| Causative Drug | SCAR Type | HLA Allele | Ancestry | Region | Reference |
|---|---|---|---|---|---|
| Allopurinol | SJS/TEN/DRESS | B*58:01 | Han Chinese | Taiwan | [30] |
| Caucasian | Europe | [32] | |||
| Thai | Thailand | [31] | |||
| Japanese | Japan | [27] | |||
| Drug eruption | Aw33, B17/Bw58 | Southern Chinese | Singapore | [40] | |
| Carbamazepine | SJS/TEN | B*15:02 | Han Chinese | Taiwan | [20,35] |
| Han Chinese | Hong Kong | [21] | |||
| Thai | Thailand | [23] | |||
| Malaysian | Malaysia | [22] | |||
| Asian | Southeastern countries | [26,41] | |||
| Indian | India | [24] | |||
| SJS/TEN | B*15:11 | Japanese | Japan | [34] | |
| SJS/TEN | B*59:01 | Japanese | Japan | [38] | |
| SJS | B44 | Korean | Korea | [38] | |
| Caucasian | Europe | [26,41] | |||
| MPE/DRESS, DRESS | A*31:01 | Han Chinese | Taiwan | [35,42] | |
| Caucasian | Europe | [28] | |||
| Japanese | Japan | [29,43] | |||
| Oxcarbazepine | SJS/TEN | B*15:02, B*15:18 | Han Chinese, Taiwanese | Taiwan | [36,44] |
| Phenytoin | SJS/TEN | B*15:02 | Han Chinese, Thai | Hong Kong, Thailand, Taiwan | [5,21,23] |
| Abacavir | HSS/MPE | B*57:01 | Western Australian, Caucasian | Australia, United States | [37,45,46,47] |
| Nevirapine | DRESS | DRB1*01:01 Cw8-B14 | Hispanics, African Caucasian | Africa Italy | [48] [49] |
| Dapsone | HSS | A*13:01 | Han Chinese | China | [33] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.-C.; Hung, S.-I.; Fan, W.-L.; Dao, R.-L.; Chung, W.-H. Severe Cutaneous Adverse Reactions: The Pharmacogenomics from Research to Clinical Implementation. Int. J. Mol. Sci. 2016, 17, 1890. https://doi.org/10.3390/ijms17111890
Su S-C, Hung S-I, Fan W-L, Dao R-L, Chung W-H. Severe Cutaneous Adverse Reactions: The Pharmacogenomics from Research to Clinical Implementation. International Journal of Molecular Sciences. 2016; 17(11):1890. https://doi.org/10.3390/ijms17111890
Chicago/Turabian StyleSu, Shih-Chi, Shuen-Iu Hung, Wen-Lang Fan, Ro-Lan Dao, and Wen-Hung Chung. 2016. "Severe Cutaneous Adverse Reactions: The Pharmacogenomics from Research to Clinical Implementation" International Journal of Molecular Sciences 17, no. 11: 1890. https://doi.org/10.3390/ijms17111890
APA StyleSu, S.-C., Hung, S.-I., Fan, W.-L., Dao, R.-L., & Chung, W.-H. (2016). Severe Cutaneous Adverse Reactions: The Pharmacogenomics from Research to Clinical Implementation. International Journal of Molecular Sciences, 17(11), 1890. https://doi.org/10.3390/ijms17111890







